Submitted:
20 March 2025
Posted:
20 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Serum and Tissues Samples
2.2. Serum Concentrations of HE4
2.3. RNAscope In Situ Hybridization (ISH) Assay
2.4. Immunohistochemistry
2.5. Clinicopathological Characteristics of DCIS Patients
2.6. HE4 as Prognostic Biomarker in Patients with Breast Cancer Using BreastMark
2.7. Statistical Analysis
3. Results
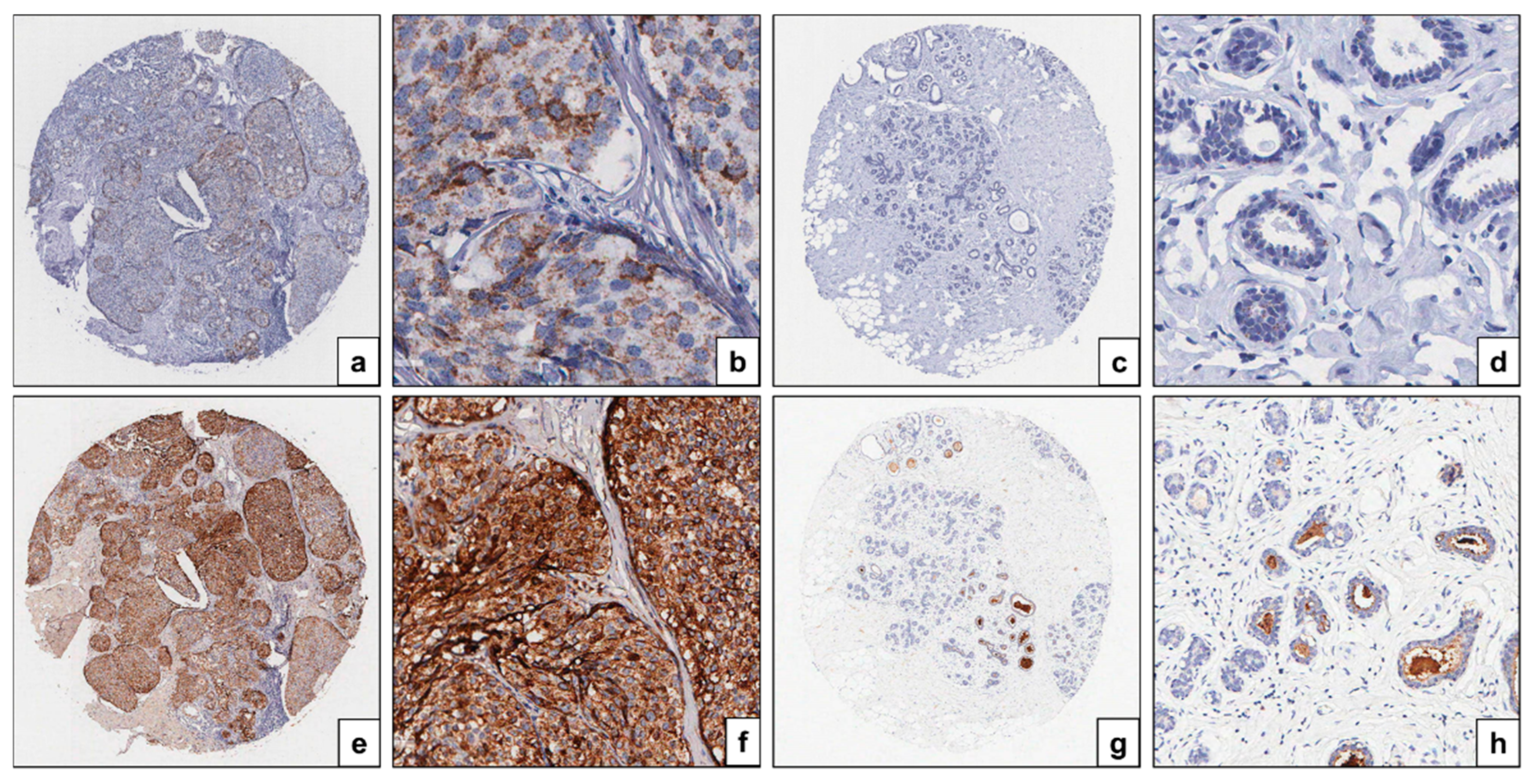
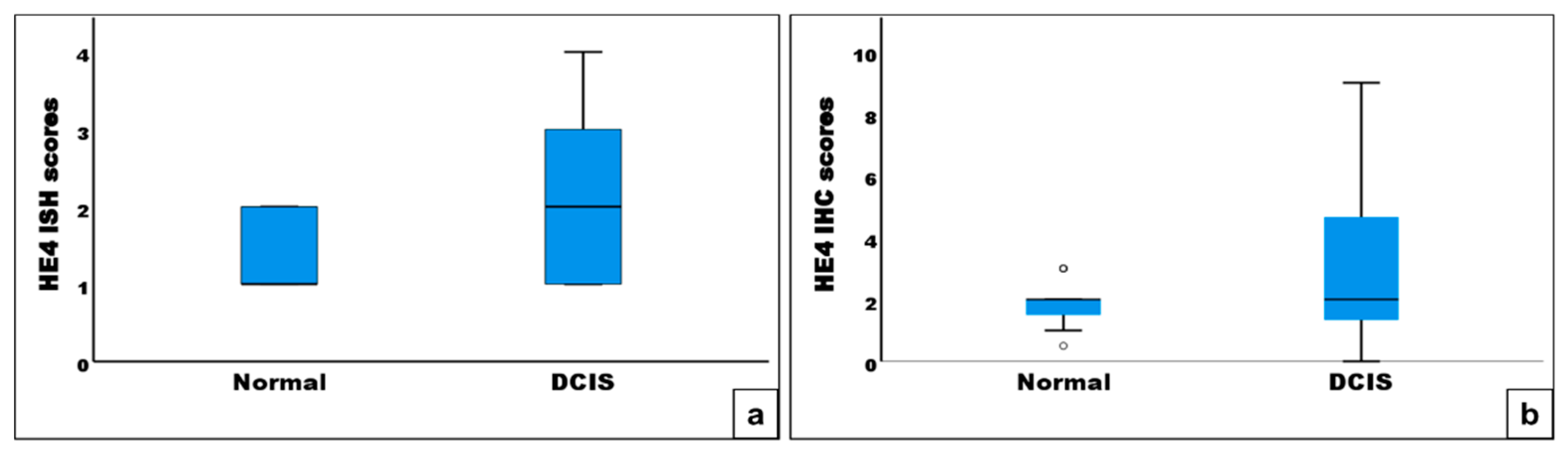
3.1. HE4 Serum Level in Patients with DCIS and HE4 mRNA and Protein Expression in DCIS Tissues and Their Adjacent Normal Breast Tissues
3.2. Expression of HE4 mRNA and Protein in DCIS Tissues and Correlation with Clinicopathological Features
3.2.1. Clinical Characteristics of DCIS Patient Cohorts
3.2.2. Expression of HE4 mRNA and Protein in DCIS Tissues
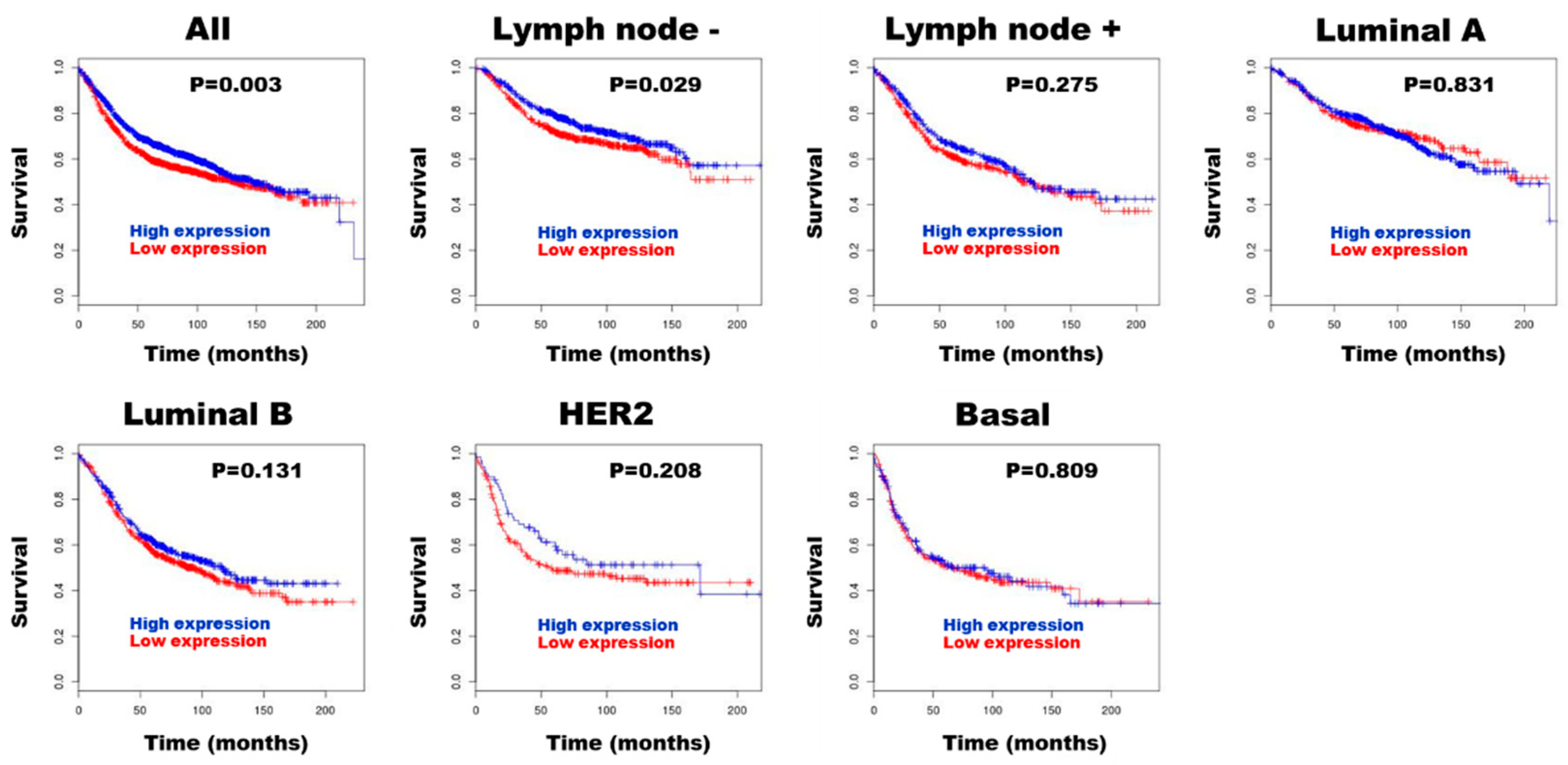
3.3. HE4 for Prognostic Biomarker in Patients with Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HE4 | Human epididymis protein 4 |
| DCIS | Ductal carcinoma in situ |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| WAP | Whey acidic protein |
| ISH | In situ hybridization |
| FFPE | Formalin-fixed-paraffin-embedded |
| CNUHH | Chonnam National University Hwasun Hospital |
| TILs | Tumor-infiltrating lymphocytes |
References
- Pinder, S.E.; Collins, L.C.; Fox, S.B.; Schnitt, S.J.; van Deurzen, C.H.M.; Weaver, D.L.; Wesseling, J. Ductal carcinoma in situ. In WHO Classification of Tumors Editorial Board. Breast Tumours. WHO Classification of Tumors, 5th ed.; Allison, K.H., Brogi, E., Ellis, I.O., Fox, S.B., Morris, E.A., Sahin, A., Salgado, R., Sapino, A., Sasano, H., Schnit, S.J., Sotiriou, C., van Diest, P.J., Eds.; IARC: Lyon, France, 2019; pp. 76–81. [Google Scholar]
- Hoda, S.A. Ductal carcinoma in situ. In Rosen’s Breast Pathology, 5th ed.; Hoda, S.A., Brogi, E., Koerner, F.C., Rosen, P.P., Eds.; Wolters Kluwer: Philadelphia, 2021; pp. 363–450. [Google Scholar]
- Cha,C.D.; Park, C.S.; Shin, H.C.; Han, J.; Choi, J.E.; Kim, J.H.; Jung, K.W.; Lee, S.B.; Nam, S.E.; Yoon, T.I.; et al. Breast cancer statistics in Korea, 2021. J. Breast. Cancer 2024, 27, 351–361.
- Lacombe, J.; Mangé, A.; Bougnoux, A.C.; Prassas, I.; Solassol, J. A multiparametric serum marker panel as a complementary test to mammography for the diagnosis of node-negative early-stage breast cancer and DCIS in young women. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1834–1842. [Google Scholar] [CrossRef]
- Bingle, L.; Singleton, V.; Bingle, C.D. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene 2002, 21, 2768–2773. [Google Scholar] [CrossRef] [PubMed]
- Bingle, L.; Cross, S.S.; High, A.S.; Wallace, W.A.; Rassl, D.; Yuan, G.; Hellstrom, I.; Campos, M.A.; Bingle, C.D. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir. Res. 2006, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Chichester, C.; Ribeiro, J.R. Beyond the biomarker: understanding the diverse roles of human epididymis protein 4 in the pathogenesis of epithelial ovarian cancer. Front. Oncol. 2018, 8, 124. [Google Scholar] [CrossRef]
- Capriglione, S.; Plotti, F.; Miranda, A.; Lopez, S.; Scaletta, G.; Moncelli, M.; Luvero, D.; De Cicco Nardone, C.; Terranova, C.; Montera, R.; et al. Further insight into prognostic factors in endometrial cancer: the new serum biomarker HE4. Expert Rev. Anticancer Ther. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- He, Y.P.; Li, L.X.; Tang, J.X.; Yi, L.; Zhao, Y.; Zhang, H.W.; Wu, Z.J.; Lei, H.K.; Yu, H.Q.; Nian, W.Q.; Gan, L. HE4 as a biomarker for diagnosis of lung cancer: A meta-analysis. Medicine (Baltimore). 2019, 98, e17198. [Google Scholar] [CrossRef]
- Cao, H.; You, D.; Lan, Z.; Ye, H.; Hou, M.; Xi, M. Prognostic value of serum and tissue HE4 expression in ovarian cancer: a systematic review with meta-analysis of 90 studies. Expert Rev. Mol. Diagn. 2018, 18, 371–383. [Google Scholar] [CrossRef]
- Zhu, L.; Gou, R.; Guo, Q.; Wang, J.; Liu, Q.; Lin, B. High expression and potential synergy of human epididymis protein 4 and Annexin A8 promote progression and predict poor prognosis in epithelial ovarian cancer. Am. J. Transl. Res. 2020, 12, 4017–4030. [Google Scholar]
- Uno, M.; Matsuo, R.; Maezawa, N.; Kato, T. Evaluation of follow-up observation using human epididymis protein 4, a tumor marker, in patients with ovarian cancer. Obstet. Gynecol. Sci. 2023, 66, 290–299. [Google Scholar] [CrossRef]
- Hertlein, L.; Stieber, P.; Kirschenhofer, A.; Krocker, K.; Nagel, D.; Lenhard, M.; Burges, A. Human epididymis protein 4 (HE4) in benign and malignant diseases. Clin. Chem. Lab. Med. 2012, 50, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Durur-Karakaya, A.; Durur-Subasi, I.; Karaman, A.; Akcay, M.N.; Palabiyik, S.S.; Erdemci, B.; Alper, F.; Acemoglu, H. The use of breast magnetic resonance imaging parameters to identify possible signaling pathways of a serum biomarker, HE4. J. Comput. Assist. Tomogr. 2016, 40, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, U.R.; Gunaldi, M.; Isiksacan, N.; Gündüz, S.; Okuturlar, Y.; Kocoglu, H. A new marker for breast cancer diagnosis, human epididymis protein 4: A preliminary study. Mol. Clin. Oncol. 2016, 5, 355–360. [Google Scholar] [CrossRef]
- Lu, M.; Ju, S.; Shen, X.; Wang, X.; Jing, R.; Yang, C.; Chu, H.; Cong, H. Combined detection of plasma miR-127-3p and HE4 improves the diagnostic efficacy of breast cancer. Cancer Biomark. 2017, 18, 143–148. [Google Scholar] [CrossRef]
- Sai Baba, K.S.S.; Rehman, M.A.; Pradeep Kumar, J.; Fatima, M.; Raju, G.S.N.; Uppin, S.G.; Mohammed, N. Serum human epididymis protein-4 (HE4) - a novel approach to differentiate malignant from benign breast tumors. Asian Pac. J. Cancer Prev. 2021, 22, 2509–2507. [Google Scholar] [CrossRef]
- Mirmohseni Namini, N.; Abdollahi, A.; Movahedi, M.; Emami Razavi, A.; Saghiri, R. HE4, A new potential tumor marker for early diagnosis and predicting of breast cancer progression. Iran J. Pathol. 2021, 16, 284–296. [Google Scholar] [CrossRef]
- Abdelrazek, M.A.; Nageb, A.; Barakat, L.A.; Abouzid, A.; Elbaz, R. BC-DETECT: combined detection of serum HE4 and TFF3 improves breast cancer diagnostic efficacy. Breast Cancer 2022, 29, 507–515. [Google Scholar] [CrossRef]
- Galgano, M.T.; Hampton, G.M.; Frierson, H.F. Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod. Pathol. 2006, 19, 847–853. [Google Scholar] [CrossRef]
- Kamei, M,; Yamashita, S.; Tokuishi, K.; Hashioto, T.; Moroga, T.; Suehiro, S.; Ono, K.; Miyawaki, M.; Takeno, S.; Yamamoto, S.; et al. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010, 30, 4779–4783.
- Akoz, G.; Diniz, G.; Ekmekci, S.; Ekin, Z.Y.; Uncel, M. Evaluation of human epididymal secretory protein 4 expression according to the molecular subtypes (luminal A, luminal B, human epidermal growth factor receptor 2-positive, triple-negative) of breast cancer. Indian J. Pathol. Microbiol. 2018, 61, 323–329. [Google Scholar]
- Kim, G.E.; Kim, N.I.; Park, M.H.; Lee, J.S. B7-H3 and B7-H4 expression in phyllodes tumors of the breast detected by RNA in situ hybridization and immunohistochemistry: association with clinicopathological features and T-cell infiltration. Tumour Biol. 2018, 40, 1010428318815032. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Kim, J.H.; Lee, K.H.; Choi, Y.D.; Lee, J.S.; Lee, J.H.; Nam, J.H.; Choi, C.; Park, M.H.; Yoon, J.H. Stromal matrix metalloproteinase-14 expression correlates with the grade and biological behavior of mammary phyllodes tumors. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch. Pathol. Lab. Med. 2010, 134, 907–922. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Madden, S.F.; Clarke, C.; Gaule, P.; Aherne, S.T.; O'Donovan, N.; Clynes, M.; Crown, J.; Gallagher, W.M. BreastMark: an integrated approach to mining publicly available transcriptomic datasets relating to breast cancer outcome. Breast Cancer Res. 2013, 15, R52. [Google Scholar] [CrossRef]
- Bignotti, E.; Ragnoli, M.; Zanotti, L.; Calza, S.; Falchetti, M.; Lonardi, S.; Bergamelli, S.; Bandiera, E.; Tassi, R.A.; Romani, C.; et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br. J. Cancer 2011, 104, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, R.; Ryan, N.A.J.; Barr, C.E.; Derbyshire, A.E.; Wan, Y.L.; Maskell, Z.; Stocking, K.; Pemberton, P.W; Bolton, J.; McVey, R.J.; et al. Baseline serum HE4 but not tissue HE4 expression predicts response to the Levonorgestrel-releasing intrauterine system in atypical hyperplasia and early stage endometrial cancer. Cancers (Basel) 2020, 12, 276. [Google Scholar] [CrossRef]
- Hanna, W.M.; Parra-Herran, C.; Lu, F.I.; Slodkowska, E.; Rakovitch, E.; Nofech-Mozes, S. Ductal carcinoma in situ of the breast: an update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod. Pathol. 2019, 32, 896–915. [Google Scholar] [CrossRef]
| Characteristics | Serum HE4 levels Number (Mean ± S.D.) |
p value |
|---|---|---|
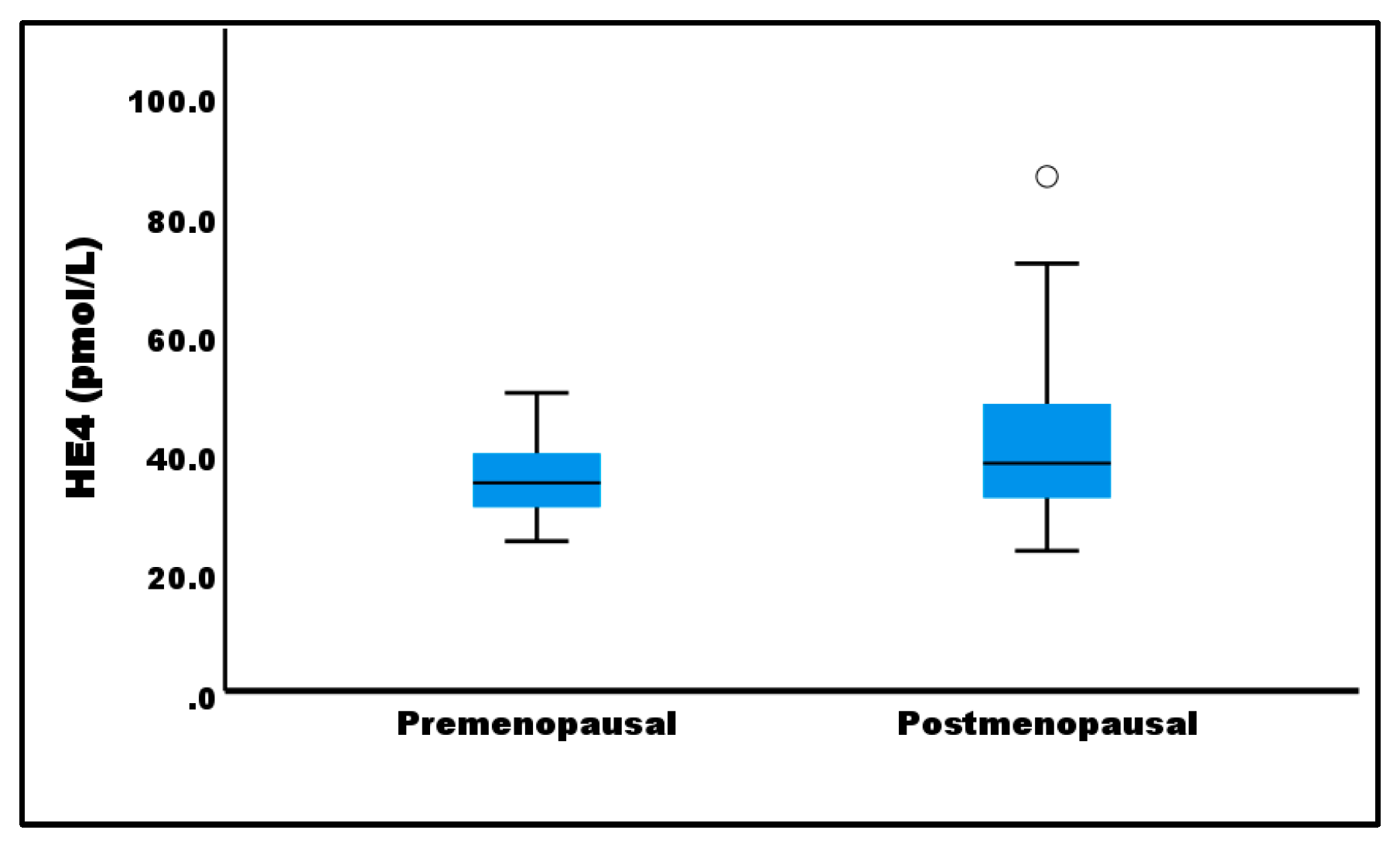
| Menopause | < 0.05 | |
| Pre | 27 (35.3 ± 6.4) | |
| Post | 32 (42.9 ± 14.4) | |
| Size (cm) | 0.881 | |
| < 2.8 | 36 (39.6 ± 12.8) | |
| ≥ 2.8 | 23 (39.1 ± 10.8) | |
| Nuclear grade | 0.516 | |
| 1 | 1 (31.3) | |
| 2 | 27 (37.9 ± 9.1) | |
| 3 | 31 (40.9 ± 14.1) | |
| Comedo-type necrosis | 0.594 | |
| No | 10 (37.5 ± 10.3) | |
| Yes | 49 (39.8 ± 12.3) | |
| Stromal TILs density score | 0.716 | |
| 1 | 39 (40.3 ± 11.6) | |
| 2 | 19 (37.6 ± 13.0) | |
| 3 | 1 (41.7) | |
| Estrogen receptor- α | 0.868 | |
| Negative | 25 (39.1 ± 14.5) | |
| Positive | 34 (39.7 ± 9.9) | |
| HER-2 | 0.692 | |
| Negative | 31 (38.8 ± 11.5) | |
| Positive | 28 (40.1 ± 12.6) | |
| Molecular subtypes | 0 | |
| HR+/HER2- | 27 (38.5 ± 10.3) | |
| HR+/HER2+ | 11 (41.2 ± 7.3) | |
| HR-/HER2+ | 17 (39.4 ±15.3) | |
| Triple-negative | 4 (41.4 ± 20.2) | |
| Recurrence | 0.222 | |
| No | 53 (40.1 ± 12.3) | |
| Yes | 6 (33.8 ± 6.9) |
| RNAscope in situ hybridization | Immunohistochemistry | Total | Concordance | κ value | p value | |
| Low | High | |||||
| Low High Total |
64 1 65 |
10 24 34 |
77 25 99 |
88.9 | 0.658 | < 0.001 |
| Characteristics | High mRNA HE4 expression N/total N (%) | p value | High protein HE4 expression N/total N (%) | p value |
| Menopause | 0.525 | 0.942 | ||
| Pre | 14/53 (26.4) | 18/53 (34.0) | ||
| Post | 11/46 (23.9) | 16/46 (34.8) | ||
| Size (cm) | 0.689 | 0.108 | ||
| < 2.8 | 15/56 (26.8) | 23/56 (41.1) | ||
| ≥2.8 | 10/43 (23.3) | 11/43 (25.6) | ||
| Nuclear grade | 0.129 | 0.068 | ||
| 1 | 1/3 (33.3) | 1/3 (33.3) | ||
| 2 | 17/55 (30.9) | 25/55 (45.5) | ||
| 3 | 7/41 (17.1) | 8/41 (19.5) | ||
| Comedo-type necrosis | 0.080 | < 0.05 | ||
| No | 9/23 (39.1) | 13/23 (56.5) | ||
| Yes | 16/76 (21.1) | 21/76 (27.6) | ||
| Stromal TILs density score | < 0.05 | < 0.05 | ||
| 1 | 22/67 (32.8) | 28/67 (41.8) | ||
| 2 | 3/30 (10.0) | 6/30 (20.0) | ||
| 3 | 0/2 (0) | 0/2 (0) | ||
| Estrogen receptor- α | < 0.01 | < 0.001 | ||
| Negative | 4/40 (10.0) | 5/40 (12.5) | ||
| Positive | 21/59 (35.6) | 29/59 (49.2) | ||
| HER-2 | < 0.05 | < 0.001 | ||
| Negative | 18/54 (33.3) | 27/54 (50.0) | ||
| Positive | 7/45 (15.6) | 7/45 (15.6) | ||
| Molecular subtypes | 0.099 | < 0.01 | ||
| HR+/HER2- | 17/48 (35.4) | 25/48 (52.1) | ||
| HR+/HER2+ | 4/16 (25.0) | 4/16(25.0) | ||
| HR-/HER2+ | 3/29 (10.3) | 3/29 (10.3) | ||
| Triple-negative | 1/6 (16.7) | 2/6 (33.3) | ||
| Recurrence | 0.688 | 0.489 | ||
| No | 22/90 (24.4) | 30/90 (33.3) | ||
| Yes | 3/9 (33.3) | 4/9 (44.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





