Submitted:
20 March 2025
Posted:
20 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Characteristics of Raw Material Prior to Agglomeration
3. Agglomeration of Zeolite Powder
4. Results and Discussion
5. Conclusions
- Agglomeration and deagglomeration in a single integrated system using HPGR (high-pressure grinding rolls) for powder zeolite with a solid binder enhanced the petroleum sorption capacity, primarily due to the formation of a secondary porosity network,
- The proposed zeolite dust agglomeration approach significantly increased the specific surface area (Sᴮᴱᵀ) of the final product compared to the raw zeolite powder. While this increase has a negligible impact on petroleum sorption, it can be a key factor in the sorption efficiency of other substances,
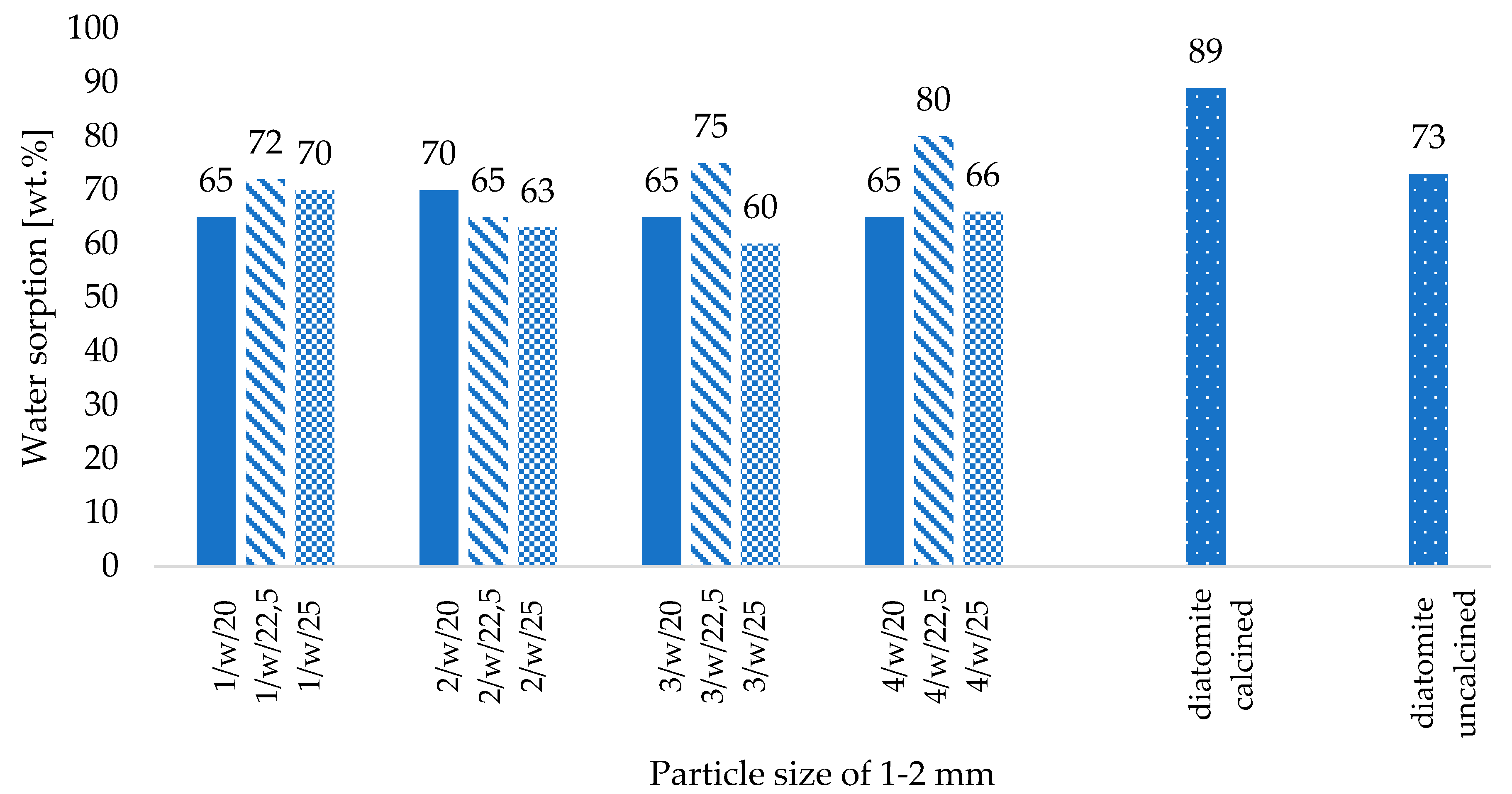
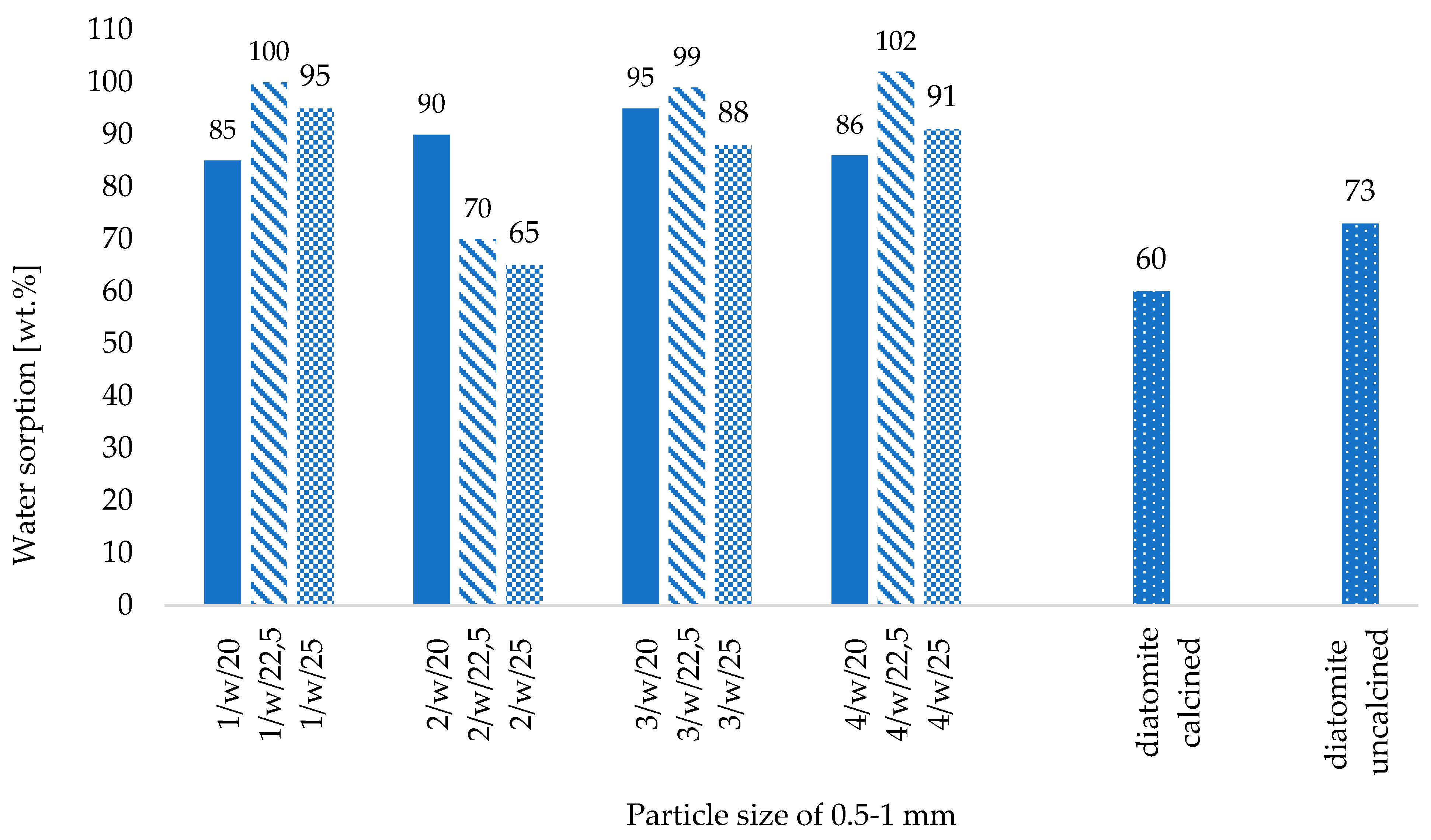
- The correlation between binder and water content and the physical properties of the fabricated zeolite agglomerates is not straightforward, particularly concerning water content. However, a higher binder content reduced powdering of the produced ribbons and improved the effectiveness of the roll compaction process, as indicated by the lower yield of fine particles (below 0.5 mm),
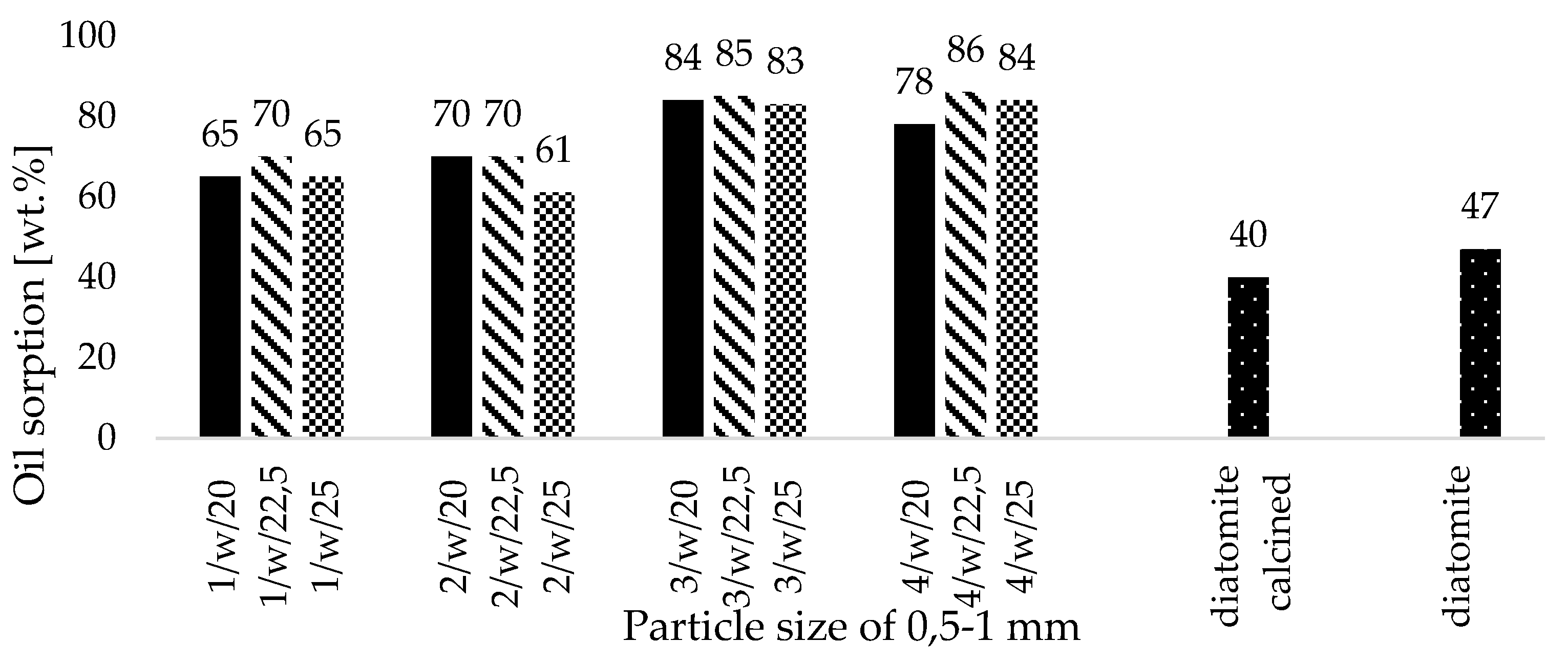
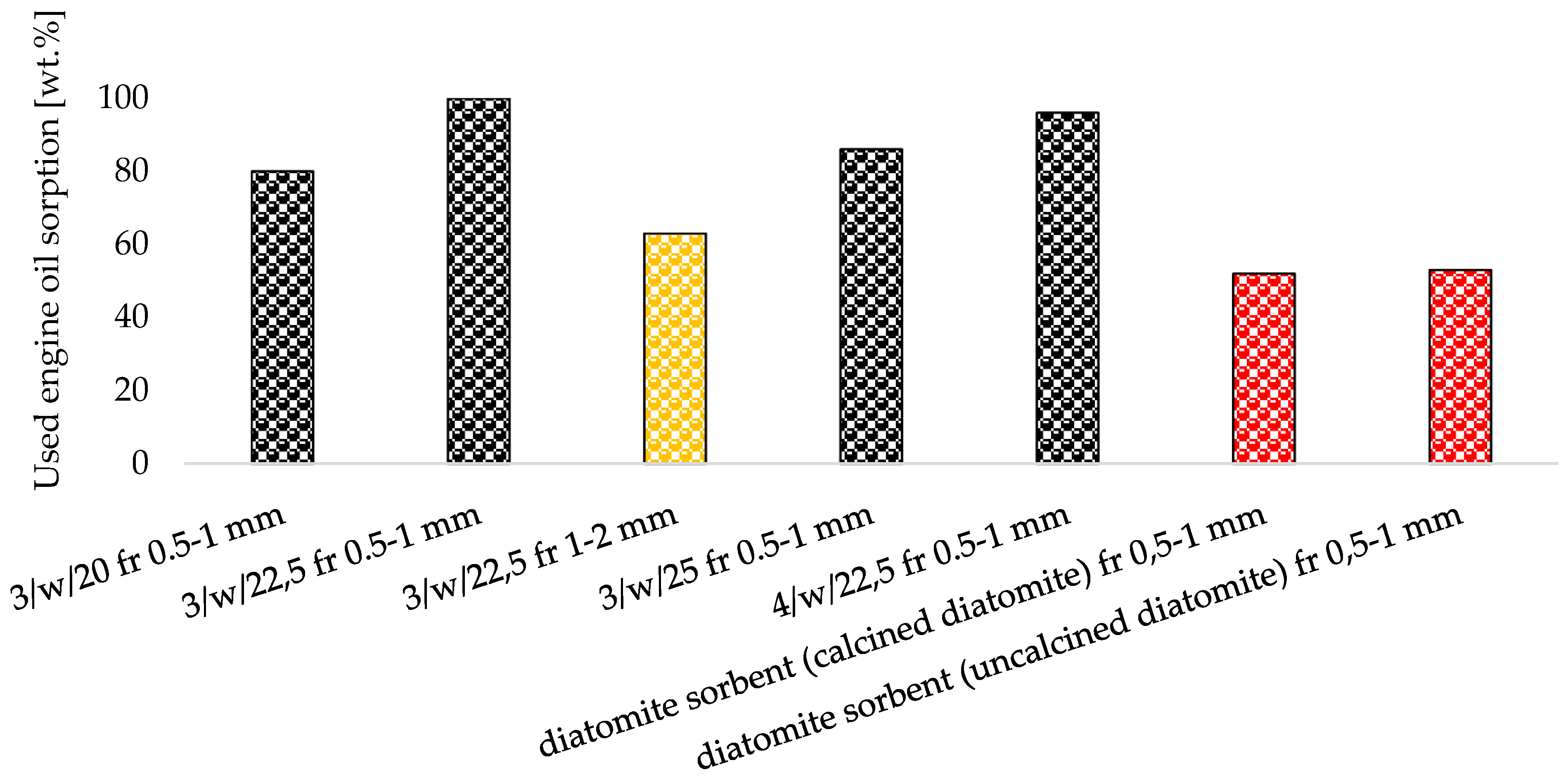
- All fabricated zeolite-based sorbents with a particle size of 0.5–1 mm met the required oil absorbency threshold of 50 wt.%, as specified for petroleum spill cleanup materials used on roads and pavements by fire departments in Poland,
- Binder contents up to 7.5 wt.% improved both the sorption capacity and mechanical strength of the fabricated zeolite particles (0.5–1 mm). However, exceeding this threshold did not further enhance these properties,
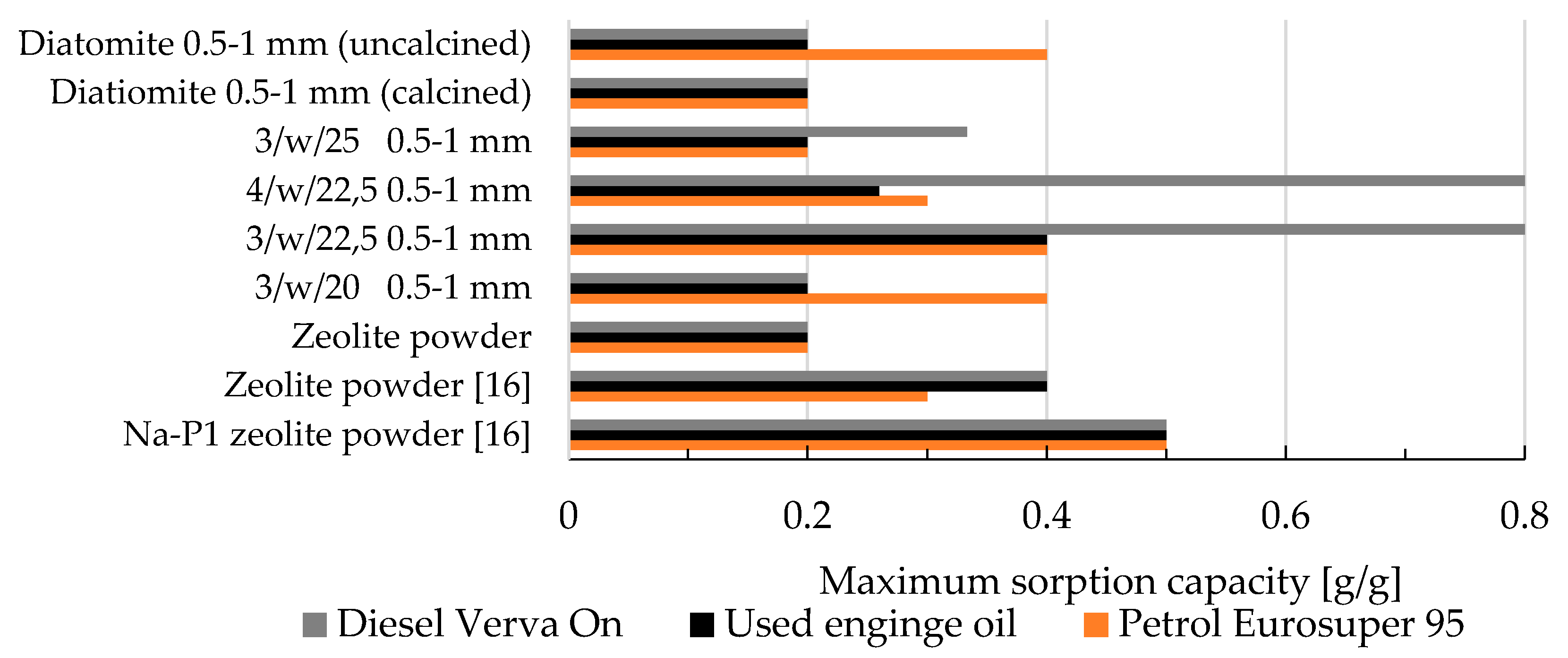
- The agglomeration process via roll compaction of zeolite powder with binder and water altered the maximum petroleum sorption capacity of the zeolite agglomerates compared to natural zeolite powder in nearly all cases, except for sample 3/w/20, where sorption efficiency remained comparable. Additionally, some manufactured zeolite-based sorbents exhibited superior sorption efficiencies compared to synthetic Na-P1 zeolite powder. Na-P1 showed sorption capacities of 0.5g/g for petrol, diesel, and used engine oil, whereas the 3/w/22.5 and 4/w/22.5 samples achieved capacities of 0.8g/g for used engine oil,
- Zeolite-based sorbents fabricated via roll compaction outperformed commercial diatomite sorbents in their affinity for petrochemical compounds,
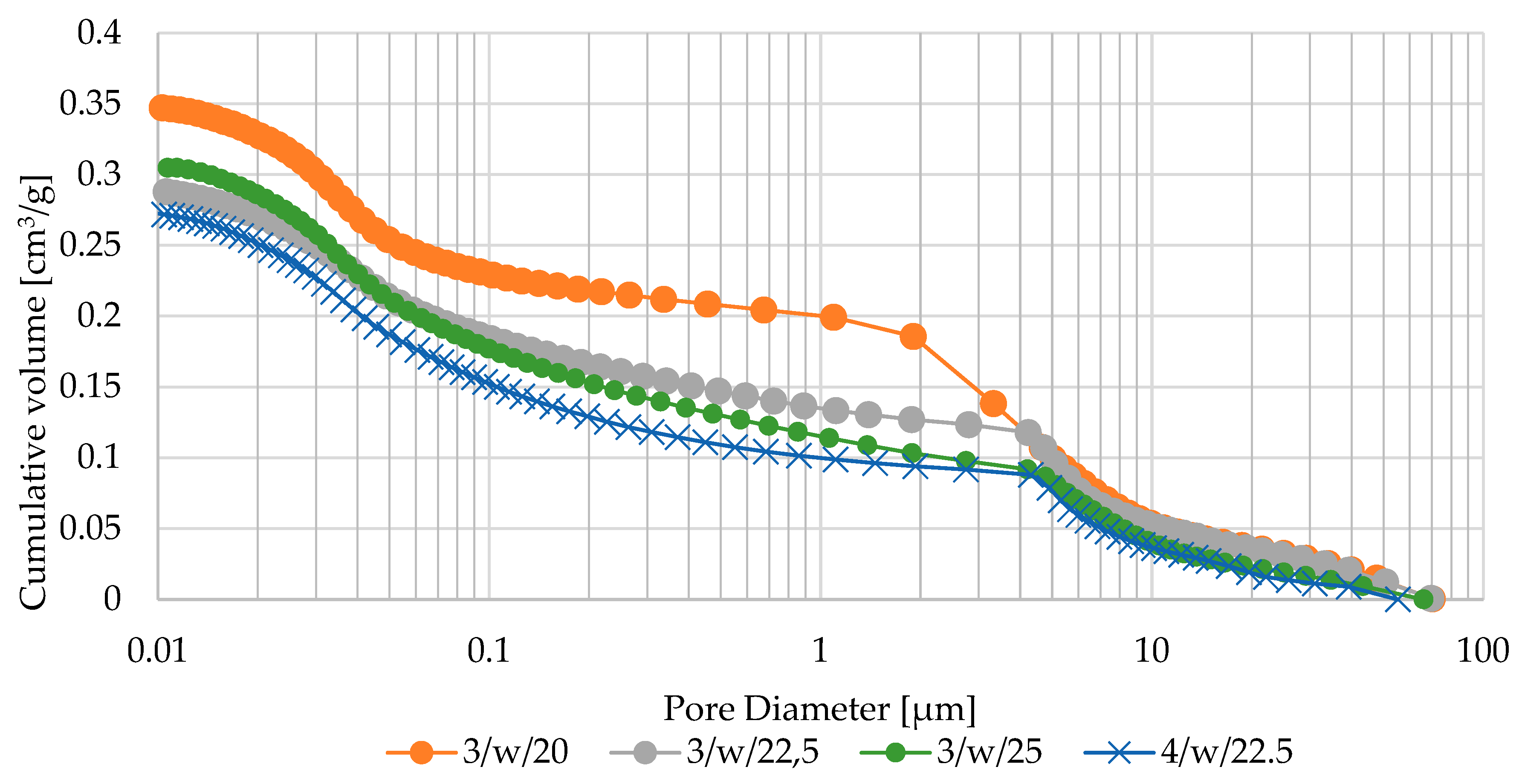
- Sample 3/w/22.5 surpassed commercial diatomite sorbents (produced via traditional pan granulation) in maximum sorption capacity for used engine oil and diesel, while demonstrating comparable efficiency for petrol sorption. This is likely due to the well-developed macro- and mesoporous structure of 3/w/22.5, which was more advanced than in other produced agglomerates,
- SEM observations of the fabricated zeolite-based agglomerates revealed that binder particles are embedded within the primary zeolite powder particles. The random distribution of feed particles of varying sizes resulted in structural heterogeneity, leading to areas with different packing densities, which directly influenced the total porosity of the granules. In contrast, particle coating through wet pan granulation created a denser, more compact structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- R. Bingre, B. Louis, P. Nguyen, An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts 2018, 8, 16. [Google Scholar] [CrossRef]
- D. A. Fungaro, T. C. R. Bertolini, Optimization Of Pelleting Parameters For Producing Composite Pellets Using Zeolitic Material From Fly Ash. Applied Materials and Technology 2023, 3, 13–23. [Google Scholar] [CrossRef]
- K. Shams, S.J. Mirmohammadi, Preparation of 5A zeolite monolith granular extrudates using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Microporous and Mesoporous Materials 2007, 106, 268–277. [Google Scholar] [CrossRef]
- M. Senila, O. Cadar, Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- E. Pérez-Botella, S. Valencia, F Rey, Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chemical Reviews 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- J. Shi, M. Zhang, L. Zhu, Q. Wu, X. Meng, F.S. Xiao, Recent advances in sustainable synthesis of zeolites. Materials Today Sustainability 2025, 29, 101065. [Google Scholar] [CrossRef]
- Verified Market Research, Global Zeolite Powder Market By Type (Zeolite A, Zeolite Y), By Application (Industrial Off-Gas Purification, Automotive Emission Control), By Geographic Scope And Forecast, February 2023.
- M. K. Pietre,, J.C.C Freitas, Fundamental studies on zeolite–adsorbate interactions: designing a better aluminosilicate adsorbent for pollutants’ removal. Environmental Earth Sciences 2022, 81, 17. [Google Scholar] [CrossRef]
- P. Mueller, A.Russell, J. Seidenbecher, J. Tomas, Progressive weakening of zeolite granules due to cyclic moisture loading and unloading. Microporous and Mesoporous Materials 2015, 211, 88–96. [Google Scholar] [CrossRef]
- E. Pabiś-Mazgaj, P. Pichniarczyk, A.Stempkowska, T. Gawenda, Possibility of Using Natural Zeolite Waste Granules Obtained by Pressure Agglomeration as a Sorbent for Petroleum Substances from Paved Surfaces. Materials 2022, 15, 6871. [Google Scholar] [CrossRef]
- M. A. Hoaghia, I. Aschilean, V. Babalau-Fuss, A. Becze, O. Cadar, C. Roman, M. Roman, M. Senila, E. Kovacs, Activated natural zeolites for petroleum hydrocarbons adsorption. Studia UBB Chemia 2021, 2, 95–104. [Google Scholar] [CrossRef]
- B. Muir, M. Wołowiec, T. Bajda, P. Nowak, P. Czupryński, The Removal of Organic Compounds by Natural and Synthetic Surface-Functionalized Zeolites: A Mini-Review. Mineralogia 2017, 48, 145–156. [Google Scholar] [CrossRef]
- B. Szala, P. Turek, A. Jeleń, T. Bajda, Synthesis and sorption properties of organo-zeolites. Zeszyty Naukowe. Inżynieria Środowiska / Uniwersytet Zielonogórski 2013, 150, 15–12. [Google Scholar]
- B. Muir, T. Bajda, Organically modified zeolites in petroleum compounds spill cleanup - Production, efficiency, utilization. Fuel Processing Technology 2016, 149, 153–162. [Google Scholar] [CrossRef]
- [bandura], L. Bandura, M. Franus, G. Józefaciuk, W. Franus, Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- B. Muir, T. Bajda, Organically modified zeolites in petroleum compounds spill cleanup — Production, efficiency, utilization. Fuel Processing Technology 2016, 149, 153–162. [Google Scholar] [CrossRef]
- M. Król, P. Rożek, Sorption of oil products on the synthetic zeolite granules. Mineralogi 2020, 51, 1–7. [Google Scholar] [CrossRef]
- K. Sharma, G. Shah, H. Singh, U. Bhatt, K. Singhal, V. Soni, Advancements in natural remediation management techniques for oil spills: Challenges, innovations, and future directions. Environmental Pollution and Management 2024, 1, 128–146. [Google Scholar] [CrossRef]
- W. Kamal, D. W. Kamal, D. Essam, A. A. Allam, H. E. Alfassam, D. abd el tawab, S. A. Moaty, R. Mahmoud, Natural Egyptian zeolite ore as a novel layered adsorbent for petroleum wastewater treatment. Chinese Journal of Analytical Chemistry 2025, 100490. [Google Scholar] [CrossRef]
- B. Szala, T. Bajda, J.Matusik, K. Zięba, B. Kijak, BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA. Microporous and Mesoporous Materials 2015, 2015 202, 115–123. [Google Scholar] [CrossRef]
- B. Muir, J. Matusik, T. Bajda, New insights into alkylammonium-functionalized clinoptilolite and Na-P1 zeolite: Structural and textural features. Applied Surface Science 2016, 361, 242–250. [Google Scholar] [CrossRef]
- B. Szala, T. Bajda, J.Matusik, K. Zięba, B. Kijak, BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA. Microporous and Mesoporous Materials 2015, 2015 202, 115–123. [Google Scholar] [CrossRef]
- M. L. Feo, M. Frattoni, E. Paoloacci, M. Masiello, G. Esposito, R. Gonzalez-Olmos, E. Tempesta, F. Trapasso, E. Zampetti, M. Torre, E. Guerriero, V. Paolini, Assessing the efficiency of zeolites in BTEX adsorption: Impact of pore structure and humidity in single and multicomponent systems. Microporous and Mesoporous Materials 2025, 384, 113462. [Google Scholar] [CrossRef]
- L. Bandura, M. Franus, G. Józefaciuk, W. Franus, Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- M. Król, A. Mikuła, Synthesis of the zeolite granulate for potential sorption application. Microporous and Mesoporous Materials 2017, 243, 201–205. [Google Scholar] [CrossRef]
- R. V. Jasra, B. Tyagi, Y.M. Badheka, V.N. Choudary T. S.G. Bhat, Effect of Clay Binder on Sorption and Catalytic Properties of Zeolite Pellets. Industrial & Engineering Chemistry Research 2003, 42, 3263–3272. [Google Scholar] [CrossRef]
- Z. Asgar Pour, M.M. Abduljawad, Y.A. Alassmy, L. Cardon, P.H.M. Van Steenberge, K.O. Sebakhy, A Comparative Review of Binder-Containing Extrusion and Alternative Shaping Techniques for Structuring of Zeolites into Different Geometrical Bodies. Catalysts 2023, 13, 656. [Google Scholar] [CrossRef]
- P. Müller, A. Russell, J. Tomas, Influence of binder and moisture content on the strength of zeolite 4A granules. Chemical Engineering Science 2015, 126, 204–215. [Google Scholar] [CrossRef]
- P. Rajniak, C. Mancinelli, R.T. Chern, F. Stepanek, L. Farber, B.T. Hill, Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology. International Journal of Pharmaceutics 2007, 334, 92–102. [Google Scholar] [CrossRef]
- S. Hibare, R, Sivanathan, S. Nadakatti, Behavior of soft granules under compression: Effect of reactive and non-reactive nature of the binder on granule properties. Powder Technology 2011, 210, 241–247. [Google Scholar] [CrossRef]
- N. L. Michels, S. Mitchell, J.Pérez-Ramírez, Effects of Binders on the Performance of Shaped Hierarchical MFI Zeolites in Methanol-to-Hydrocarbons. ACS Catalysis 2014, 4. [Google Scholar] [CrossRef]
- J. García-Martínez, D. Cazorla-Amorós, A. Linares-Solano, Y.S. Lin, Synthesis and Characterization of MFI-Type Zeolites Supported on Carbon Materials. Microporous Mesoporous Mater. 2001, 42, 255–268. [Google Scholar] [CrossRef]
- K. Gleichmann, B. K. Gleichmann, B. Unger, A. Brandt, Industrial zeolite molecular sieves, ZeolitesUseful Minerals (2016).
- H. J. Lee, J.H. Kim, D.W Park, S.J. Cho, Effect of base binder, flash calcined hydrotalcite, in MFI zeolite granule: Catalytic activity over 1-butene isomerization and MTO reaction. Applied Catalysis A : General 2015, 502, 1. [Google Scholar] [CrossRef]
- K. Schumann, B. Unger, A. Brandt, F. Scheffler, Investigation on the pore structure of binderless zeolite 13× shapes. Microporous and Mesoporous Materials 2012, 154, 119–123. [Google Scholar] [CrossRef]
- J. Kim, M. Choi, R. Ryoo, Effect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process. Journal of Catalysis 2010, 269, 219–228. [Google Scholar] [CrossRef]
- M. Bjørgen, F. Joensen, M. Spangsberg Holm, U. Olsbye, K.P. Lillerud, S. Svelle, Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Applied Catalysis A: General 2008, 345, 43–50. [Google Scholar] [CrossRef]
- P. N.R. Vennestrøm, M. Grill, M. Kustova, K. Egeblad, L. F. Lundegaard, F. Joensen, C. H. Christensen, P. Beato, Hierarchical ZSM-5 prepared by guanidinium base treatment: Understanding microstructural characteristics and impact on MTG and NH3-SCR catalytic reactions. Catalysis Today 2011, 168, 71–79. [Google Scholar] [CrossRef]
- Z. X. Qin, L. Lakiss, L. Tosheva, J.P. Gilson, A. Vicente, C. Fernandez, V. Valtchev, Comparative study of nano-ZSM-5 catalysts synthesized in OH- and F- media. Advanced Functional Materials 2014, 24, 257–264. [Google Scholar] [CrossRef]
- St. Palzer, Agglomeration of pharmaceutical, detergent, chemical and food powders — Similarities and differences of materials and processes. Powder Technology 2011, 206, 2–17. [Google Scholar] [CrossRef]
- B.K.G. Theng, Formation and Properties of Clay-Polymer Complexes, Elsevier Science, 2nd ed., 2012 .
- L. M. Tavares, Breakage of single particles: Quasi-static. Handbook of Powder Technology 2007, 12, 1–68. [Google Scholar] [CrossRef]
- J. Fu, M.J. Adams, G.K. Reynolds, A.D. Salman, M.J. Hounslow, Impact deformation and rebound of wet granules. Powder Technology 2004, 140, 248–257. [Google Scholar] [CrossRef]
- Y. Saito, J. Nyumura, Y. Zhang, S. Tanaka, N. Uchida, K. Uematsu, Kinetics of property change associated with atmospheric humidity changes in alumina powder granules with PVA binder. Journal of the European Ceramic Society 2022, 22, 2835–2840. [Google Scholar] [CrossRef]
- P. Müller, S. Antonyuk, J. Tomas, Influence of moisture content on the compression behavior of granules. Chemical Engineering Technology 2011, 34, 1–9. [Google Scholar] [CrossRef]
- US 2973327 e W.J. Mitchell, W.F. Moore, Bonded molecular sieves.
- US 3287281 e F.J. Dzierzanowski, W.L. Haden Jr., Zeolite agglomerates and the preparation thereof.
- US 5079201 e, P. Chu, W.E. Garwood, Zeolite-clay composition and uses thereof.
- US 6107354 e, T.J. Shaniuk, R.V. Russo, Composite material, preparation and use thereof.
- V. R. Choudhary, P. Devadas, A.K. Kinage, M. Guisnet, Influence of binder on the acidity and performance of H-Gallosilicate (MFI) zeolite in propane aromatization. Applied Catalysis A: General 1997, 162, 223–233. [Google Scholar] [CrossRef]
- P. Cañizares, A. Durán, F. Dorado, M. Carmona, The role of sodium montmorillonite on bounded zeolite-type catalysts. Applied Clay Science 2000, 16, 273–287. [Google Scholar] [CrossRef]
- J. M. Fougerit, N.S. Gnep, M. Guisnet, P. Amigues, J.L. Duplan, F. Hugues, Effect of the binder on the properties of a mordenite catalyst for the selective conversion of methanol into light olefins. Studies in Surface Science and Catalysis 1994, 84, 1723–1730. [Google Scholar] [CrossRef]
- J. Freiding, F.C. Patcas, B. Kraushaar-Czarnetzki, Extrusion of zeolites: Properties of catalysts with a novel aluminium phosphate sintermatrix. Applied Catalysis A: General 2007, 328, 210–218. [Google Scholar] [CrossRef]
- G. T. Whiting, S.H. Chung, D. Stosic, A.D. Chowdhury, L.I. van der Wal, D. Fu, J. Zecevic, A. Travert, K. Houben, M. Baldus, Multiscale Mechanistic Insights of Shaped Catalyst Body Formulations and Their Impact on Catalytic Properties. ACS Catalysis 2019, 9, 4792–4803. [Google Scholar] [CrossRef]
- V. G. Baldovino-Medrano, M.T. Le, I. Van Driessche, E. Bruneel, C. Alcázar, M.T. Colomer, R. Moreno, A. Florencie, B. Farin, E.M. Gaigneaux, Role of shaping in the preparation of heterogeneous catalysts: Tableting and slip-casting of oxidation catalysts. Catalysis Today 2015, 246, 81–91. [Google Scholar] [CrossRef]
- S. Y. Devyatkov, A. Al. Zinnurova, A. Aho, D. Kronlund, J. Peltonen, N. V. Kuzichkin, N.V. Lisitsyn, D. Y. Murzin, Shaping of Sulfated Zirconia Catalysts by Extrusion: Understanding the Role of Binders. Industrial & Engineering Chemistry Research 2016, 55, 6595–6606. [Google Scholar] [CrossRef]
- M. Absi-Halabi, A. Stanislaus, H. Al-Zaid, Effect of acidic and basic vapors on pore size distribution of alumina under hydrothermal conditions. Applied Catalysis A: General 1993, 101, 117–128. [Google Scholar] [CrossRef]
- Rogala, K.A. Tarach, L. Lakiss, A. Kordek, V. Valtchev, J.P Gilson, K. Góra-Marek, Shaped zeolites Y for polypropylene cracking. Applied Catalysis B: Environment and Energy 2025, 365, 124893. [Google Scholar] [CrossRef]
- M. W. Kasture, P.S. Niphadkar, V.V. Bokade, P.N. Joshi, On the catalytic performance in isopropylation of benzene over H/β zeolite catalysts: Influence of binder. Catalysis Communications 2007, 8, 1003–1008. [Google Scholar] [CrossRef]
- X. Kong, J. Liu, Influence of alumina binder content on catalytic performance of Ni/HZSM-5 for hydrodeoxygenation of cyclohexanone. PLoS ONE 2014, 9, 5–10. [Google Scholar] [CrossRef]
- D. Bazer-Bachi, B. Harbuzaru, E. Lecolier, Zeolite Formed by Extrsion and Pelleting with a Hydraulic Binder Having Improved Mechanical Properties and Process and Preparing Same. U.S. Patent 20,160,288,109A1, 6 October 2016.
- D. Bika, G.I. Tardos, S. Panmai, L. Farber, J. Michaels, Strength and morphology of solid bridges in dry granules of pharmaceutical powders. Powder Technology 2005, 150, 104–116. [Google Scholar] [CrossRef]
- Amir Charkhi, M. Kazemeini, S. J. Ahmadi, H. Kazemian, Fabrication of granulated NaY zeolite nanoparticles using a new method and study the adsorption properties. Powder Technology 2012, 231, 1–6. [Google Scholar] [CrossRef]
- N. Li, W. Liu, L. Liu, P. Gao, Q. Wu, X. Ma, G. K. Li, Evaluation of gas adsorption and separation performance of binder-free Y zeolite particles prepared by the rotary pelletization method. Separation and Purification Technology 2025, 361, 3–131520. [Google Scholar] [CrossRef]
- E. Luzzi, P. Aprea, M. Salzano de Luna, D. Caputo, G. Filippone, Mechanically Coherent Zeolite 13X/Chitosan Aerogel Beads for Effective CO2 Capture. ACS Applied Materials & Interfaces 2021, 13, 20728–20734. [Google Scholar] [CrossRef]
- S. Krishnamurthy, R. Roelant, R. Blom, B. Arstad, Z. Li, M. Rombouts, V. Middelkoop, A. B. Borras, L. Naldoni, Scaling up 3D printed hybrid sorbents towards (cost) effective post-combustion CO2 capture: A multiscale study. International Journal of Greenhouse Gas Control 2024, 132, 104069. [Google Scholar] [CrossRef]
- S. Wang, P. Bai, M. Sun, W. Liu, D. Li, W. Wu, W. Yan, J. Shang, J. Yu, Fabricating mechanically robust binder-free structured zeolites by 3D printing coupled with zeolite soldering: a superior configuration for CO2 capture. Advanced Science 2019, 6, 1901317. [CrossRef]
- [Wears, Kedar Bharat Jivrakh et []]K. B. Jivrakh, A. M. Varghese, S. Ehrling, S. Kuppireddy, K. Polychronopoulou, R. K. Abu Al-Rub, N. Alamoodi, G. N. Karanikolos, 3D-printed zeolite 13X gyroid monolith adsorbents for CO2 capture. Chemical Engineering Journal 2024, 497, 1385–8947. [Google Scholar] [CrossRef]
- Rianjanu, T. Taher, F. Desriani, R.O. Delmita, A.G.N. Sianturi, S.A. Muhtar, B. Ariwahjoedi, N. I. Khamidy, D.R. Adhika, M.F. Arif, Examining the influence of sintering temperatures on the efficiency of 3D-printed natural zeolite for methylene blue dye adsorption. Results in Surfaces and Interfaces 2024, 17, 2666–8459. [Google Scholar] [CrossRef]
- D. M. D'Alessandro, B. Smit, J.R. Long, Carbon Dioxide Capture: Prospects for New Materials. A Jornal of the German Chemisal Society 2010, 49, 35–6058. [Google Scholar] [CrossRef]
- Michaiko, E. Method for Producing Molecular Sieves Zeolite Particles. U.S. Patent 3,348,911A, 24 December 1967. [Google Scholar]
- Michaiko, E. Preparation of Crystalline Zeolite Particles. U.S. Patent 3,359,068A, 19 December 1967. [Google Scholar]
- P. Vasiliev, F. Akhtar, J. Grins, J. Mouzon, C. Andersson, J. Hedlund, L. Bergstrom, Strong hierarchically porous monoliths by pulsed current processing of zeolite powder assemblies. ACS Appl. Mater. Interfaces 2010, 2, 732–737. [Google Scholar] [CrossRef] [PubMed]
- B. T Holland, L. Abrams, A. Stein, Dual Templating of Macroporous Silicates with Zeolitic Microporous Frameworks. Journal of the American Chemical Society 1999, 121, 4308–4309. [Google Scholar] [CrossRef]
- Dong, Y. Wang, Y. Tang, Y. Zhang, N. Ren, Z. Gao, Mechanically Stable Zeolite Monoliths with Three-Dimensional Ordered Macropores by the Transformation of Mesoporous Silica Spheres. Advanced Materials 2002, 14, 1506–1510. [Google Scholar] [CrossRef]
- W. C Li, A.H. Lu, R. Palkovits, W. Schmidt, B. Spliethoff, F. Schüth, Hierarchically Structured Monolithic Silicalite-1 Consisting of Crystallized Nanoparticles and Its Performance in the Beckmann Rearrangement of Cyclohexanone Oxime. Journal of the American Chemical Society. 2005, 127, 12595–12600. [Google Scholar] [CrossRef]
- US 3114603 e, P.A. Howell, Process for synthetic zeolite A.
- US 3515511 e, W.H. Flank, Faujasite production.
- US 5001098 e, B. Pacaud, M. Mercier, 5A zeolite/kaolinite adsorbent for gas purification.
- D. P. Costa, A. Fernandes, E. F. S.-Aguiar, C. Alves, P. Ferreira, J. C.B. Lopes, B. F. Machado, M. F. Ribeiro, Exploring the influence of zeolite textural properties on the production of sustainable fuels through the Fischer-Tropsch process. Applied Catalysis A: General 2025, 692, 120095. [Google Scholar] [CrossRef]
- B. J Zeng, S.M Wu, M. Antonietti, C. Janiak, O. Terasaki, X.Y. Yang, Hierarchy design of zeolite mesocrystals. Chemical Engineering Journal 2025, 505, 159282. [Google Scholar] [CrossRef]
- X. Gao, A. Feng, L. Mi, Y. Yu, Y. Yu, Fabrication and adsorption properties of hierarchical NaY zeolite with mesopores in the range of 2–5 nm. Chemical Physics Letters 2025, 858, 141732. [Google Scholar] [CrossRef]
- D. Bazer-Bachi, L. Assié, V. Lecocq, B. Harbuzaru, V. Falk,Towards industrial use of metal-organic framework: Impact of shaping on the MOF properties. Powder Technology 2014, 255, 52–59. [Google Scholar] [CrossRef]
- R. Panek, M. Wdowin, L. Bandura, E. Wisła-Walsh, P. Gara, W. Franus, Changes in the Textural Parameters of Fly Ash-Derived Na-P1 Zeolite During Compaction Processes. Mineralogia 2017, 40, 1–4. [Google Scholar] [CrossRef]
- N. Marinko, I. Sedlářová, S. Römerová, M. Gajdošová, V. Zvoníček, P.r Zámostný, Method to determine envelope density for roller compacted ribbons by solid displacement of glass microspheres. Powder Technology 2024, 444, 120014. [Google Scholar] [CrossRef]
- P. Kleinebudde, Improving Process Understanding in Roll Compaction. Journal of Pharmaceutical Sciences 2022, 111, 552–558. [Google Scholar] [CrossRef]
- H. L. Reimer, P. Kleinebudde, Hybrid modeling of roll compaction processes with the Styl'One Evolution. Powder Technology 2019, 341, 66–74. [Google Scholar] [CrossRef]
- A. K. Vadaga, S.S. Gudla, G.S.K. Nareboina, H. Gubbala, B. Golla, Comprehensive review on modern techniques of granulation in pharmaceutical solid dosage forms. Intelligent Pharmacy 2024, 2, 5–609. [Google Scholar] [CrossRef]
- N. Patil, S.C. Khadse, P. P. Ige, Review on novel granulation techniques. World Journal of Pharmaceutical Research 2016, 5, 7. [Google Scholar]
- R.W. Miller, Roller Compaction Technology, Handbook of Pharmaceutical Granulation Technology 2005, 32. [CrossRef]
- Y. Teng, Z. Qiu, H. Wen, Systematical approach of formulation and process development using roller compaction. European Journal of Pharmaceutics and Biopharmaceutics 2009, 72. [Google Scholar] [CrossRef]
- Y. A. Yusof, A. C. Smith, B. J. Briscoe, Roll compaction of maize powder. Chemical Engineering Science 2005, 60, 3919–3931. [Google Scholar] [CrossRef]
- P. García-Triñanes, M. Morgeneyer, J.J. Casares, M. Bao, Use of organic byproducts as binders in the roll compaction of caustic magnesia. Powder Technology 2012, 226, 173–179. [Google Scholar] [CrossRef]
- S. H Lim, S.I Kim, J.S Song, K.H Kim, A study on the pelletizing condition for roll compaction of powdered radioactive wastes. Powder Technology 2021, 284, 554–563. [Google Scholar] [CrossRef]
- ISO 13320 Particle size analysis — Laser diffraction methods.
- PN-EN 933-1 Examination of Geometric Properties of Aggregates; Part 1 Determination of Grain Composition.
- EN 1097-7 Tests for mechanical and physical properties of aggregates - Part 7: Determination of the particle density of filler - Pyknometer method.
- EN 1097-3, Tests for mechanical and physical properties of aggregates - Part 3: Determination of loose bulk density and voids.
- Pabiś-Mazgaj, E.; Gawenda, T. Ciśnieniowa aglomeracja pyłu zeolitowego w prasie walcowej wysokociśnieniowej. Surowce I Masz. Bud. 2021, 1, 68–73. [Google Scholar]
- EN 933-1, Tests for geometrical properties of aggregates - Part 1: Determination of particle size distribution - Sieving method.
- E. Pabiś-Mazgaj, T. Gawenda, P. Pichniarczyk, A. Stempkowska, Mineral Composition and Structural Characterization of the Clinoptilolite Powders Obtained from Zeolite-Rich Tuffs. Minerals 2021, 11, 1030. [Google Scholar] [CrossRef]
- M. Thommes, K. Kaneko, A. Neimark, J. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (UPAC Technical Report). Pure and Applied Chemistry. 2015, 87, 9–10. [Google Scholar] [CrossRef]
- Journal of Laws of the Republic of Poland, No. 85, item 553. Polish Public Procurement Office: Warsaw, Poland, 2010.
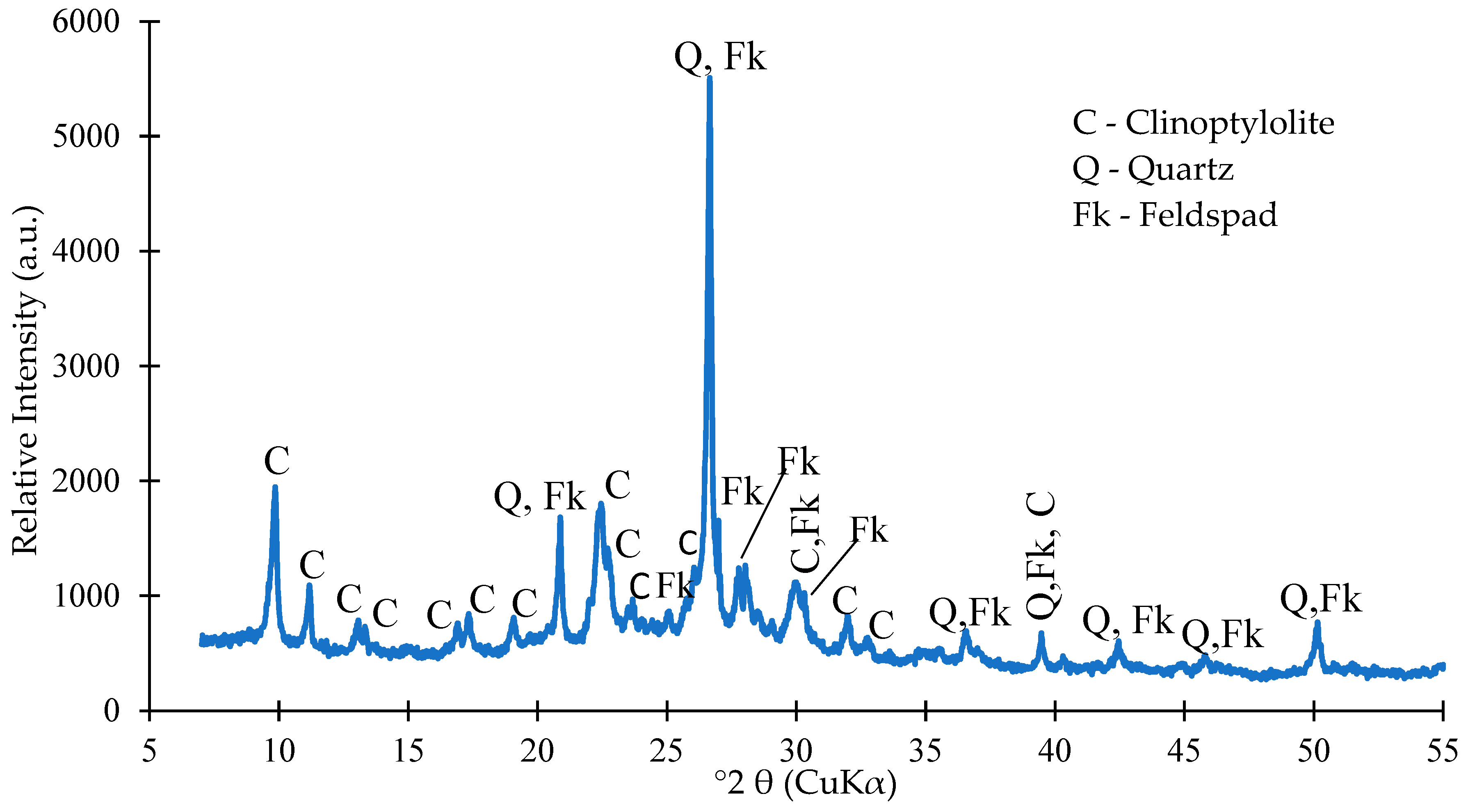
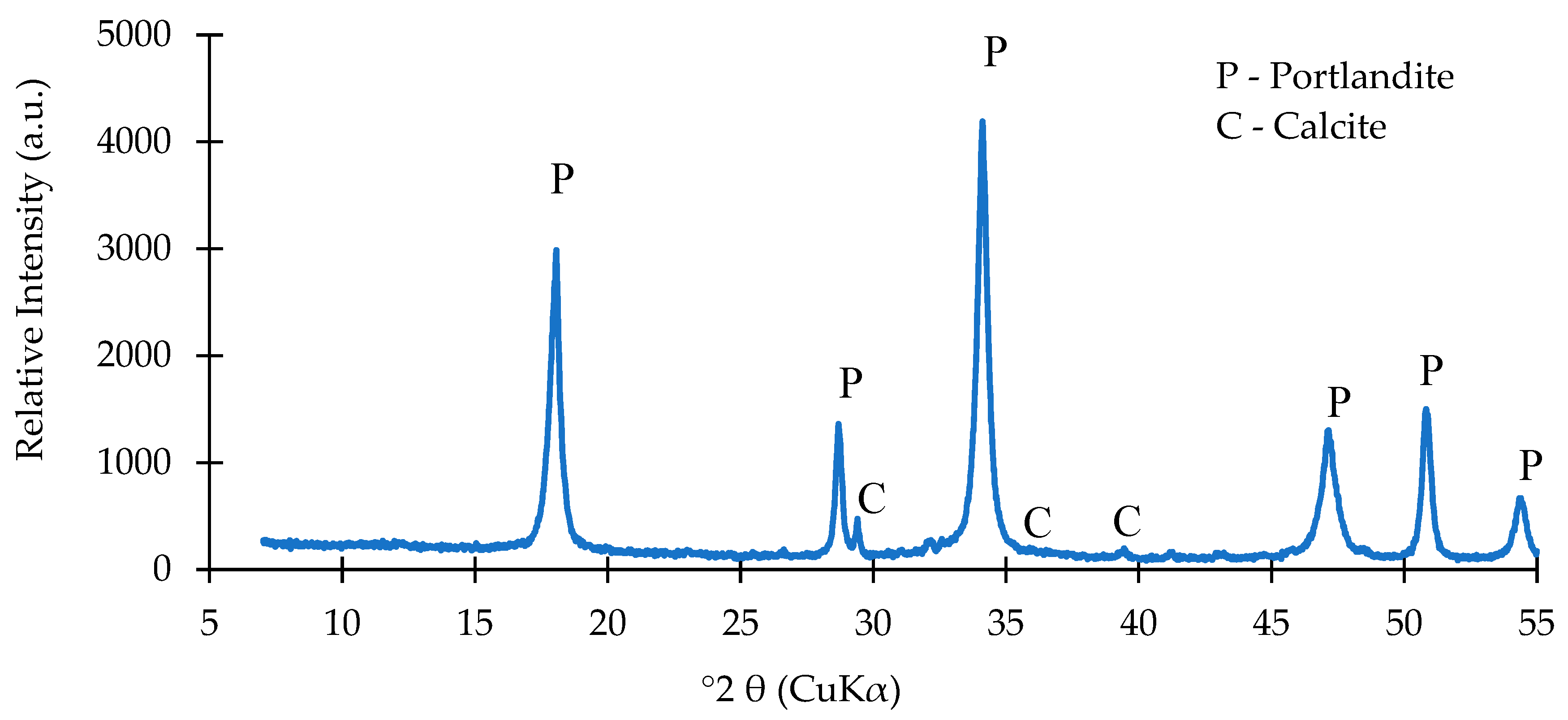
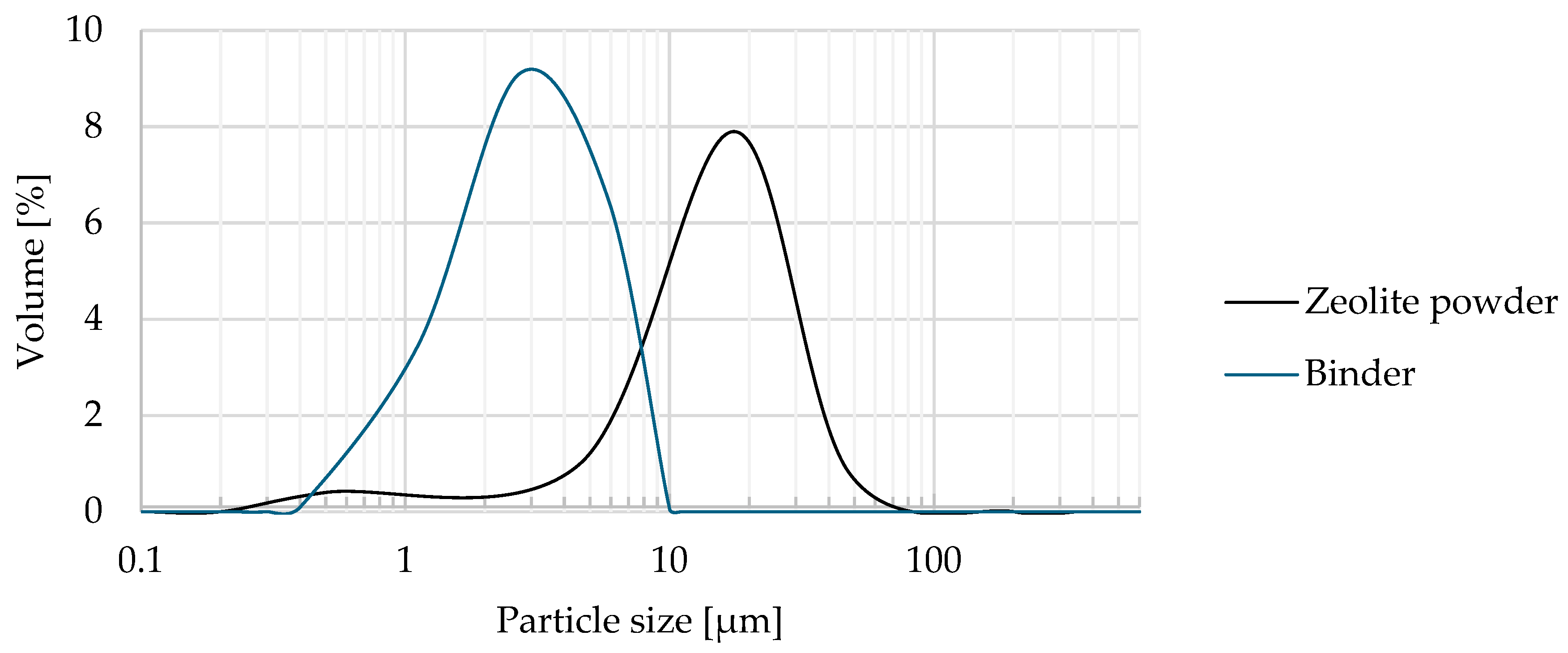

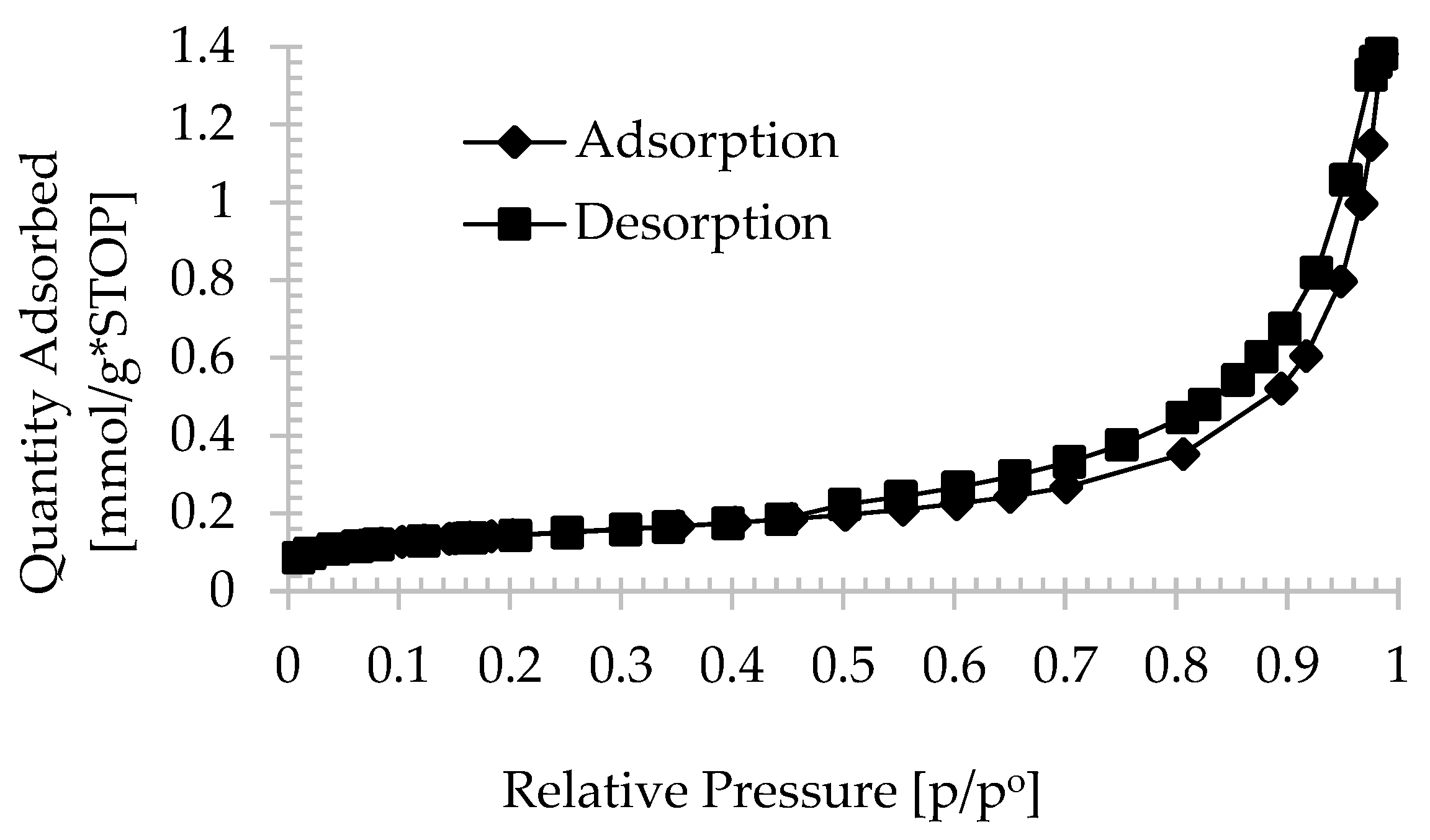

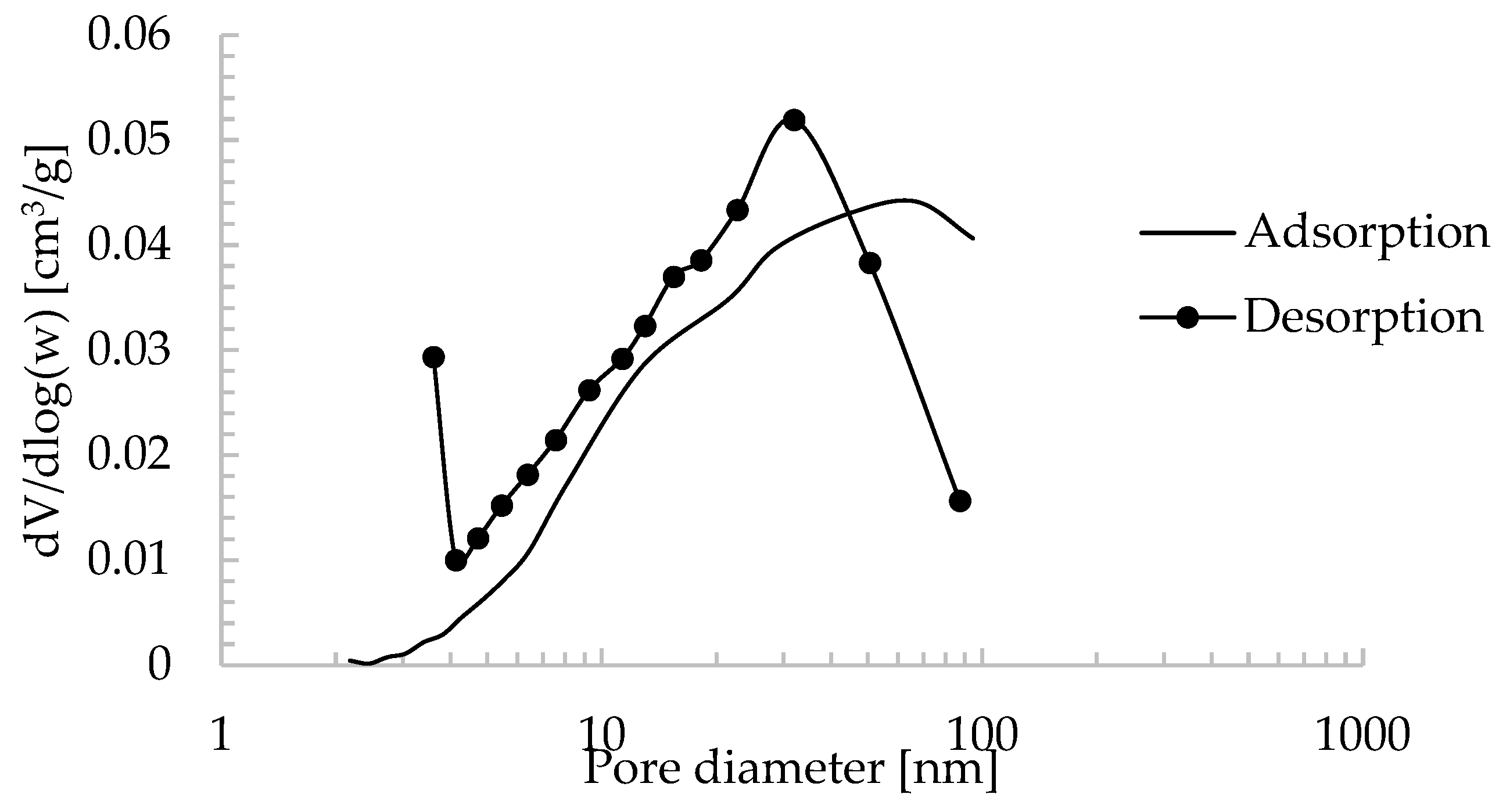
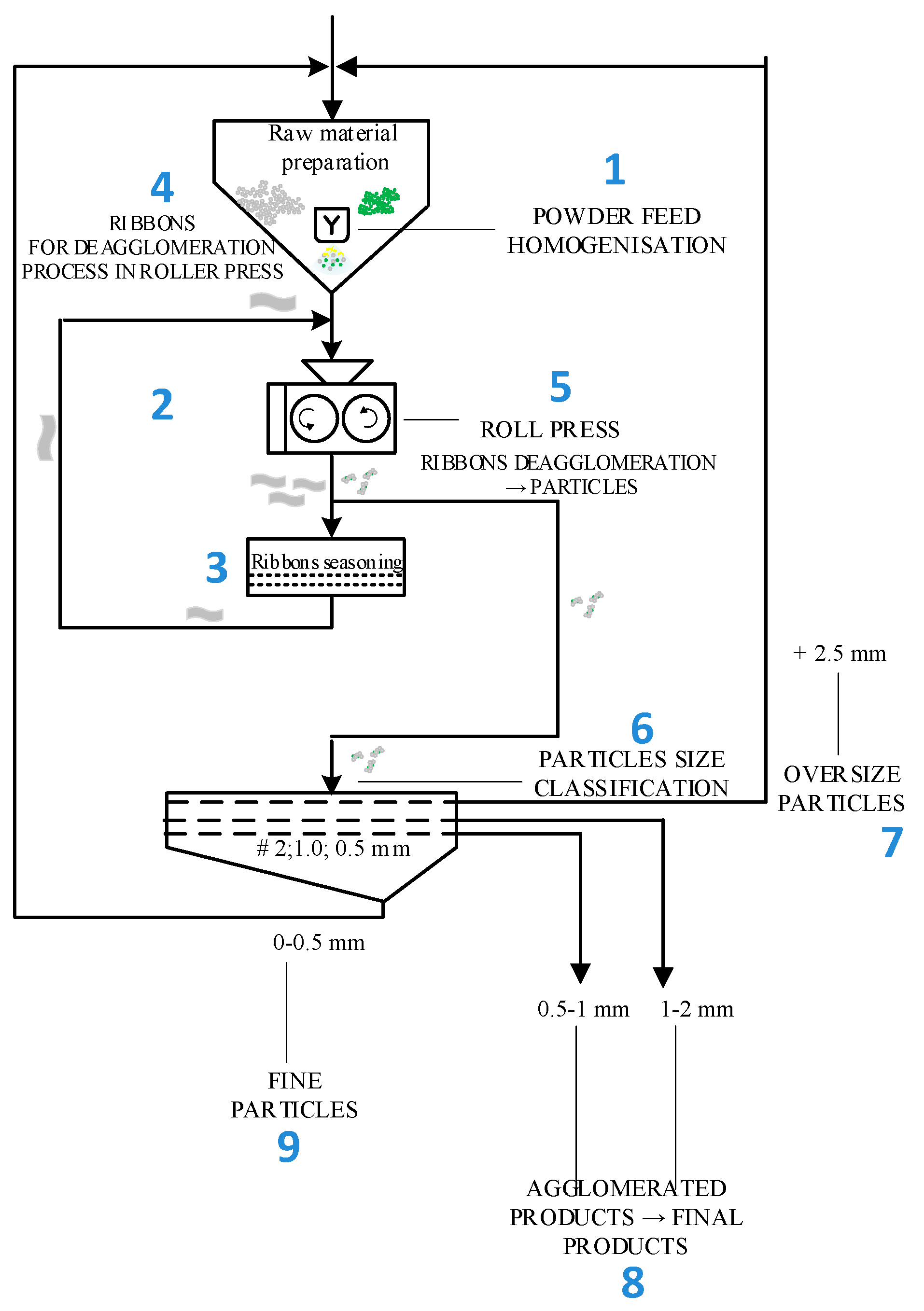





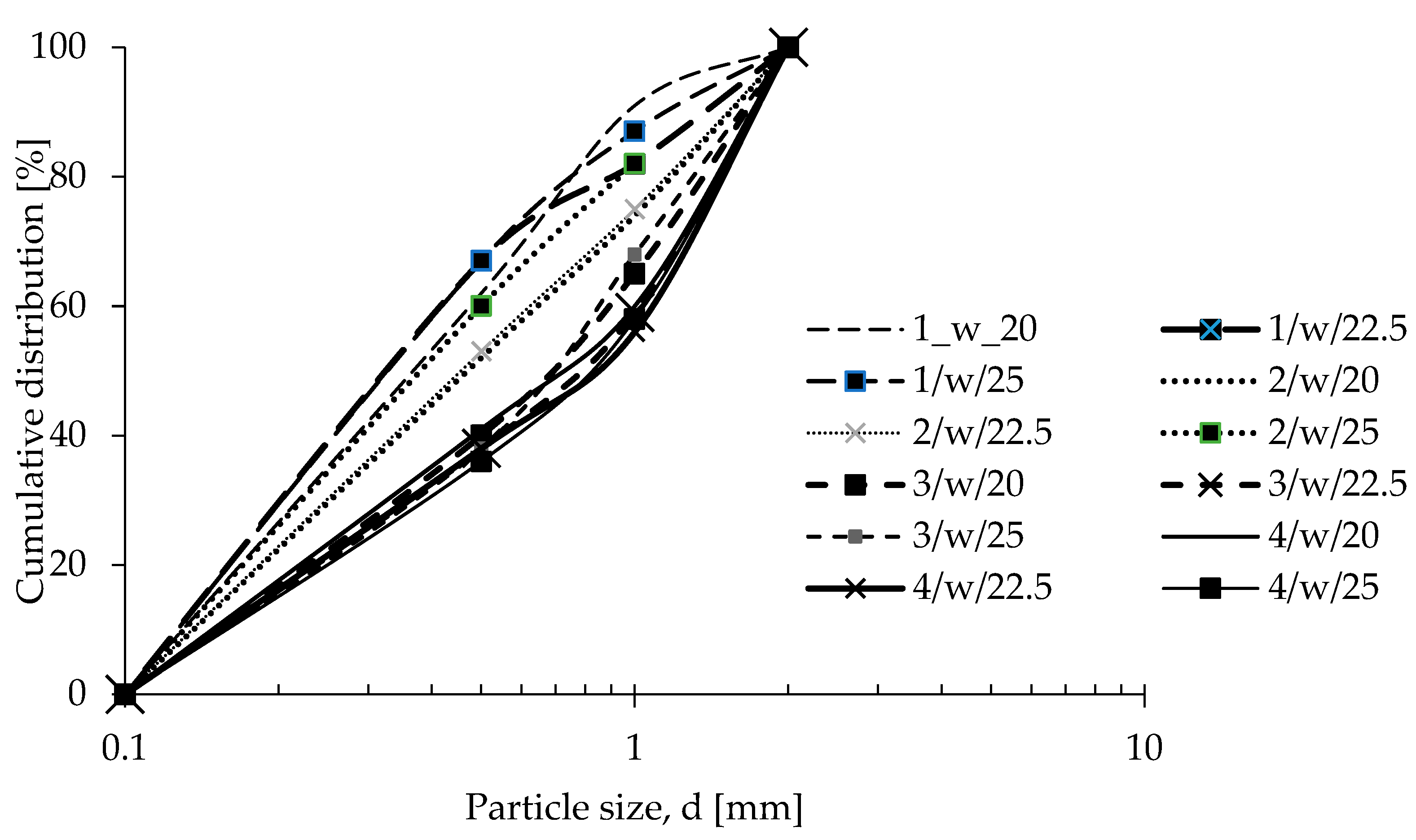
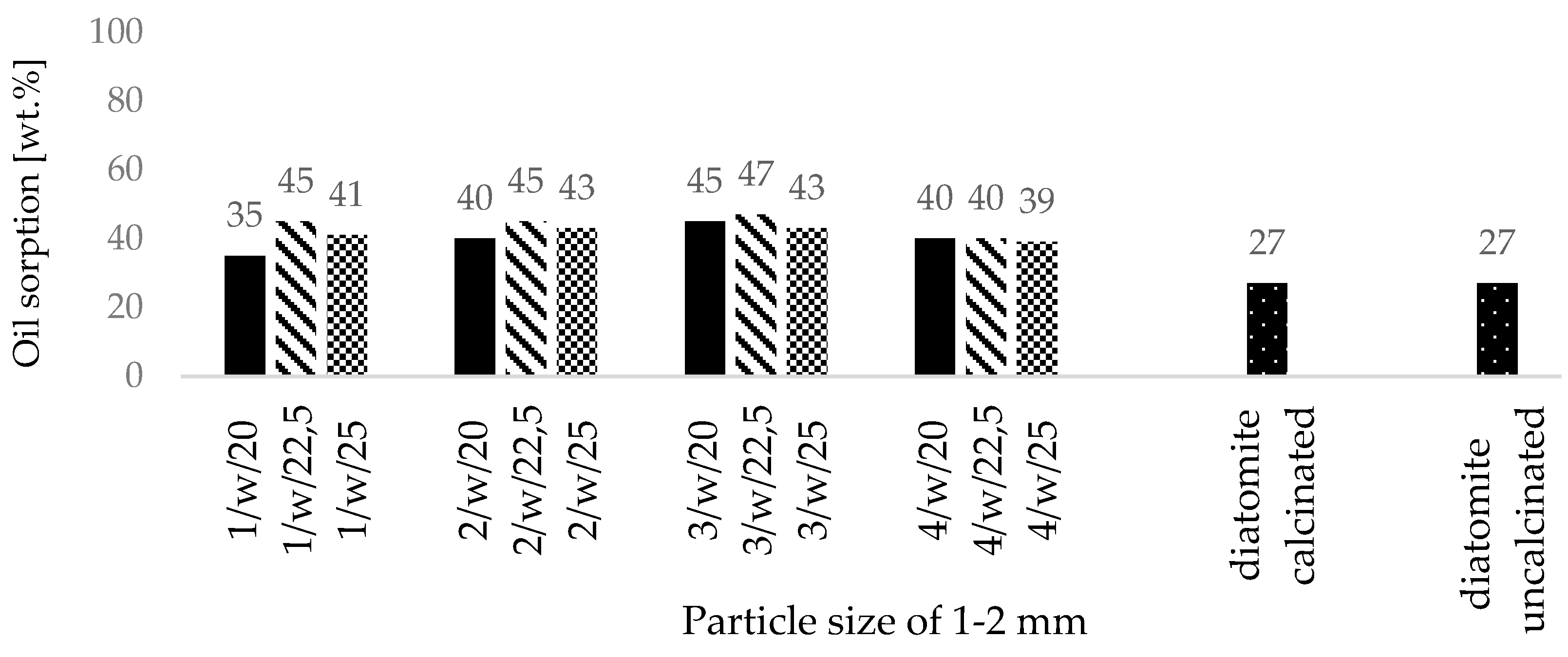
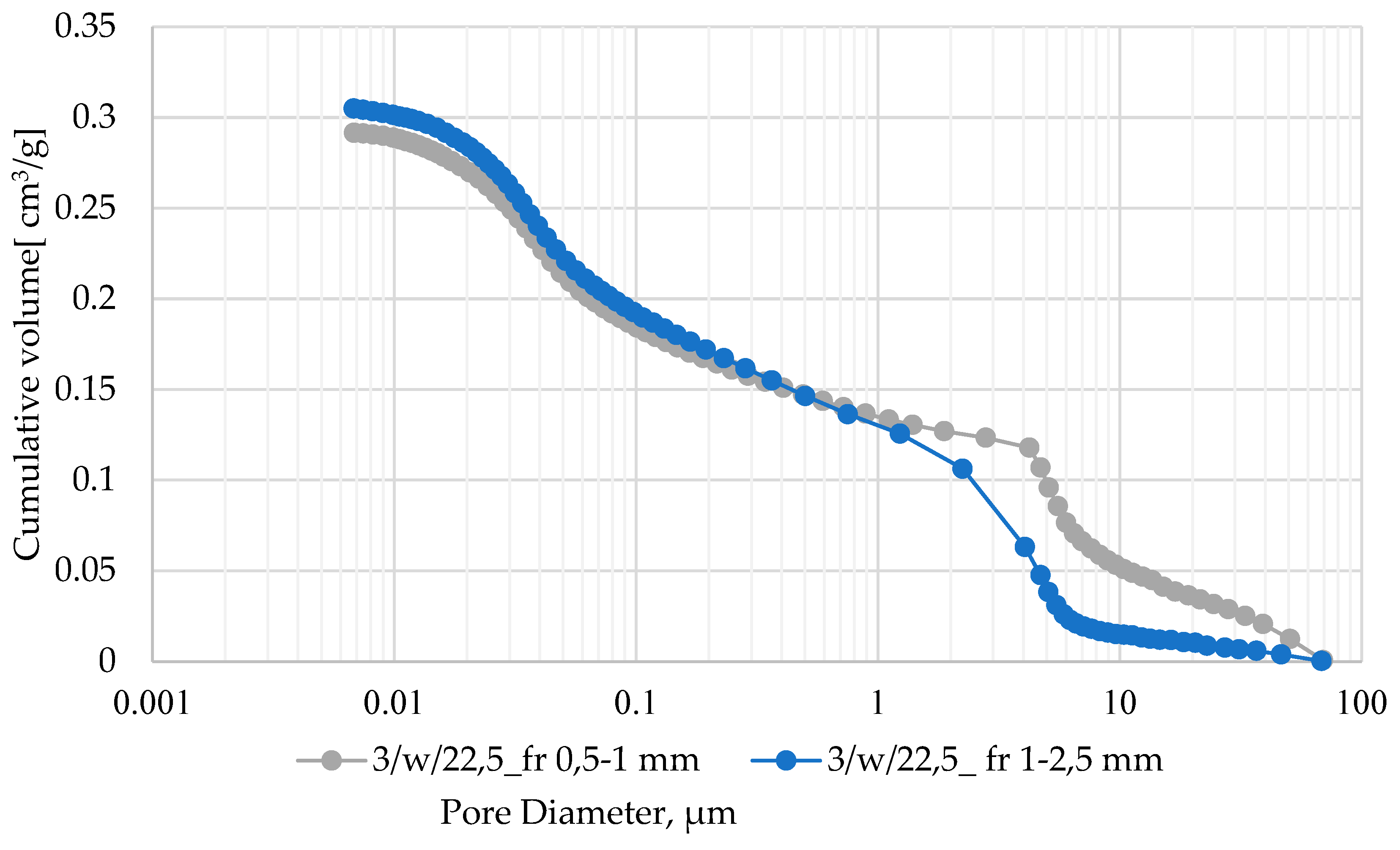
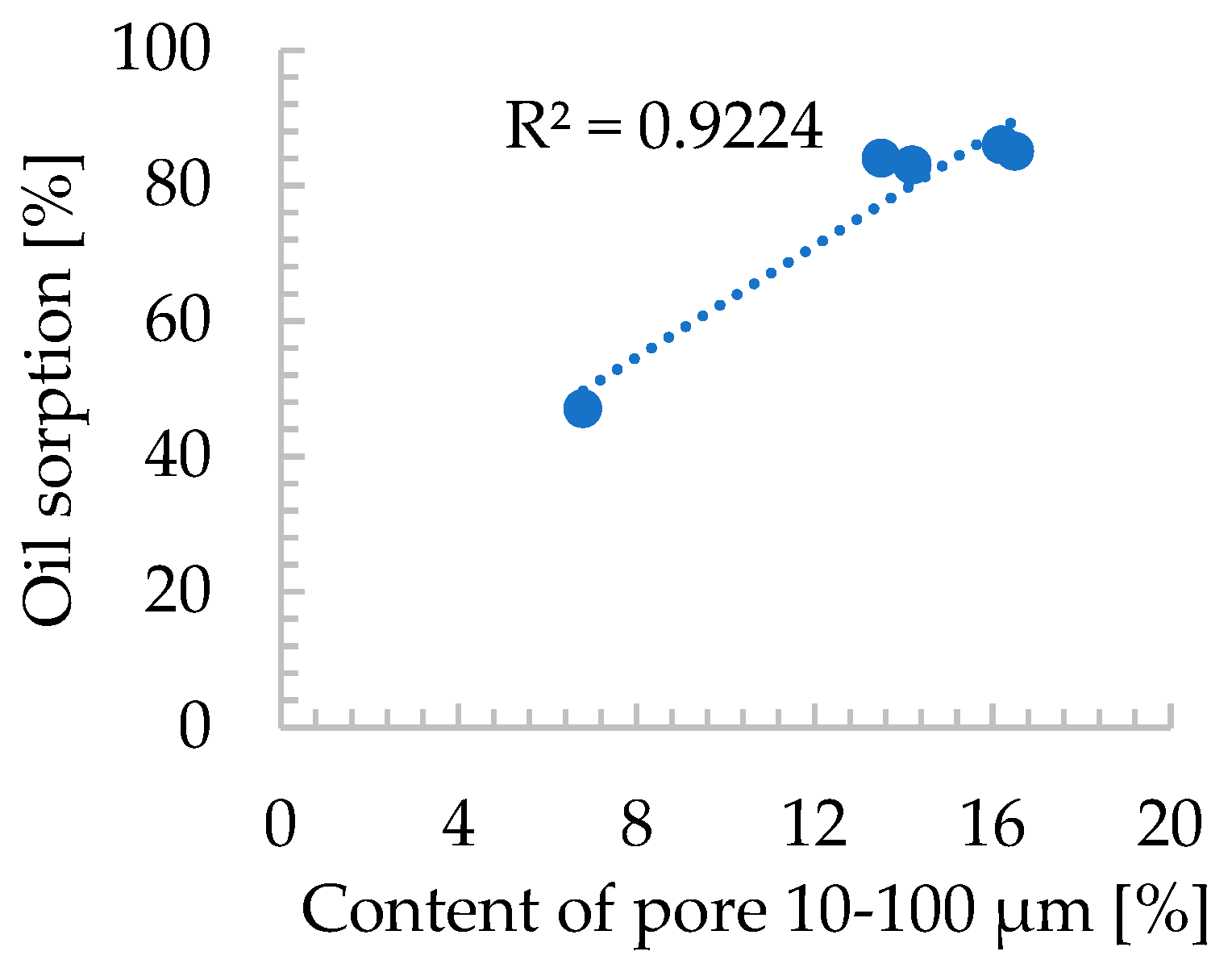
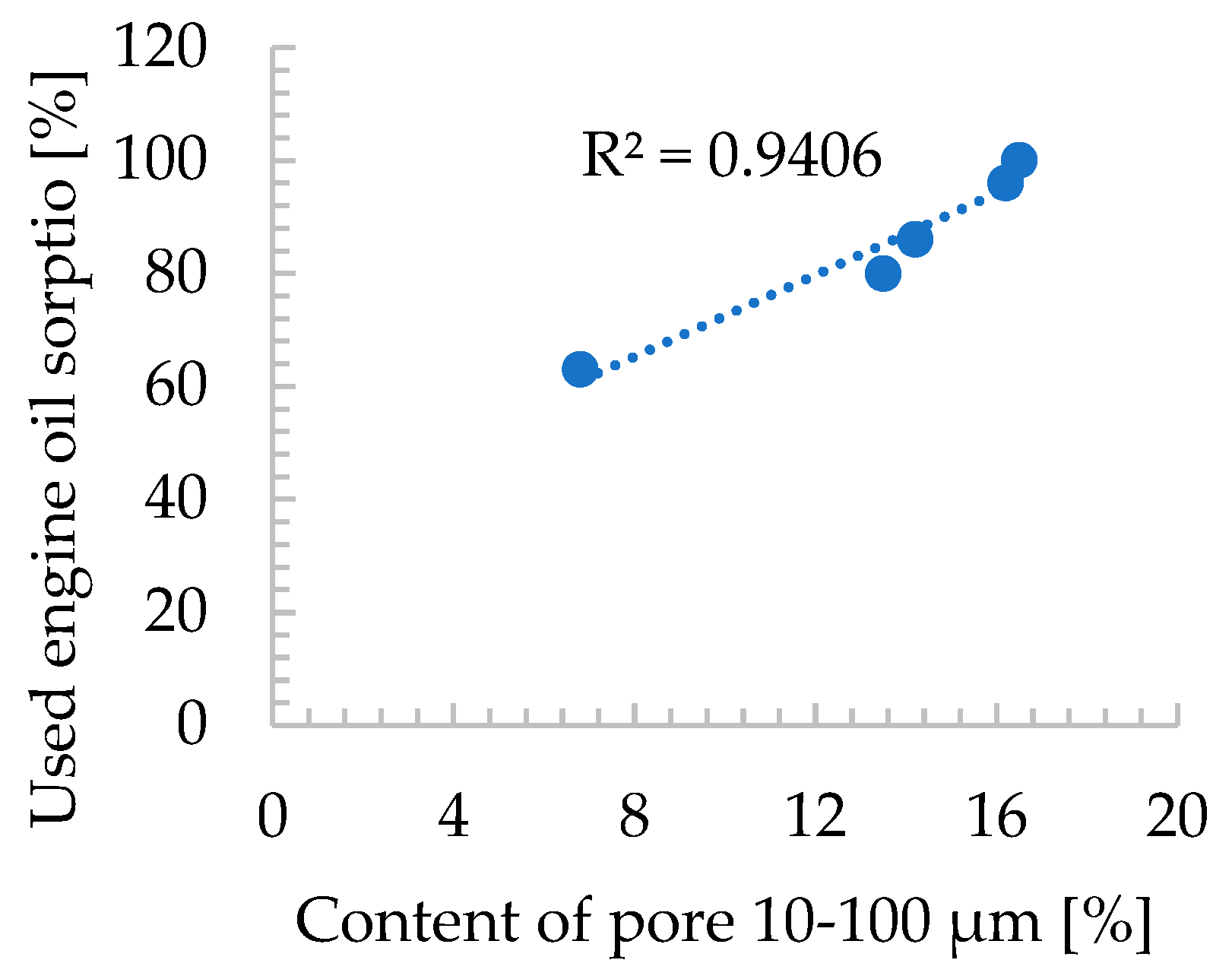
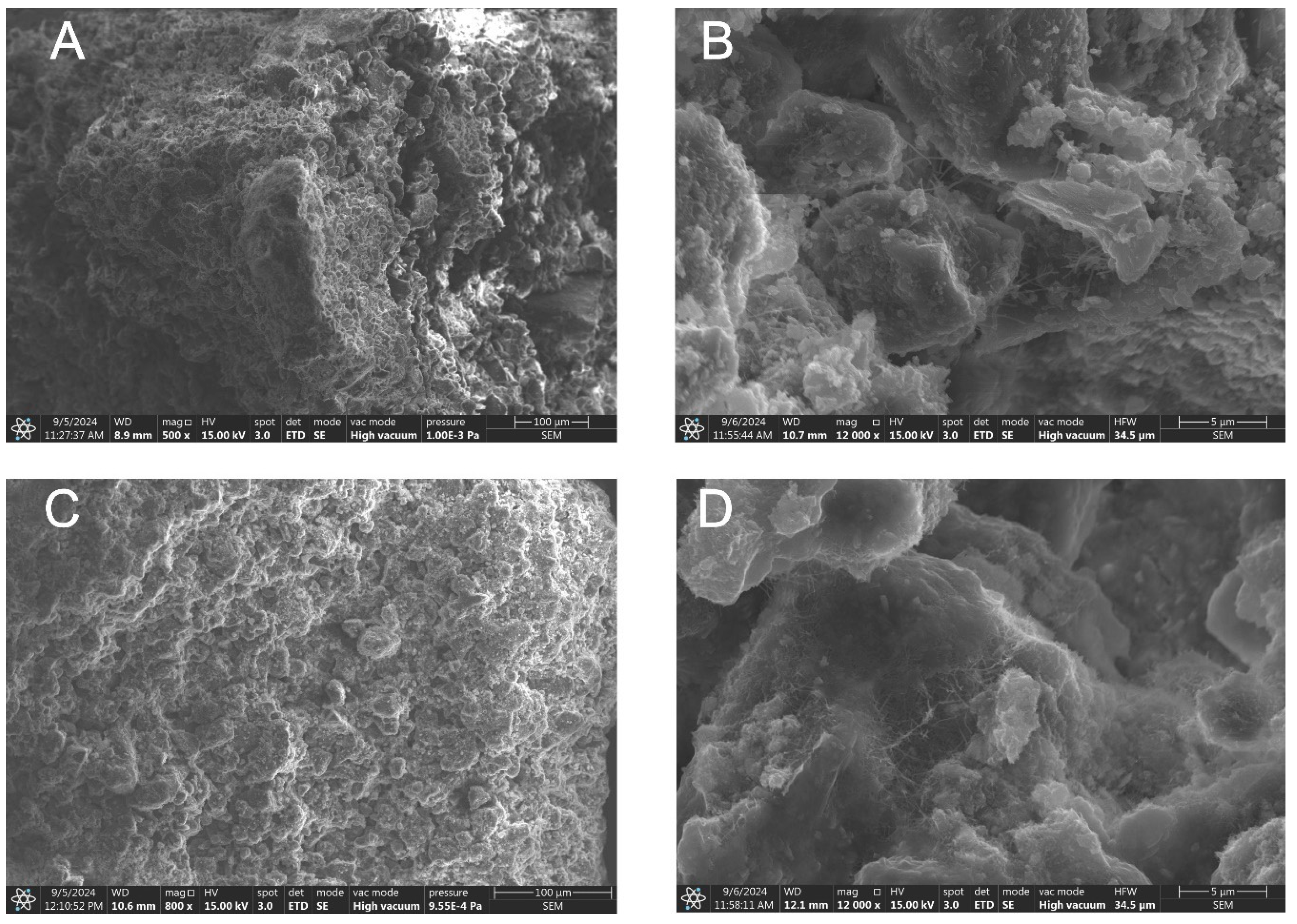
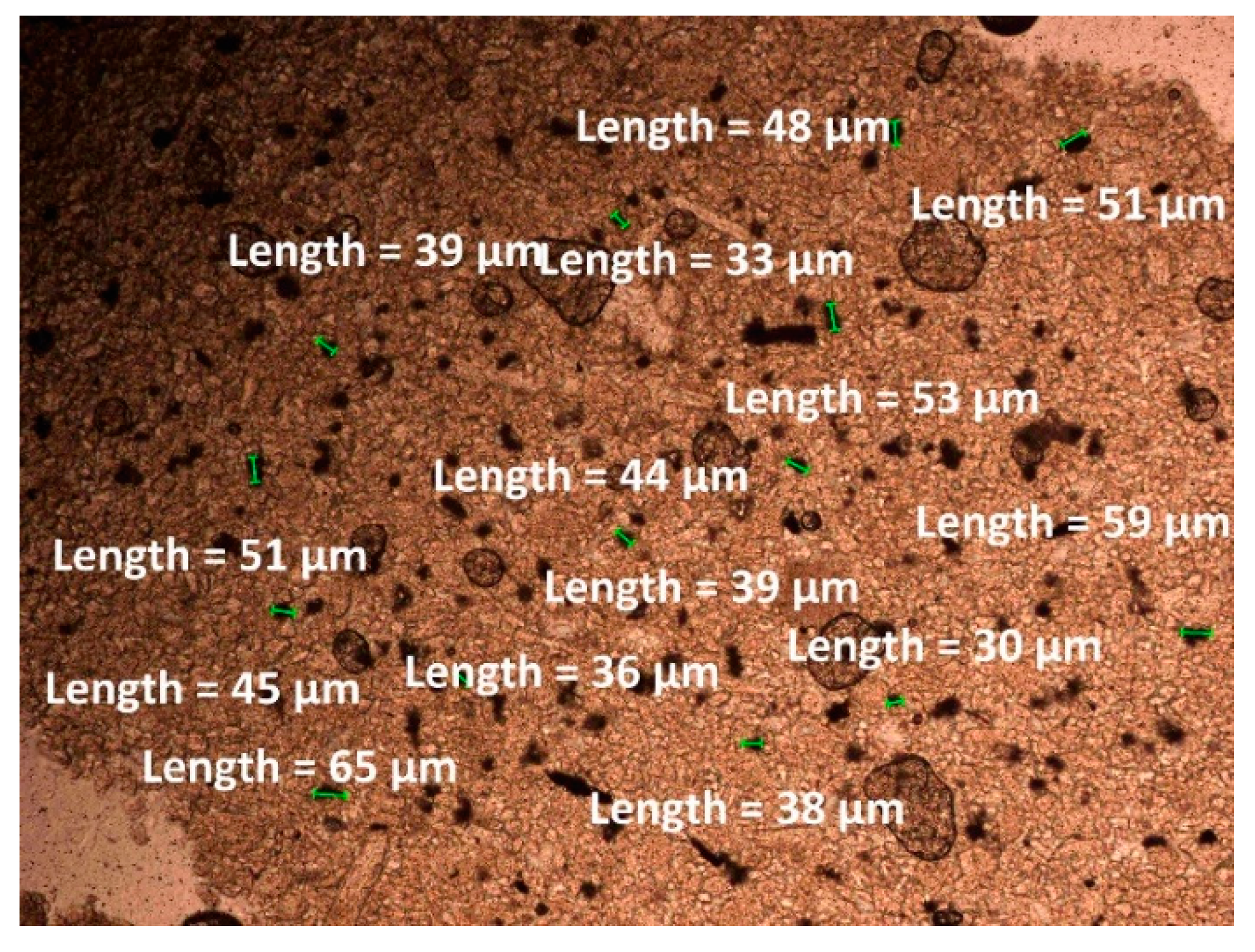
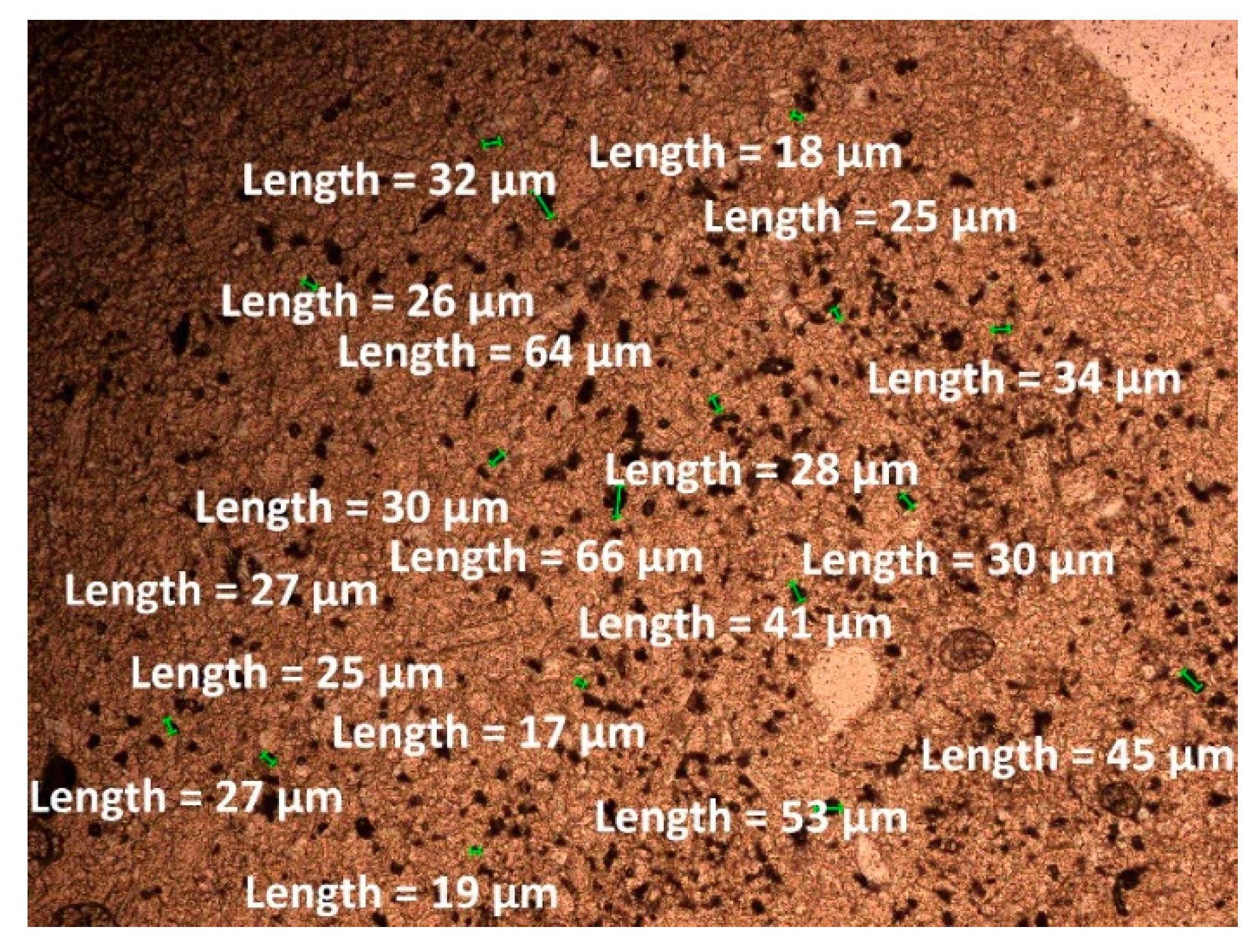
| Oxide composition (wt %) | |||||||||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | TiO2 | Mn2O3 | SrO | ZnO | LOI |
| 68,76 | 11,7 | 1,98 | 2,58 | 0,72 | 0,25 | 3,28 | 1,21 | 0,02 | 0,16 | 0,01 | 0,04 | 0,01 | 9,28 |
| Powder Type | BET (m2/g) | Specific gravity (g/cm3) | Loos bulk density (g/cm3) |
Tapped bulk density (g/cm3) |
Compaction (%) |
Moisture content (%) |
Avg. grain size (µm) |
|---|---|---|---|---|---|---|---|
| Zeolite | 11.58 | 2.31 | 0.97 | 1.190 | 18.5 | 0.70 | 17.75 |
| Binder | 9.94 | 2.2 | 0.47 | 0.78 | 39.7 | 0.53 | 2,91 |
| Sample | SBET (m2/g) | Vtot 0.99 (cm3/g) |
V BJHmes (cm3/g) |
VmicBJH (cm3/g) |
Vmac (cm3/g) |
Average pore diameter 4V/A by BET (nm) |
Average pore width 4V/A by BJH (nm) |
| Zeolite powder | 11.58 | 0.048 | 0.043 | 0.001 | 0.0041 | 1.01 | 20.11 |
| Fabricated Zeolite-based agglomerates |
Binder (%) |
Zeolite Powder (%) |
Water (%) |
|---|---|---|---|
| 1/w/20 | 2,5 | 97,5 | 20 |
| 2/w/20 | 5,0 | 95,0 | |
| 3/w/20 | 7,5 | 92,5 | |
| 4/w/20 | 10,0 | 90,0 | |
| 1/w/22,5 | 2,5 | 97,5 | 22,5 |
| 2/w/22,5 | 5,0 | 95,0 | |
| 3/w/22,5 | 7,5 | 92,5 | |
| 4/w/22,5 | 10,0 | 90,0 | |
| 1/w/25 | 2,5 | 97,5 | 25 |
| 2/w/25 | 5,0 | 95,0 | |
| 3/w/25 | 7,5 | 92,5 | |
| 4/w/25 | 10,0 | 90,0 |
| Fabricated Zeolite-based agglomerates |
Ribbon moisture (%) | Ribbons Fragility | Ribbons powdering | Ribbons Water resistant |
|---|---|---|---|---|
| 1/w/20 | 17.6 | + | P | Ribbons Water resistance |
| 2/w/20 | 16.7 | ++ | P | |
| 3/w/20 | 19.5 | ++++ | PP | |
| 4/w/20 | 17.6 | ++++ | PPP | |
| 1/w/22,5 | 19.4 | + | P | |
| 2/w/22,5 | 18.9 | +++ | P | |
| 3/w/22,5 | 19.0 | +++++ | PP | |
| 4/w/22,5 | 18.2 | +++++ | PPP | |
| 1/w/25 | 22.0 | + | P | |
| 2/w/25 | 22.9 | ++ | P | |
| 3/w/25 | 23.3 | ++++ | PP | |
| 4/w/25 | 23.1 | ++++ | PPP |
|
Fabricated Zeolite-based agglomerates |
Loose bulk density | Drop strength (%) | |
| Granule size (mm) | |||
| 0.5-1 | 1-2.5 | ||
| 1/w/20 | 881 | 87 | 84 |
| 2/w/20 | 860 | 95 | 93 |
| 3/w/20 | 853 | 99 | 98 |
| 4/w/20 | 824 | 98 | 98 |
| 1/w/22,5 | 875 | 88 | 86 |
| 2/w/22,5 | 850 | 95 | 93 |
| 3/w/22,5 | 921 | 98 | 97 |
| 4/w/22,5 | 854 | 99 | 98 |
| 1/w/25 | 879 | 90 | 88 |
| 2/w/25 | 865 | 95 | 95 |
| 3/w/25 | 877 | 98 | 98 |
| 4/w/25 | 850 | 98 | 98 |
|
Uncalcined granular diatomite |
- | 100 | 94 |
| Calcined granular diatomite | - | 100 | 100 |
| Fabricated Zeolite-based agglomerates |
Particle Fraction (mm) |
Total volume of intrusion (cm3/g) |
Total pore Surface (m2/g) |
Average Pore radius (2V/A) |
Apparent density (g/cm3) |
Specific surface area (m2/g) |
|---|---|---|---|---|---|---|
| 3/w/20 | 0.5-1 | 16.1056 | 7.8984 | 4.0782 | 1.23 | 18.33 |
| 3/w/25 | 13.5428 | 7.0563 | 3.8385 | 1.17 | 19.59 | |
| 4/w/22.5 | 12.1479 | 6.788 | 3.579 | 1.31 | 18.72 | |
| 3/w/22.5 | 13.1967 | 5.6687 | 4.656 | 1.20 | 16.15 | |
| 3/w/22.5 | 2-1 | 11.952 | 6.8599 | 3.485 | 1.31 | 17.12 |
|
Fabricated Zeolite-based agglomerates |
Particle Fraction [mm] |
Porosity | Pore size | ||||
| 1-10 nm | 10-100 nm |
100nm-1µm | 1-10 µm |
10-100µm | |||
| Pore content (%) | |||||||
| 3/w/20 | 0,5-1 | 43.09 | 2,1 | 33,6 | 7,8 | 42,9 | 13,5 |
| 3/w/25 | 36.02 | 1,2 | 41,3 | 20,4 | 24,2 | 14,2 | |
| 4/w/22.5 | 36.26 | 1,5 | 39,8 | 18,8 | 23,7 | 16,2 | |
| 3/w/22.5 | 35.10 | 2,3 | 35,6 | 16,3 | 29,4 | 16,5 | |
| 3/w/22.5 | 2-1 | 40.07 | 0,7 | 35,1 | 17,5 | 39,9 | 6,8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





