1. Introduction
Intramyocardial bridge (MB) is a specific coronary anomaly, often asymptomatic, but potentially associated with inducible ischemic events [
1]. It is largely considered a benign variant; however, in sports medicine, and particularly in the context of Italian sports eligibility, it has been a cause for disqualification for many years, as suggested by COCIS 2017 [
2]. The relevance and attention are due to the demonstration of the potential link with acute events, as in the case of MINOCA [
3]. With the revision of COCIS in 2023 the approach has changed. MB forms that are not considered significant in terms of morphology (depth and extension) may be compatible with eligibility. However, significant forms (depth > 3 mm and extension > 1 mm) must undergo specific testing to demonstrate the absence of reduced perfusion and the potential impact on the hemodynamic component. The most commonly used tests to identify inducible ischemia are CPET, physical ECOSTRESS, myocardial scintigraphy with exercise, and coronary angiography. All these are included in the sports medicine protocol suggestions; however, they are not proposed in a sequential manner, as they are considered equally important, without any priority, and therefore without any substantial difference in their specific contribution to prognostic value.
Previous studies have reported the role of nuclear scintigraphy stress testing as consistent and indicative of the presence of an intramyocardial bridge (MB) in the left anterior descending (LAD) coronary artery [
4;
5], although with low specificity in identifying a simple perfusion deficit [
6;
7], due to the "Venturi mechanisms" in the first phase of the diastolic time. On the contrary, the cardiopulmonary test is often used in cases of suspected reduced myocardial capacity [
8], despite the test itself not offering a specific evaluation of segmental myocardial behavior. In this context, echocardiographic deformation parameters, like myocardial strain analysis and the study of torsion, could be helpful to highlight eventual myocardial dysfunction.
The aim of this study was to evaluate the role, in a selected group of asymptomatic athletes with a confirmed diagnosis of "significant" MB (at least for extension) and ST-T alterations suggestive of inducible ischemia, of a non-invasive combined provocative test that integrates CPET data on exercise capacity and myocardial deformation registered during the exercise test. Particularly, the "twist" variant has been tested, as it is the most sensitive parameter for globally evaluating myocardial function in addition to the ejection fraction, to highlight functional deficits in the territory supplied by the left anterior descending artery, such as the LV apex. The group was compared with a control sample of healthy, trained subjects.
2. Materials and Methods
2.1. Material
This is a retrospective observational study that included a sample of 18 participants (9 cases and 9 controls), aged 18 to 78 years, who underwent the competitive certification process at our center between 2022 and 2024. The study was conducted following the Declaration of Helsinki and in accordance with privacy protection regulations. All subjects gave formal consent to participate in the investigation. No specific ethical committee approval was required; as a result, the data are available in the database of the sports medicine center and were normally acquired for sports eligibility purposes. The case group, consisting of 9 athletes (mean age 41.6 years), had a confirmed diagnosis of "significant" intramyocardial bridge based on at least one morphological criterion of extension or depth. The control group consisted of 9 healthy, trained subjects with a mean age of 27.7 years. The case group had undergone CT coronary angiography due to ST-T alterations suggestive of ischemia during exercise testing in previous assessments. The mean age at diagnosis was 39.67 years. All subjects were male, Caucasian, and asymptomatic for chest pain, dyspnea, and syncope. Seven out of nine athletes had a bridge located on the left anterior descending artery (LAD, II-III section), while two had a bridge on the right coronary artery (RCA).
Inclusion criteria included:
A confirmed coronary anomaly diagnosis (intramyocardial bridge).
A uniform level of training and/or physical activity between the groups, estimated using the IPAQ (with IPAQ > 2500 METS/week).
Willingness to participate in the required diagnostic and physical tests.
Exclusion criteria included:
Severe cardiac diseases (e.g., advanced heart failure), cardiological symptoms, or conditions that would prevent eligibility.
Severe chronic renal insufficiency.
Use of medications that affect cardiovascular parameters (e.g., beta-blockers).
Inability to perform the exercise test (CPET) due to physical or neurological limitations.
According to the study protocol, and prior to conducting the CPET test, all subjects were screened for demographic characteristics and cardiovascular risk factors. The case group had also previously undergone myocardial scintigraphy to either demonstrate or exclude coronary flow perfusion induced by physical exercise. Only for the case group with MB was a specific 24-hour Holter ECG (3 leads) performed.
The initial investigation showed that the case population had a higher cardiovascular risk profile (
Table 1), particularly regarding lifestyle factors, compared to the controls.
Methods
2.2.1. Standard Echocardiography
Following the study protocol, the subjects underwent a standard 2D echocardiography. The main systolic and diastolic parameters were measured in accordance with the ASE guidelines [
9]. For the standard parameters, the following measurements were considered: diameters (left ventricle end-diastolic diameter, LVEDD) and thicknesses (interventricular septum, IVS, and posterior wall, PW) of the left ventricle, LV cardiac mass, and indexed left atrial volume (LA volume).
Regarding diastolic function, the mitral inflow pattern (E/A ratio, deceleration time), annular mitral septal and lateral TDI with the E/e′ ratio were considered. The mitral E/e′ ratio was calculated and regarded as a reliable index of LA and filling pressures. Diastolic function was classified as normal or abnormal, with impaired relaxation (grade 1), pseudo-normal (grade 2), or restrictive (grade 3).
2.2.2. Strain Analysis
Deformation parameters were obtained using the speckle tracking method. Specifically, the global longitudinal strain (GLS), radial and circumferential strain, and ventricular twist (twist) at rest and during exercise were considered. For the correct acquisition of the cycles during the CPET, the heart rate range for capturing images was established between the 1st and 2nd thresholds, as measured by CPET. This choice was made in order to avoid and reduce potential errors caused by artifacts due to elevated heart rates. A dedicated software, included in the MyLab Echo (X-Strain) (XStrain TM - ESAOTE - Genoa, Italy), was used for specific post-processing analysis. According to the criteria established in the EACVI/ASE consensus document [
10], it was possible to obtain the overall study and the average myocardial deformation by acquiring the images in a full cycle at a high frame rate. According to the EACVI/ASE guidelines and based on the estimated reproducibility of the data [
11], normal GLS values were considered to range from -24% to -16%, and normal left ventricular twist values ranged from 10° to 20°.
2.2.3. Cardiopulmonary Exercise Test (CPET)
The CPET was conducted based on the guidelines [12; 13; 14]. The test was performed using an electromagnetic brake cycle ergometer (Ergoline) and a specific gas measurement machine (COSMED Quark CPET, Albano Laziale, Rome, Italy). Measurements of VO2 max, peak oxygen pulse, and VE/VCO2 slope were taken. ECG parameters were monitored during the CPET. Each participant was instructed to avoid strenuous physical activity the day before the test and to abstain from consuming solid foods or carbohydrate-rich drinks for three hours prior to the test. The test was performed in the morning under controlled conditions (temperature: 18–24°C; humidity: 30–60%). The ramp protocol for cardiopulmonary testing was tailored based on gender and body composition, aiming for muscle exhaustion between 8 and 12 minutes. An oro-facial mask connected to a gas-measuring device was used. Exhaled CO2 and O2 consumption were measured breath by breath. The smallest possible increase in watts (1, 2, or 5 watts) was set for each ramp to achieve the most linear increase in load and, therefore, a more physiological response. After 3 minutes of warm-up cycling without load at 50 rpm, the test proceeded as follows: at the start of the actual effort, participants were required to cycle at a cadence between 60 and 80 rpm until muscle exhaustion. The test concluded when the participant could no longer maintain their cycling cadence despite verbal encouragement. The test was considered maximal if at least two of the following criteria were met: Respiratory Exchange Ratio (RER) > 1.10, maximum heart rate > 85% of the age-predicted maximum, and a plateau in oxygen consumption (increase < 150 mL·min−1) in the last 30 seconds of the test. The test was stopped early in the presence of cardiovascular signs and symptoms (complex ventricular arrhythmias, drops in systolic blood pressure, dizziness, etc.). Continuous monitoring included a 12-lead ECG and oxygen saturation. During the test, various parameters were measured, including oxygen consumption (VO2), carbon dioxide production (VCO2), tidal volume (VT), respiratory rate (RF), minute ventilation (VE), heart rate (HR), and workload (WR). The lactate threshold was determined using the V-slope and ventilatory equivalents approach. Other variables analyzed included the relationship between oxygen consumption and heart rate (VO2/HR, a measure of stroke volume), the relationship between oxygen consumption and workload (VO2/W slope, a measure of circulatory efficiency), and the product of VO2 peak (mL/kg/min) and systolic blood pressure (a measure of circulatory strength). For the present investigation, the VO2 max value was considered as an expression of normal heart performance in the two groups.
2.2.4. Specific Exams for Cases Group
24-Hour Holter ECG: To analyze the presence of arrhythmias and repolarization abnormalities.
Myocardial Scintigraphy at Rest and Under Stress: To assess myocardial perfusion.
Coronary CT (Coro-TC): For the analysis of coronary anomalies.
During the cardiopulmonary exercise test, between the first and second thresholds, identified by the VO2 max graph and/or the ventilatory threshold, cineloop images were acquired for subsequent analysis of deformation parameters, particularly GLS and Twist.
2.3. Study Design
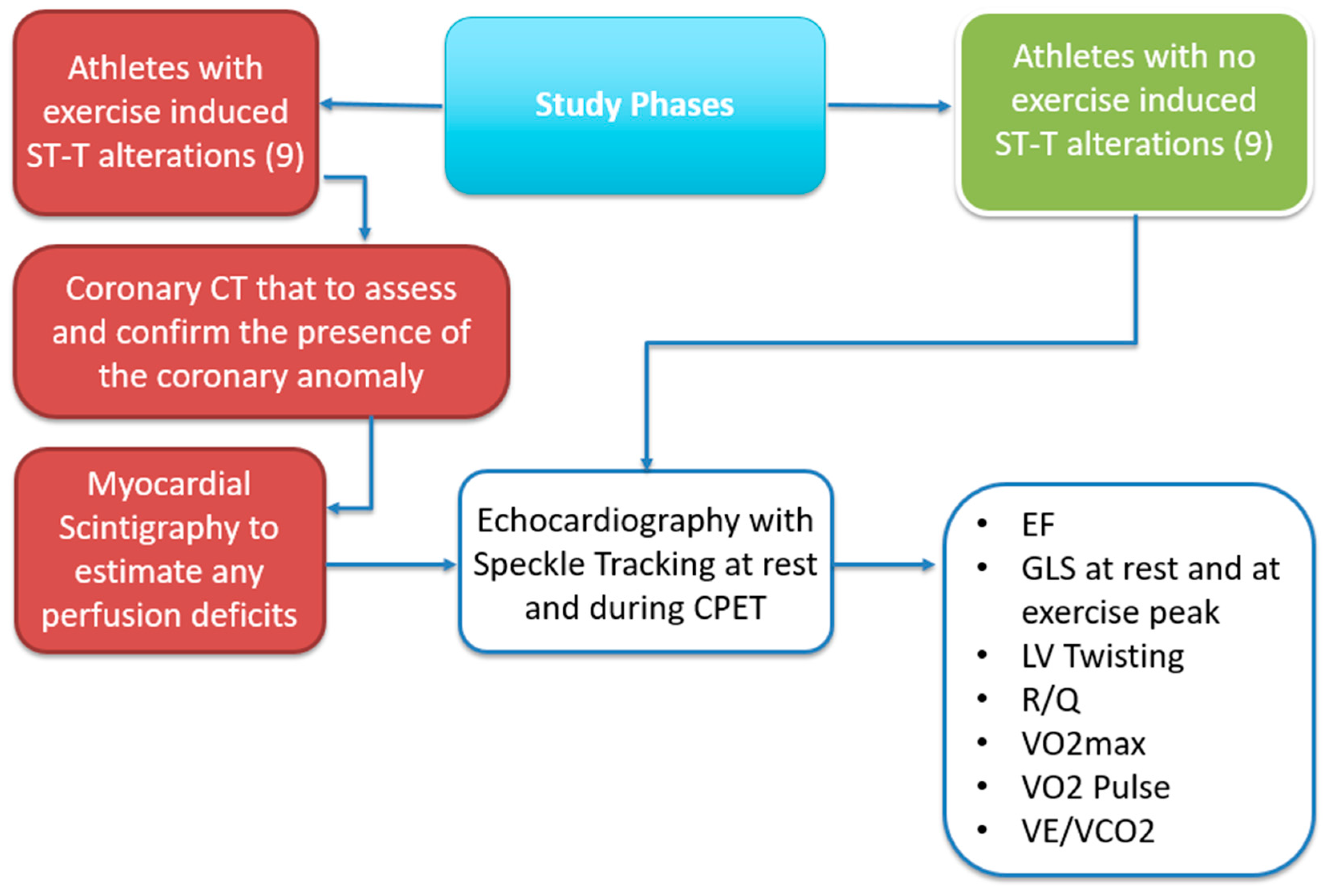
The study was observational and retrospective, aimed at evaluating both athletes with an intramyocardial bridge and controls. The study assessed their training level and both regional and global cardiovascular function using the diagnostic tools mentioned above.
2.4. Study Phases
Participants were selected from the sports medical center based on inclusion and exclusion criteria and underwent under evaluations as showed in
Figure 1. Clinical, anthropometric, and diagnostic parameters were recorded. Each participant completed the following:
Coronary CT (Coro-TC) for cases, to assess and confirm the presence of the coronary anomaly in the presence of ST-T alterations suggestive of exercise-induced ischemia.
24-Hour Holter ECG to assess the progression of ST-segment changes and any associated, asymptomatic arrhythmias.
Myocardial Scintigraphy to estimate any perfusion deficits.
Echocardiography with Speckle Tracking at rest and during CPET.
Cardiopulmonary Exercise Test (CPET) to evaluate exercise capacity and respiratory function.
The data were first analyzed based on the ESC guidelines, and specifically COCIS 2023 guidelines, formulated for competitive sports medical certification, with a focus on strain behavior, twist, and cardiovascular performance during physical exertion.
2.5. Statistical Analysis
The Shapiro-Wilk test was used to assess the normal distribution of the variables, and due to their skewness, the Mann-Whitney U test was applied to compare non-normally distributed continuous and discrete variables. The Chi-square test with Yates continuity correction and Fisher's exact test were used to compare categorical variables. Descriptive statistics were performed to calculate the demographic and prognostic factors of the patients. All statistical analyses were performed using IBM-SPSS® version 26.0 (IBM Corp., Armonk, NY, USA, 2019). In all analyses, a two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. SPECT
Myocardial perfusion tomography (SPECT) was performed (only in cases) to assess the presence of myocardial ischemia in the group of subjects diagnosed with significant intramyocardial bridge. The results were analyzed using the standard ventricular subdivision into 17 segments according to the QPS report to determine the distribution and frequency of exercise-induced ischemia.
Distribution of Ischemic Segments by Patient:
Patient 1: No ischemia detected.
Patient 2: No ischemia detected.
Patient 3: 2 ischemic segments (RCA territory, mild under stress).
Patient 4: No ischemia detected.
Patient 5: No ischemia detected.
Patient 6: No ischemia detected.
Patient 7: 1 ischemic segment (LAD territory, mild under stress).
Patient 8: No ischemia detected.
Patient 9: 3 ischemic segments (2 CX: 1 mild under stress and 1 moderate under stress; 1 RCA: mild under stress).
These results reflect the pathophysiology of intramyocardial bridge, which typically limits coronary blood flow under conditions of increased hemodynamic demand. The presence of moderate ischemia, observed in only one patient (Patient 9), highlights the variability of the hemodynamic impact among the subjects analyzed.
3.2. Echocardiography
All data are expressed as mean as SD. The standard resting echocardiographic analysis shows no significant difference between the case and control groups for some parameters. The ejection fraction (EF) was slightly lower in the cases (mean: 65.1%) compared to the controls (mean: 67.6%), with a difference of -2.4%. Regarding the thickness of the interventricular septum (IVS) and the posterior wall (PW), the average values in the cases were 9.39 mm and 9.20 mm, slightly higher than in the controls (9.12 mm and 9.12 mm). The left atrial diameter (LA diameter) was smaller in the cases (32.1 mm) compared to the controls (34.1 mm), with a difference of -1.94 mm. Finally, the ventricular volumes (LVEF) showed no significant difference, with slightly lower average values in the cases (49.1 mL) compared to the controls (50.2 mL). These data show that the two populations studied are homogeneous at least for the standard echocardiographic parameters.
3.3. Analysis of Strain and Twist Parameters Related to Myocardial Deformation
The data related to the strain and twist parameters, both at rest and during stress, were analyzed in the two groups:
GLS (Global Longitudinal Strain): At rest, the cases showed a mean value of -18.09% vs. controls (-18.92%), with no significant differences. However, during stress, the difference was significant, with mean values of -15.38% in the cases and -21.44% in the controls (p :< 0.01), suggesting a reduction in the longitudinal contractile capacity (reduced longitudinal contribution) in the cases under physical stress.
Circumferential Strain: The contribution of apical reserve estimated with circumferential strain at the apex was similar between the two groups at rest (cases: 6.28%, controls: 6.30%). However, the mean values of the base rotation of the heart in the cases were more negative (-6.03%) and thus better than in the controls (-4.43%). During stress, the apical rotation (GCS) significantly increased in the controls, but not in the cases. This behavior suggests a loss of the characteristic athlete-like behavior in the cases with myocardial bridge.
Ventricular twist: At rest was greater in the cases, with a mean of 10.64° ± 3.29°, compared to the controls, with a mean of 9.49° ± 2.03°. This behavior reverses under stress, with a mean value of 8.27° ± 1.78° for the cases and 16.89° ± 3.34° for the controls. This behavior indicates a loss of apical reserve in subjects with myocardial bridge. Upon analyzing the myocardial deformation during stress (Twist) in the two groups, significant differences were found in the apical reserve of the left ventricle, which does not increase while decreases in the cases compared to the controls. This finding is potentially related to reduced perfusion and should be considered atypical, particularly in athletes. It is noteworthy that in the cases, the Twist value remains within the normal range or increases as in the controls group.
CPET (Cardiopulmonary Exercise Testing): the data collected during the cardiopulmonary exercise test (CPET) show significant differences between the cases and the controls. VO2max, indicative of cardiorespiratory capacity, particularly exercise capacity, was lower in the cases (mean VO2max: 36.12 mL/kg/min) compared to the controls (mean: 52.14 mL/kg/min), with a difference of -16.02 mL/kg/min, suggesting a reduction in aerobic performance in the cases. The oxygen pulse, which reflects the efficiency of oxygen transport during exercise, was also lower in the cases (mean: 15.19 mL/beat) compared to the controls (mean: 19.76 mL/beat), with a difference of -4.57 mL/beat. In contrast, the VE/VCO2 ratio, which represents ventilatory efficiency, was slightly higher in the cases (mean: 24.56) compared to the controls (mean: 23.46), with a difference of 1.10, indicating a possible ventilatory compensation in the cases. These latter data are still under analysis and have not yet been clarified in the present study; therefore, they are currently considered outside the main scope.
Electrocardiographic (ECG) and Holter Abnormalities: Abnormalities were observed only in the cases, both during the maximal exercise test (TEM) and during Holter monitoring. Specifically:
ST >1 mm: Detected in 4 cases (44.4%).
ST <1 mm: Present in 4 cases (44.4%).
Regression of the ST wave during recovery: Found in 4 cases (44.4%).
Inferolateral alterations: Present in 4 cases (44.4%).
Right bundle branch block (RBBB): Identified in 6 cases (66.7%).
Supraventricular (>500/24h): Found in 6 cases (66.7%).
Simple ventricular arrhythmias: Present in 6 cases (66.7%).
Complex ventricular arrhythmias: Identified in 1 case (11.1%).
In the control group, no significant abnormalities were observed either during the TEM or during Holter monitoring:
ST >1 mm, ST <1 mm, regression during recovery: Absent in all controls.
Inferolateral alterations: Not detected.
Right bundle branch block (RBBB): Absent.
Supraventricular (>500/24h): Not detected.
Simple and complex ventricular arrhythmias: Not present in the controls.
These results highlight a higher incidence of electrocardiographic abnormalities in the cases compared to the controls, with particular findings of ST segment alterations, right bundle branch block, and both ventricular and supraventricular arrhythmias. This pattern suggests and confirms a correlation between the myocardial bridge and the observed electrocardiographic abnormalities.
3.4. Correlations Between Myocardial Deformation Parameters and CPET:
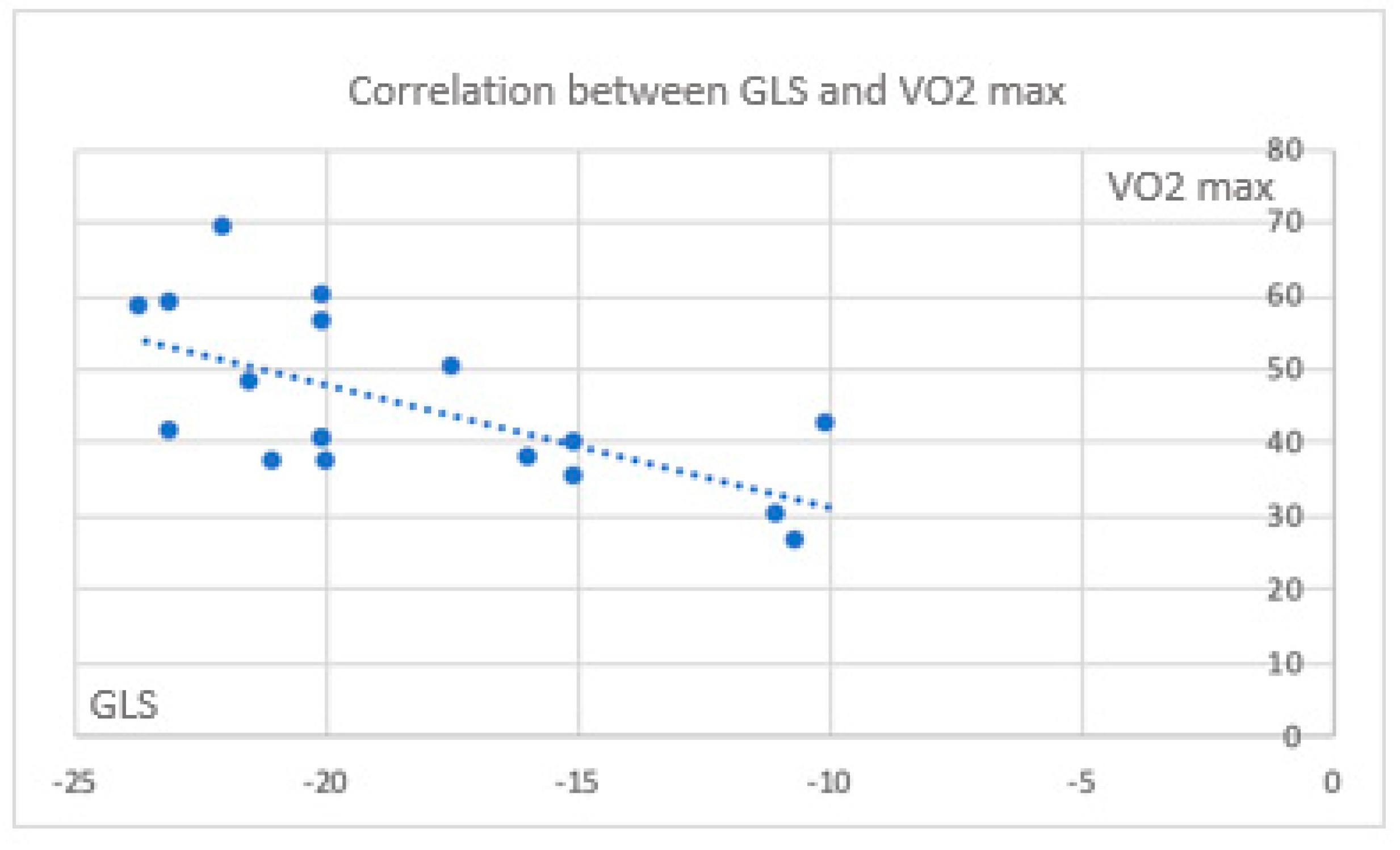
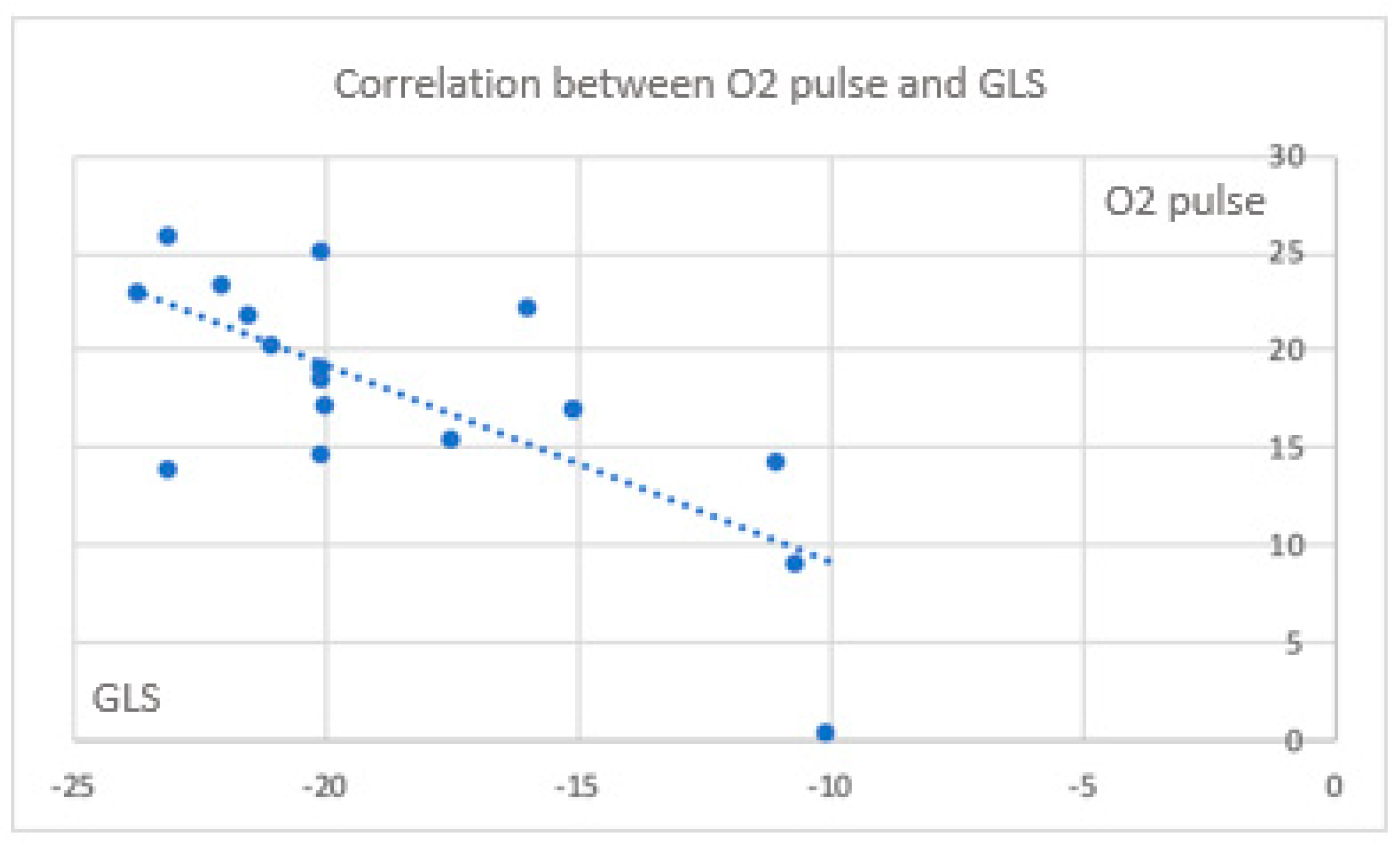
Color mapping highlights the parameters that correlate with each other, including those obtained from CPET and those measured during the stress echocardiography, with greater emphasis on myocardial deformation parameters. All echocardiographic parameters expression of myocardial deformation correlate significantly with cardiac function parameters (exercise capacity) obtained from the CPET. In the cases, the correlation between CPET parameters and deformation one, are indicative for a trend of a maintenance of exercise capacity and GLS , despite it is negative (
Figure 1 and
Figure 2).
Figure 1.
Correlation between GLS and VO2 max.
Figure 1.
Correlation between GLS and VO2 max.
Figure 2.
Correlation between O2 pulse and GLS.
Figure 2.
Correlation between O2 pulse and GLS.
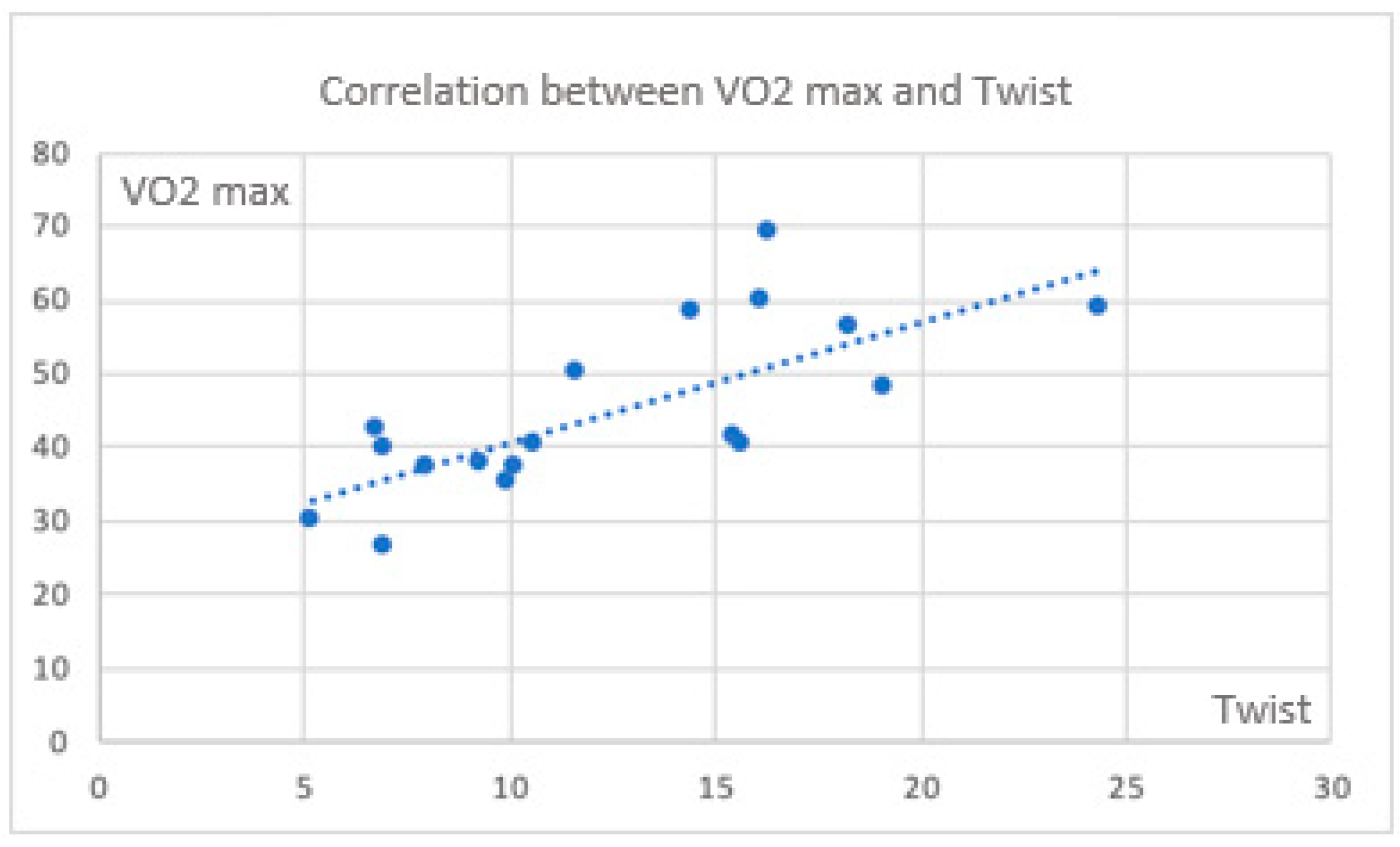
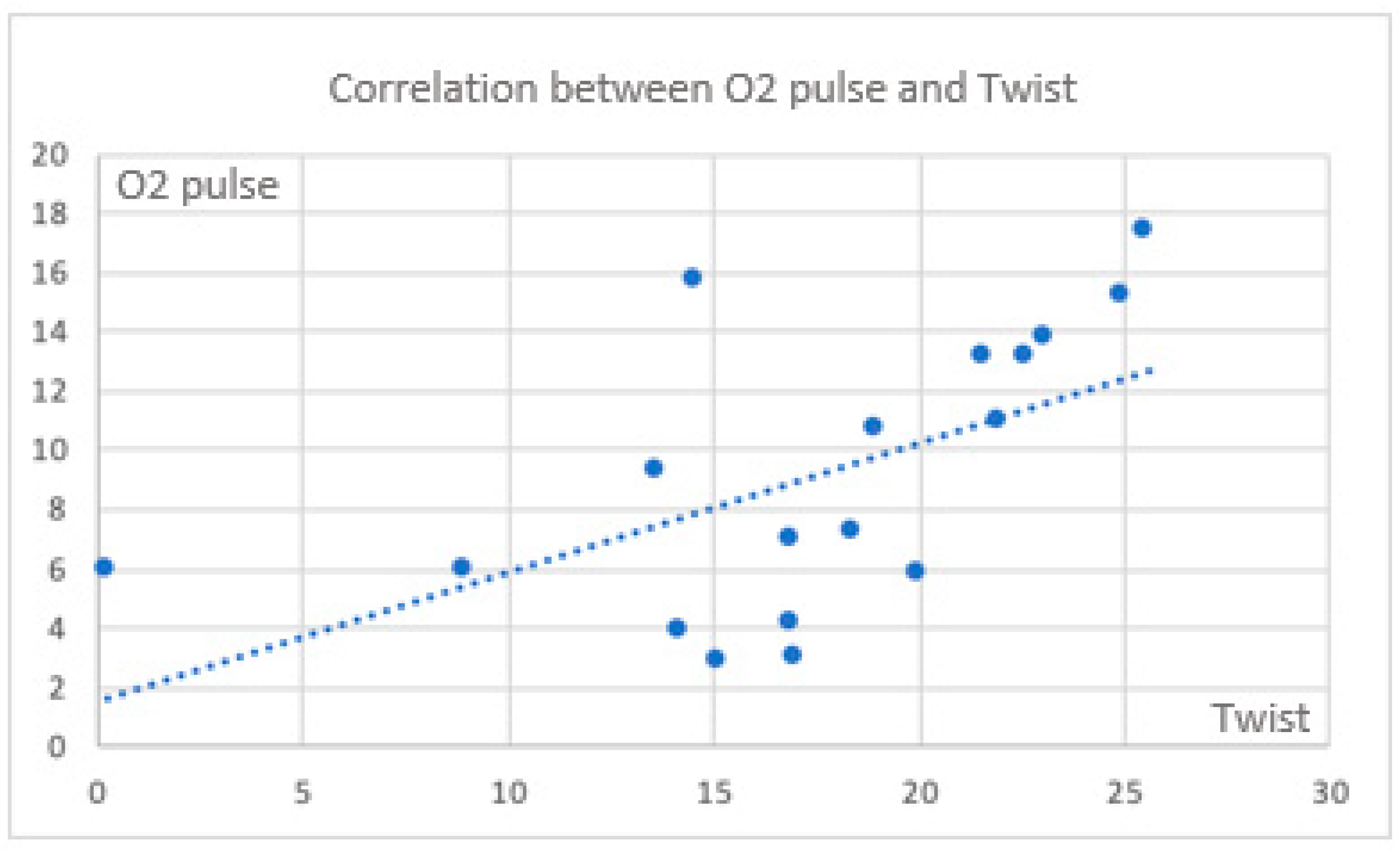
There are also significant correlations between CPET parameters and twist (
Figure 3 and
Figure 4).
Considering the torsion parameter as an expression of apical reserve and the potential for maintaining an increased contribution to the major myocardial contraction, the correlation between VO
2 max and twist is lost in some cases. On the contrary, it is evident in the control group. (
Figure 5) During exercise, a significant positive correlation between circumferential strain at the apex and the oxygen pulse is also maintained in this group.
All data suggest a specific pattern of deformation parameters in subjects with MB, which becomes evident during the exercise test. The specific correlation of the two parameters derived from different investigations (CPET and echocardiography) highlights the importance of studying these events in the acute phase. In summary, in the cases, there is a significant reduction in deformation parameters during exercise (GLS and twist), the loss of apical contribution, and a lack of correlation with VO2 max and oxygen pulse. In contrast, the controls, who maintain their apical reserve, show a significant and positive correlation in both parameters during exercise.
4. Discussion
The intramyocardial bridge refers to an anomalous course of the coronary arteries, which has traditionally been considered incompatible with eligibility for elite sports, particularly under the Italian regulations [Cocis, 2017]. The potential impairment of coronary blood flow, especially during systolic-diastolic phases of exercise, has made it an important subject of study in recent years [15; 16; 17]. Despite this, a recent review in sports cardiology, incorporating an anatomical classification of the myocardial bridge in terms of morpho-functional aspects, has led to a broader inclusion of affected individuals. Specifically, those who do not present the critical characteristics of the anomaly (Depth > 3 mm; Length > 1 cm, and the so-called "significant" features [
18]) may still be eligible for sports activities. However, the appropriate follow-up of athletes, especially those in the "grey zone" of the anatomical classification, and their eventual inclusion in regular training, have not yet been thoroughly investigated. This is particularly relevant in cases where no clinical symptoms are present, even during exercise. In such cases, where only one of the morphological criteria is met but accompanied by ECG changes, ST-T patterns suggestive of ischemia may emerge. These factors can lead to interpretative and management uncertainties, especially during the annual follow-up of athletes seeking sport eligibility, particularly for individuals over the age of 35 or those with additional comorbidities. Therefore, it is crucial in sports medicine to optimize testing methods that, following a definitive morphological diagnosis—especially if the case is borderline—can confirm the absence of ischemia in an outpatient setting. Given this context, an integrated dynamic test with additional information becomes indispensable. In line with previous studies [
15], the combination of cardiopulmonary exercise testing (CPET), supported by dynamic assessments of deformation parameters at rest and during exercise (e.g., GLS and Twist), could offer a valuable approach for this group, even in follow-up situations. In particular, the dynamic CPET, integrated with myocardial deformation data, provides enhanced interpretative possibilities for the cases studied. GLS and Twist during exercise are strongly correlated with VO2max and oxygen pulse, demonstrating that the longitudinal and torsional mechanical functions of the heart are key indicators of aerobic capacity and cardiac efficiency. Additionally, circumferential strain at the apex and base during exercise shows strong correlations with VE/VCO2 and VO2max, emphasizing the role of the heart's circumferential function in ventilation and aerobic capacity. The absence of significant differences between the two groups during rest further supports the homogeneity and robustness of the data. The results indicate that, while the cases do not differ from the controls during the resting phase, they display a unique pattern during exercise, marked by a loss of apical rotation contribution, particularly after the first threshold. This is especially evident in apical circumferential strain. This finding correlates with a reduction in longitudinal strain, which is known to contribute less to left ventricular contraction. In contrast, controls, without any coronary abnormalities, exhibit a significant increase in both parameters. Despite these differences, the cases remain within normal functional limits, suggesting that their ventricular function is preserved and that they may still be eligible for sports.
5. Conclusions
Sports medicine is often called upon to manage a diverse range of individuals from the general population, and the potential discovery of myocardial ischemia is frequent. An anomalous course of the coronary arteries has traditionally excluded individuals from eligibility, especially when reduced perfusion is evident. However, there are often borderline cases in which the anomalous course is present but normal perfusion is maintained. In this specific category, the appropriate annual follow-up to determine eligibility cannot include scintigraphy, and an alternative lean program must be implemented to provide clear evidence of normal perfusion. Cardiopulmonary exercise testing (CPET) is well-established in sports medicine as a tool for assessing cardiac function, though it lacks certain key information regarding heart contractility. In fact, deformation parameters have been increasingly utilized in sports medicine [19; 20; 21; 22] to detect subtle dysfunctions that are not typically captured by ejection fraction (EF). Previous studies have proposed the use of this complex examination, despite the technical challenges of performing echocardiographic assessments during exercise [23; 24; 25]. The data gathered support the feasibility of enhancing outpatient evaluations for athletes with a confirmed intramyocardial bridge by incorporating CPET alongside deformation parameter assessments at peak exercise [
26]. This approach seems particularly relevant in individuals with borderline coronary anomalies, at least in cases where the depth criterion is involved, as observed in the limited sample studied. This could potentially form the basis of a protocol for the annual follow-up of individuals with an intramyocardial bridge, improving the management of medical-sport eligibility criteria. Furthermore, the absence of changes in coronary function during the initial evaluation could, over time, suggest the stability of coronary flow in these individuals. However, some limitations should be acknowledged, including the lack of comparisons with other centers, the small sample size, and the absence of data concerning assessments beyond the second threshold. This opens the door for future research, including evaluations during the recovery phase and further validation of the data in individuals with anomalous coronary courses in other coronary territories (e.g., left circumflex, right coronary). Future studies are necessary to confirm the findings and provide more robust evidence.
Author Contributions
Conceptualization, F.F. and L.S.; methodology, L.S.; validation, L.S, M.O, R.P; formal analysis, V.B.; investigation, F.F.; resources, R.P.; data curation, M.O.; writing—original draft preparation, R.P. and F.F.; writing—review and editing, R.P. and L.S.; visualization, L.S.; supervision, L.S.; project administration, L.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on behalf of Prof. L Stefani.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ciliberti, G.; Laborante, R.; Di Francesco, M.; Restivo, A.; Rizzo, G.; Galli, M.; Canonico, F.; Zito, A.; Princi, G.; Vergallo, R.; Leone, A.M.; Burzotta, F.; Trani, C.; Palmieri, V.; Zeppilli, P.; Crea, F.; D'Amario, D. Comprehensive functional and anatomic assessment of myocardial bridging: Unlocking the Gordian Knot. Front. Cardiovasc. Med. 2022, 9, 970422. [Google Scholar] [CrossRef]
- Pelliccia, A.; Delise, P.; Inama, G.; et al. Protocolli cardiologici per il giudizio di idoneità allo sport agonistico. G. Ital. Cardiol. 2017, 18, 1–20. [Google Scholar]
- Sucato, G.; Galassi, V.; Damerino, A.; et al. Myocardial bridging is significantly associated with myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 501–508. [Google Scholar]
- Sucato, G.; Galassi, V.; Damerino, A.; et al. Myocardial bridging of mid-left anterior descending artery (LAD) presenting as transient left bundle branch block during nuclear stress test. Cureus 2022, 14, e23024. [Google Scholar]
- Berman, D.S.; Kang, X.; Slomka, P.J.; et al. The role of myocardial perfusion imaging in evaluating patients with myocardial bridging. J. Nucl. Cardiol. 2023, 30, 1234–1245. [Google Scholar]
- Klues, H.G.; Schwarz, E.R.; vom Dahl, J.; Reffelmann, T.; Reul, H.; Potthast, K.; et al. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation 1997, 96, 2905–2913. [Google Scholar] [CrossRef]
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; et al. Principles of Exercise Testing and Interpretation, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- 9 Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; et al. ; Standardization of Left Atrial, Right Ventricular, and Right Atrial Deformation Imaging Using Two-Dimensional Speckle Tracking Echocardiography: A Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; et al. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Liguori, G. ACSM's Guidelines for Exercise Testing and Prescription, American College of Sports Medicine (ACSM), ISBN/ISSN: 9781975150181, Publication Date: April 8, 2021.
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Ozemek, C.; Systrom, D.; Arena, R. Cardiopulmonary Exercise Testing: What is its Value? Am. Coll. Cardiol. 2017, 70, 1618–1636. [Google Scholar] [CrossRef] [PubMed]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- De Ornelas, B.; Sucato, V.; Vadalà, G.; Buono, A.; Galassi, A.R. Myocardial Bridge and Atherosclerosis, an Intimal Relationship. Curr. Atheroscler. Rep. 2024, 26, 353–366. [Google Scholar] [CrossRef]
- Murtaza G, Mukherjee D, Gharacholou SM, Nanjundappa A, Lavie CJ, Khan AA, Shanmugasundaram M, Paul TK. An Updated Review on Myocardial Bridging. Cardiovasc Revasc Med. 2020 Sep; 21(9):1169-1179. 2020, 21(9), 1169–1179.
- COCIS. Protocolli Cardiologici Idoneità Sportiva. Available from: https://www.sicsport.com/pubblicazioni/cocis-protocolli-cardiologici-idoneita-sportiva.
- D'Ascenzi, F.; Anselmi, F.; Cossu, E.; et al. Left ventricular global longitudinal strain: An early marker of subclinical myocardial dysfunction in athletes. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 973–979. [Google Scholar]
- Pencina, K.M.; Phelan, D.; Garcia, M.; et al. Utility of myocardial deformation imaging in sports cardiology. J. Am. Soc. Echocardiogr. 2018, 31, 1221–1230. [Google Scholar]
- Xie, W.; Zhang, Y.; Liao, Y.; et al. Role of deformation imaging in the assessment of myocardial dysfunction in athletes. J. Appl. Physiol. 2020, 129, 1106–1115. [Google Scholar]
- Sharma, S.; Whyte, G.; Jones, H.; et al. Myocardial deformation imaging in athletes: A critical review. Br. J. Sports Med. 2016, 50, 1050–1057. [Google Scholar]
- McMahon, C.J.; Corcoran, D.; Codd, M.; et al. The challenges and potential of exercise echocardiography in sports cardiology. Br. J. Sports Med. 2019, 53, 1502–1508. [Google Scholar]
- D'Andrea, A.; Caso, P.; Rigo, F.; et al. Feasibility of exercise echocardiography in the assessment of myocardial performance in elite athletes. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 730–736. [Google Scholar]
- Scharf, J.; Grosse, S.; Müller, H.; et al. Technical challenges and clinical applications of exercise echocardiography in sports medicine. J. Sports Med. 2017, 47, 49–55. [Google Scholar]
- Mapelli M, Cattadori G, Salvioni E, Mattavelli I, Pestrin E, Attanasio U, Magrì D, Palermo P, Agostoni P. "Under the Bridge": Looking for Ischemia in a Patient with Intramyocardial Coronary Artery Course-The Role of the Cardiopulmonary Exercise Test. J Clin Med. 2023, 12(17), 5764. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).