1. Introduction
Running capacity is a key indicator of an individual’s physical fitness and overall health status [
1,
2]. Physical activity not only enhances cardiopulmonary function, improves muscle strength and endurance, but also plays a vital role in regulating energy metabolism, alleviating oxidative stress, suppressing inflammatory responses, and promoting muscle adaptation [
3,
4,
5]. However, natural differences in running capacity exist among individuals. Previous studies have analyzed factors influencing these differences from various perspectives. For instance, from a genetic standpoint, DNA polymorphisms are significant contributors to variations in running capacity [
6]. From a psychological perspective, mental health disorders have also been found to affect running performance [
7]. Nevertheless, these factors cannot fully account for the variability in running capacity between individuals.
Metabolomics, as an essential branch of systems biology, utilizes high-throughput technologies to comprehensively analyze small-molecule metabolites within organisms, providing deep insights into metabolic networks and physiological states [
8]. running significantly alters the concentrations of various metabolites, which are small molecules generated through metabolic reactions in the body. During physical activity, the concentrations of metabolites involved in energy metabolism and other metabolic pathways undergo continuous change [
9]. With advancements in metabolomics technologies, researchers can more precisely detect molecular responses triggered by running and comprehensively uncover running -induced changes in metabolite concentrations. These altered metabolites are recognized as critical signaling molecules that influence running capacity [
10].
Metabolomics studies in mice have revealed that metabolic pathways such as linoleic acid, phenylalanine, and glycerophospholipid metabolisms significantly impact the running capacity of Alzheimer’s disease (AD) model mice, providing a theoretical basis for modulating metabolism in AD organisms through running [
11]. In human studies, metabolomics findings have demonstrated that various metabolites related to energy metabolism (e.g., glycolysis and the tricarboxylic acid cycle) and redox metabolism (e.g., the pentose phosphate pathway) significantly influence running capacity [
12].
Although numerous studies have explored factors influencing differences in running capacity from various perspectives and using different methods, few have systematically analyzed these factors from a metabolomics perspective or validated their reliability. This study aims to employ untargeted metabolomics to analyze serum metabolite differences in rats with varying running capacities, identify key metabolic pathways, and validate the pathways’ effectiveness using key molecular markers. By doing so, the study seeks to uncover the underlying metabolic mechanisms influencing running capacity and provide new insights into the biological basis of running performance.
2. Methods
2.1. Materials and Equipment
The rat treadmill (model YLS-15A) was purchased from Jinan Yiyan Technology Development Co., Ltd.; Ultra-high Performance Liquid Chromatography (model ACQUITY UPLC I-Class), High-resolution Mass Spectrometry (model XevoG2-XS QTof), and Chromatographic column (model HSS T3 column) were purchased from Waters Company; Centrifuge (model Legend Micro 17R) was purchased from Thermo Fisher Scientific; Water (LC-MS), Acetonitrile (LC-MS), and Formic acid (LC-MS) were also purchased from Thermo Fisher Scientific; Inosine Triphosphate (ITP) was purchased from Beijing Aoke Biotechnology Co., Ltd [
13].
2.2. Experimental Design
Twenty 8-10 weeks old SPF-grade male Sprague-Dawley (SD) rats were purchased from Beijing Vitohe Laboratory Animal Technology Co., Ltd. The experiments were conducted at the SPF-grade animal facility of Wannan Medical College. All procedures adhered to the relevant animal welfare and ethical guidelines outlined in the Regulations on the Management of Laboratory Animals at Wannan Medical College and were approved [WNMC-AWE-2024365] by the Institutional Animal Care and Use Committee (IACUC) of Wannan Medical College.
20 SDF rats were put through three repeated rat treadmill tests and screened into High and Low running capacity groups. Four (4) rats from each running capacity groups (High = 4, Low = 4) were then selected for the experiment.The selection criteria for running capacity were based on a rotary treadmill test. Each rat was given two exhaustion rest opportunities, with exhaustion defined as remaining stationary on the treadmill for more than 15 seconds, after which the rat received a one-minute rest period. The total running time and distance were recorded, and rats with the highest cumulative running time and distance were classified into the High running capacity group, while those with the lowest were placed in the Low running capacity group. The experiment was repeated three times to ensure result consistency and reliability.
Peripheral blood samples were then collected from the rats’ orbital regions to isolate serum, and metabolites were detected using liquid chromatography-tandem mass spectrometry (LC-MS/MS). After preprocessing the data, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were employed to assess metabolic profile differences, and significant differential metabolites were identified [
14,
15]. Subsequently, KEGG pathway enrichment analysis was performed on these metabolites to identify key metabolic pathways and validate their reliability.
2.3. Collection and Processing of Rat Serum Samples
Blood samples were collected one week after the completion of the treadmill exhaustion test. Blood was collected via orbital vein sampling. After centrifugation, serum was collected and quickly frozen for storage. A total of 8 samples were collected, divided into two groups, each with 4 replicates. Each serum sample (50 µL) was mixed with 150 µL of methanol solvent, vortexed for 1 minute at -20°C, and left to stand overnight. The mixture was then centrifuged (2000 rpm, 4°C, 20 minutes), and the supernatant was collected for subsequent mass spectrometry analysis. Quality control samples were prepared during the sample processing to ensure the stability and accuracy of the analysis.
QC samples were generated by pooling 10 μL aliquots from each experimental serum sample. These pooled QCs were analyzed every 10 experimental injections throughout the LC-MS/MS run sequence. Metabolites with a coefficient of variation (CV) >30% across QCs were excluded to ensure data reliability. Instrument stability was further validated by calculating Pearson correlation coefficients (r) between QC replicates, retaining only features with r >0.90.
Liquid Chromatography-Mass Spectrometry Analysis Conditions (LC-MS/MS)
Parameter Settings: Column Temperature: 40°C; Sample Temperature: 10°C; Liquid Phase Flow Rate: 0.30 mL/min. Mobile Phases: Phase A: Water + 0.1% formic acid (FA); Phase B: Acetonitrile + 0.1% FA.
Operating Conditions: Negative Ion Collection Mode: The IDA (Information-Dependent Acquisition) high-sensitivity scanning mode was used, with dynamic background subtraction enabled.
Ion Source Parameters: Sheath Gas Flow Rate: 30; Gas1 Flow Rate: 50; Gas2 Flow Rate: 50; Temperature: 500°C; Scanning Time: 15 minutes; Scan Range: 60–1250 m/z for the primary scan, followed by 12 secondary scans for each primary scan.
Positive Ion Collection Mode: The same scanning mode and parameter settings were used [
16].
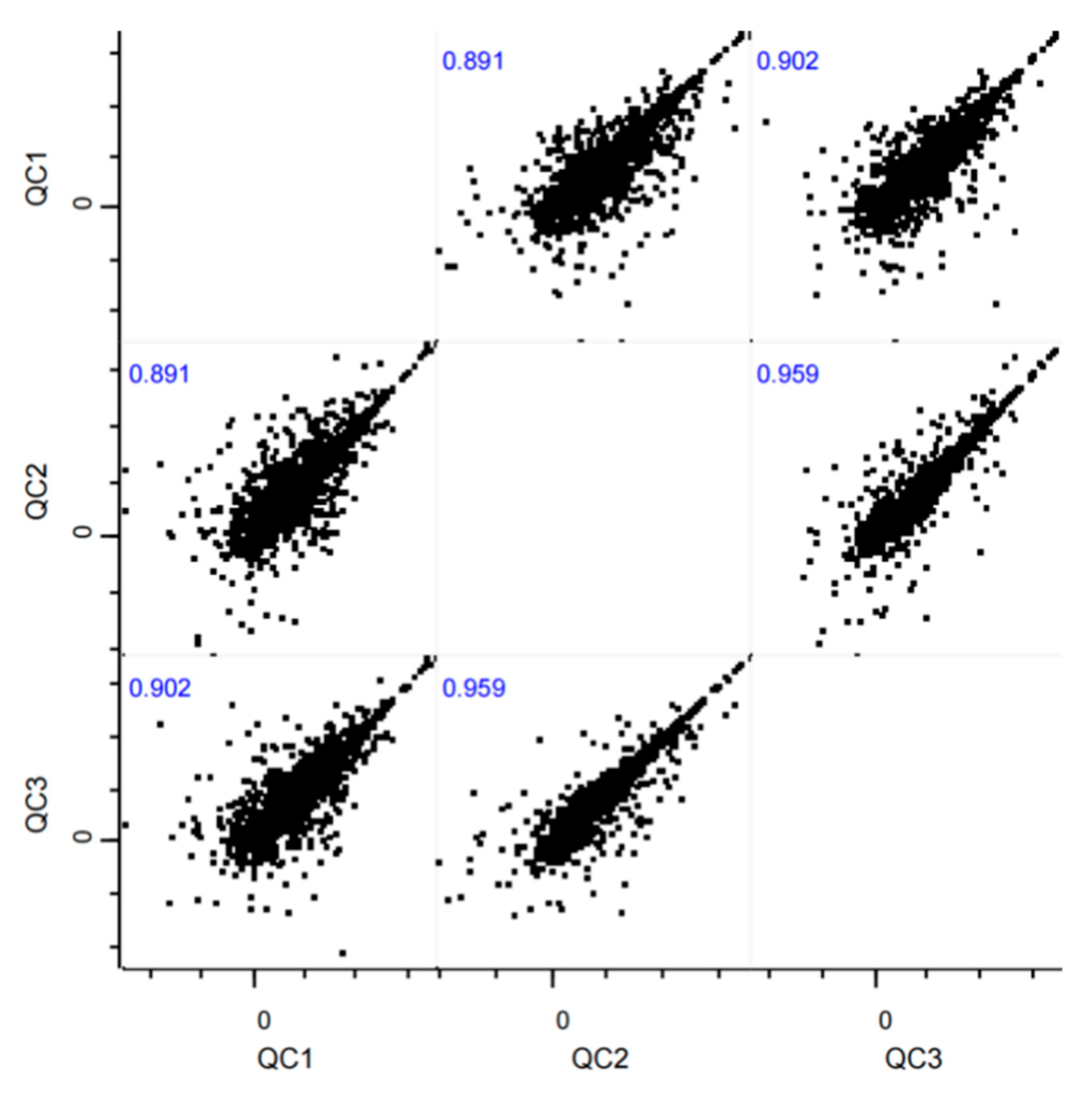
To evaluate the reproducibility of multiple biological experiments on the research samples, correlation analysis was performed based on the intensity values of commonly quantified metabolites across groups. The intensity values of metabolites for each experimental group were log
2-transformed and plotted on the x-axis and y-axis, respectively. The Pearson correlation coefficients for commonly quantified metabolites between any two repeated experiments are shown in
Figure 1. All correlation coefficients exceeded 0.80, indicating excellent consistency.
2.4. Metabolomics Data Extraction
Raw LC-MS/MS data were processed to extract metabolic features using Progenesis QI V2.3 software (Waters Corporation, USA). Initially, raw spectra were imported and aligned to correct retention time shifts across samples. Baseline noise reduction and peak detection were performed with a sensitivity threshold set to 10,000 counts, ensuring robust identification of low-abundance metabolites. Features were filtered to retain those with coefficients of variation (CV) < 30% in quality control (QC) samples, ensuring data reproducibility. Peak annotation was conducted by matching accurate mass-to-charge ratios (m/z) (±5 ppm) and retention times against the Human Metabolome Database (HMDB) and in-house spectral libraries. Isotopic peaks and adducts (e.g., [M+H]⁺, [M-H]⁻) were excluded using built-in algorithms. Data normalization was performed using total ion current (TIC) correction to minimize batch effects. A three-dimensional matrix comprising metabolite intensities, retention times, and m/z values was generated for downstream analysis. To ensure data quality, QC samples were injected at regular intervals (every 10 experimental samples) and evaluated using Pearson correlation coefficients (>0.80), confirming instrument stability. Missing values were imputed using half-minimum substitution for metabolites detected in ≥80% of samples. This rigorous workflow ensured reliable extraction of 519 differential metabolites for subsequent statistical and pathway analyses.
2.5. Metabolomics Data Analysis Methods
The serum data of rats were analyzed using Progenesis QI V2.3 software, generating a three-dimensional dataset in Excel that included peak-identified ions, peak matching, and peak alignment. Metabolites were identified using the HMDB (Human Metabolome Database,
http://www.hmdb.ca, accessed on October 20, 2024) through the native QI (Waters) data processing software. The processed data were then imported into R for orthogonal partial least squares discriminant analysis ((O)PLS-DA), which was used to visually represent metabolic changes between experimental groups.
Substances with a variable importance in projection (VIP) score >1, based on their statistical significance in influencing the OPLS-DA model, were screened. These substances underwent a t-test followed by one-way analysis of variance (ANOVA) using SPSS 26.0. To control the false discovery rate (FDR) caused by multiple comparisons, the Benjamini-Hochberg procedure was applied, and the significance threshold was set at FDR <0.05. Candidates satisfying both VIP >1 and adjusted P < 0.05 were identified as potential biomarkers. Finally, metabolic pathway enrichment analysis was performed using MetaboAnalyst 6.0.
2.6. ITP Intervention Experimental Method
Forty 8-10 weeks old Sprague-Dawley (SD) rats, weighing 300–400 grams, were selected for the experiment. The rats, evenly divided by sex, were randomly assigned to either the experimental group or the control group, with 20 rats in each group. The initial running capacity was evaluated using a rat treadmill exhaustion test to ensure no statistically significant difference between the two groups. The ITP group received intragastric administration of 24.29mg/kg.bw ITP (inosine triphosphate) daily for seven consecutive days. The control group received the solvent (saline) only. After the intervention, the running capacity of the rats were reassessed.
2.7. Data Analysis
Data were recorded and analyzed using SPSS 26.0 software. Statistical analysis was performed using the Mann-Whitney U test to determine significant differences between groups.
3. Results
3.1. Rat Treadmill Test Analysis
The high running capacity group demonstrated significantly longer running times and distances compared to that of the low running capacity group in three repeated experiments, with statistically significant differences observed, as shown in
Table 1.
3.2. Rat Serum Metabolomics Analysis
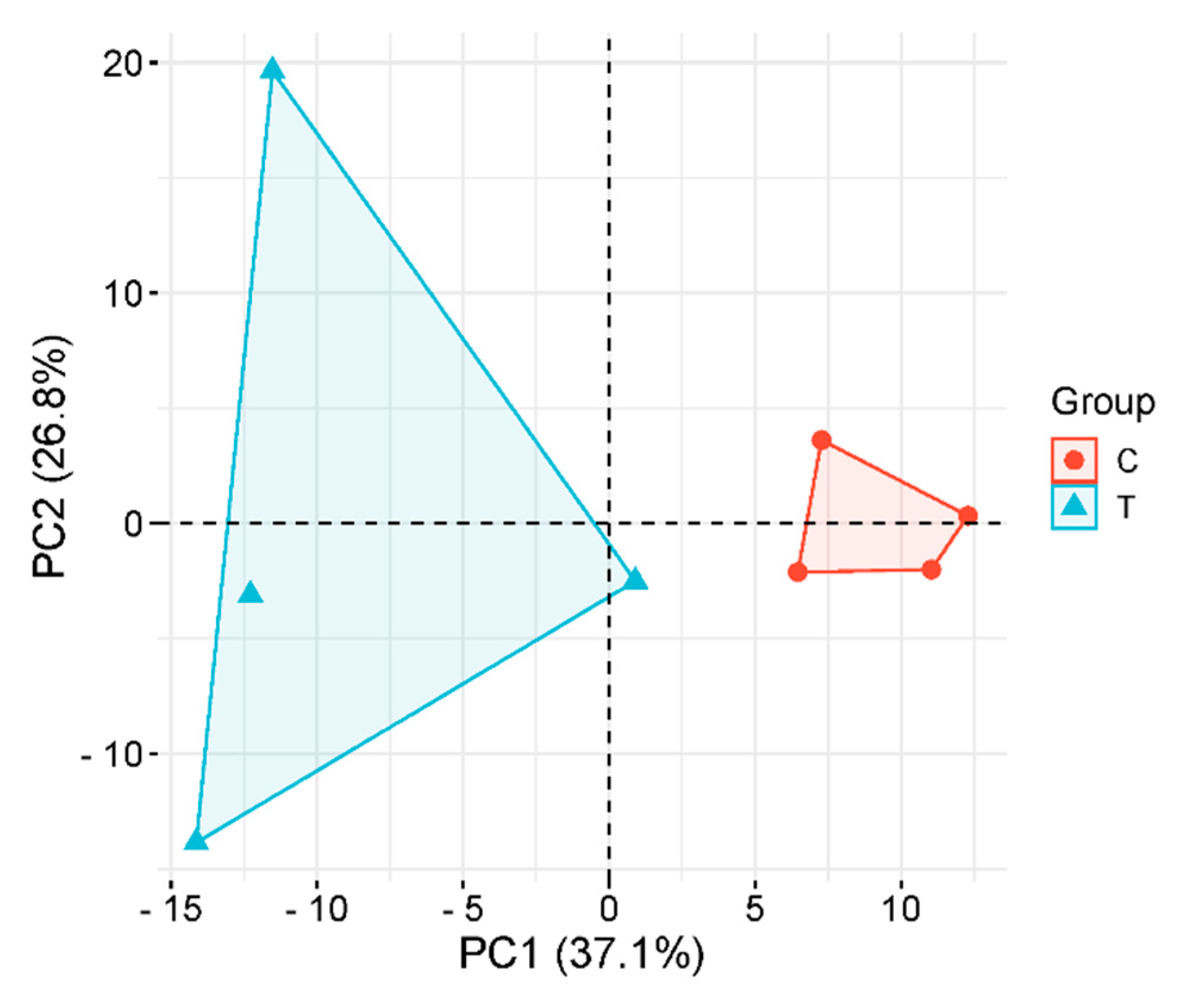
Principal component analysis (PCA) revealed a clear separation between Group C and Group T along the first two principal components (
Figure 2). The model captured 63.9% of total metabolic variance (PC1: 37.1%, PC2: 26.8%), with PC1 primarily driving inter-group discrimination. Group C specimens (red circles) clustered tightly in the positive PC1 quadrant (PC1 range: +5.2 to +9.8), demonstrating high intra-group consistency. In contrast, Group T samples (blue triangles) exhibited broader dispersion across both PC1 (-3.1 to +12.4) and PC2 (-8.6 to +15.2) dimensions, indicative of enhanced metabolic heterogeneity following experimental intervention. Permutational MANOVA confirmed significant between-group dissimilarity (
p<0.001,F=18.3), while 95%confidence ellipses showed non-overlapping distributions, substantiating treatment-induced metabolic perturbation.
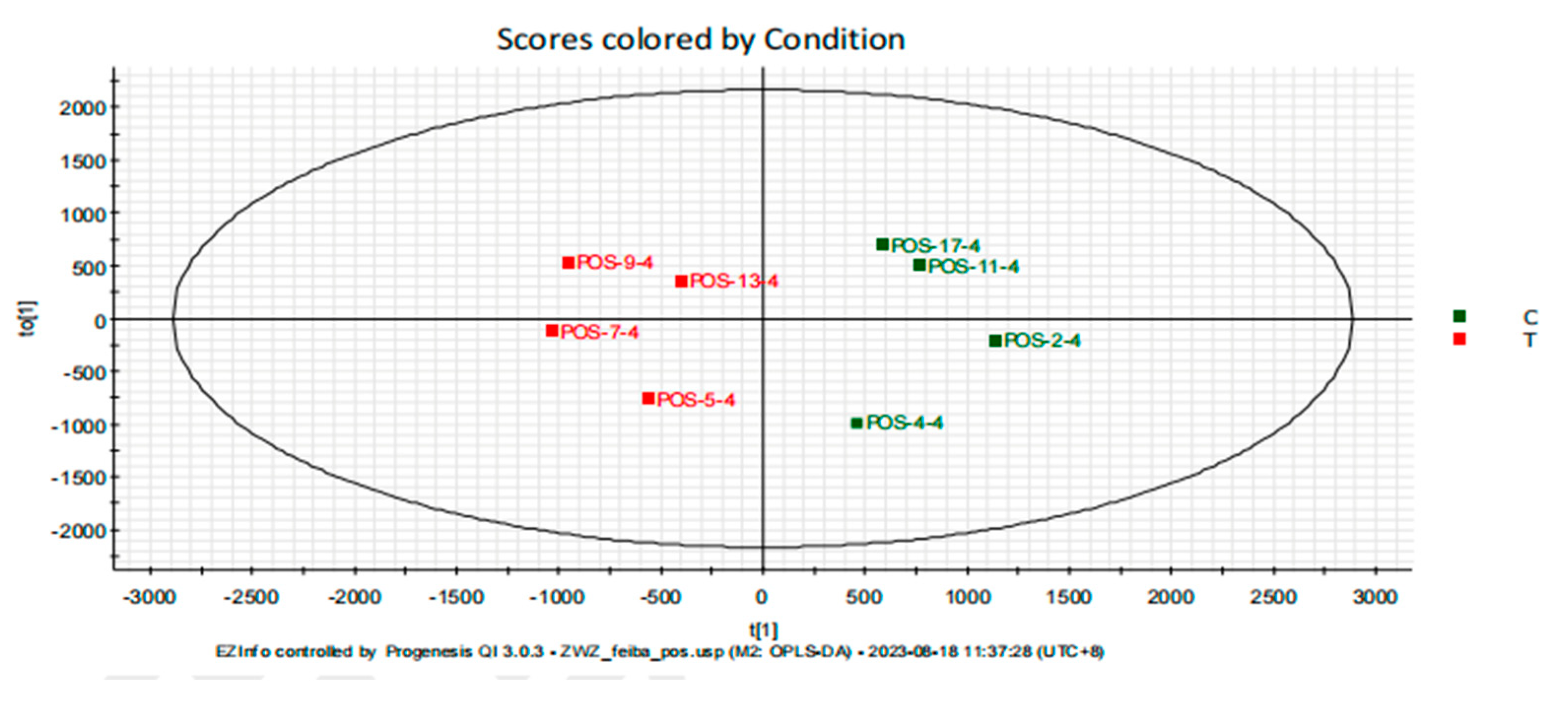
To eliminate noise information unrelated to classification and to identify reliable metabolites contributing to classification differences, orthogonal partial least squares discriminant analysis (OPLS-DA) was applied to filter out orthogonal signals unrelated to classification, generating the OPLS-DA model (
Figure 3) [
17]. The R²Y and Q² values for the OPLS-DA model were 89% and 53% respectively, indicating that the model has a certain level of predictive ability for the grouping.
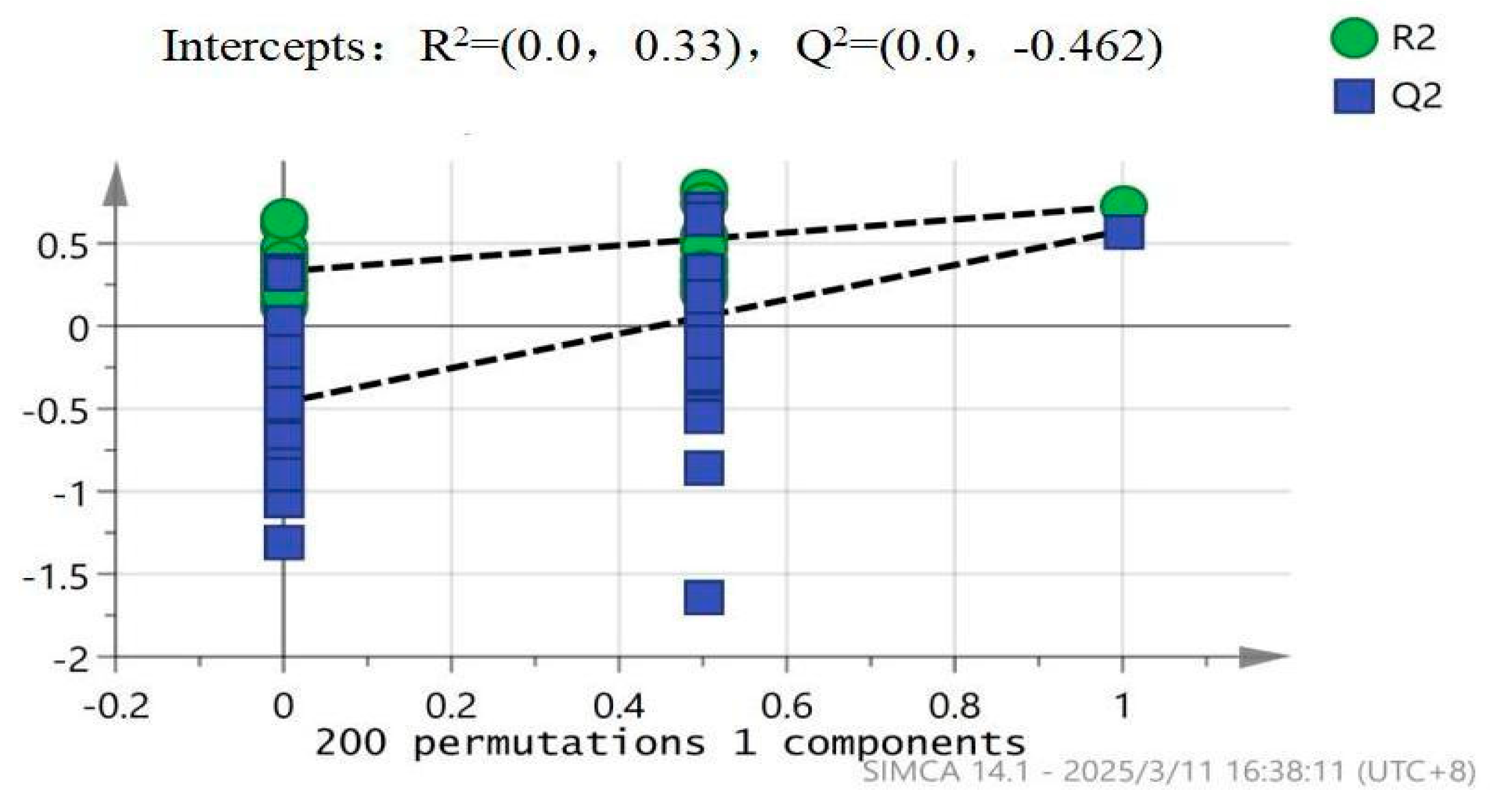
To evaluate the reliability and overfitting risk of the OPLS-DA model, a permutation test (200 permutations) was performed using SIMCA 14.1. As shown in
Figure 4, the R² (green circles) and Q² (blue squares) intercepts were calculated as 0.33 and -0.462, respectively. The distribution trends (dashed lines) indicate that the original R²and Q²values of the model are significantly higher than those from permuted datasets (plotted along the x-axis: 0–1 components). Although negative Q² values were observed (potentially due to weak inter-group discrimination or excessive model complexity), the R² intercept (0.33) remained below the empirical threshold of 0.3–0.4, suggesting no severe overfitting (per SIMCA validation criteria by Eriksson et al.). This validation supports the statistical robustness of subsequent differential metabolite screening.
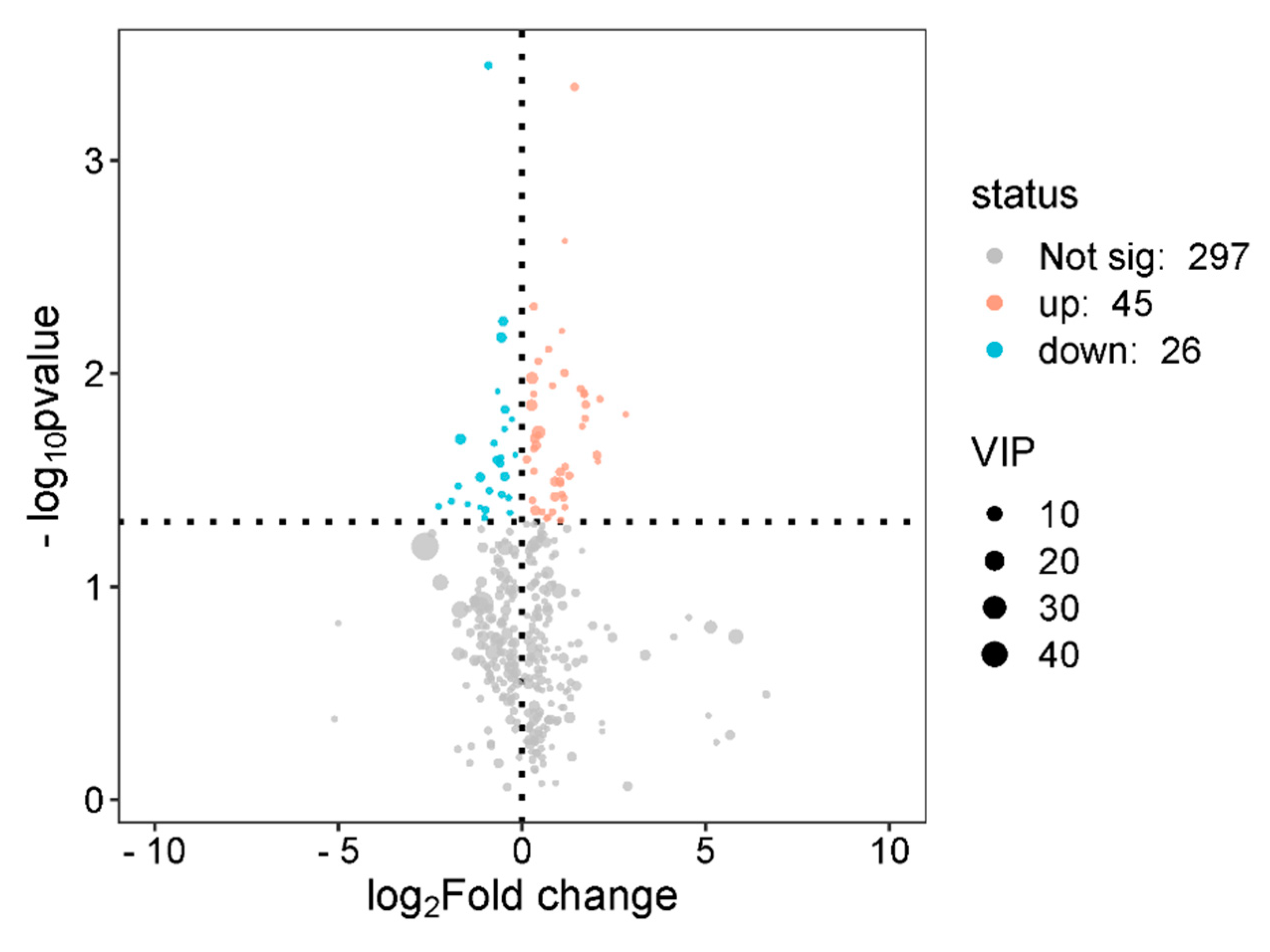
Next, a metabolite volcano plot (
Figure 5) was used to select metabolites with VIP > 1 as differential metabolites. After Benjamini-Hochberg correction for multiple testing, a total of 519 metabolites were identified, including 255 up-regulated and 264 down-regulated metabolites. Ten differential metabolites from each group were listed (
Table 2 and
Table 3). Among these, the changes in metabolites such as tryptophan, Tocladesine, tryptophanol, inosine triphosphate, and uric acid have a certain impact on rat running capacity.
3.3. KEGG Pathway Analysis
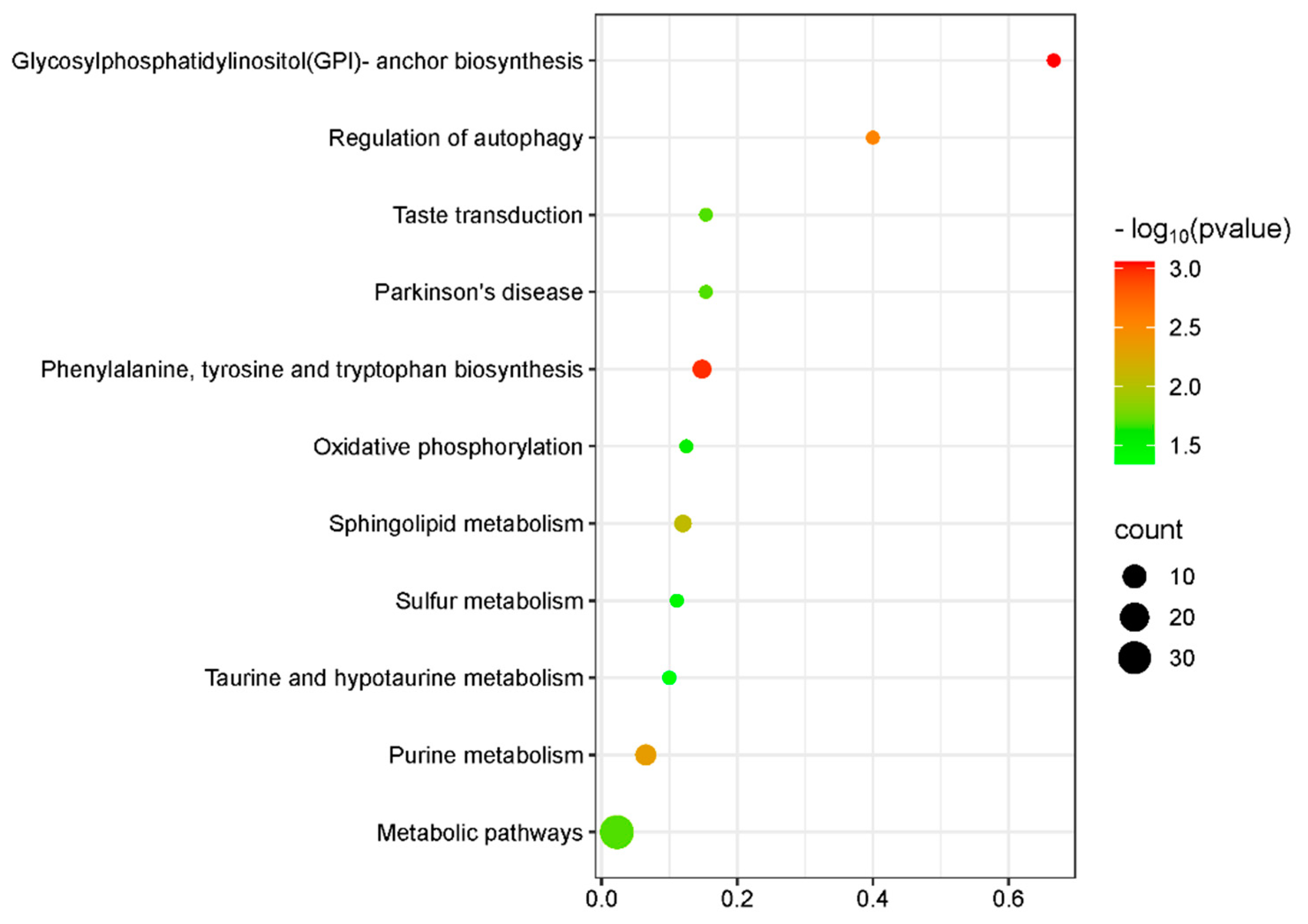
The differential metabolites were input into MetaboAnalyst 6.0 [
18] for pathway enrichment analysis, Among the metabolic pathways, significant changes were observed in glycerophospholipid, purine, and taurine and hypotaurine metabolism pathways (
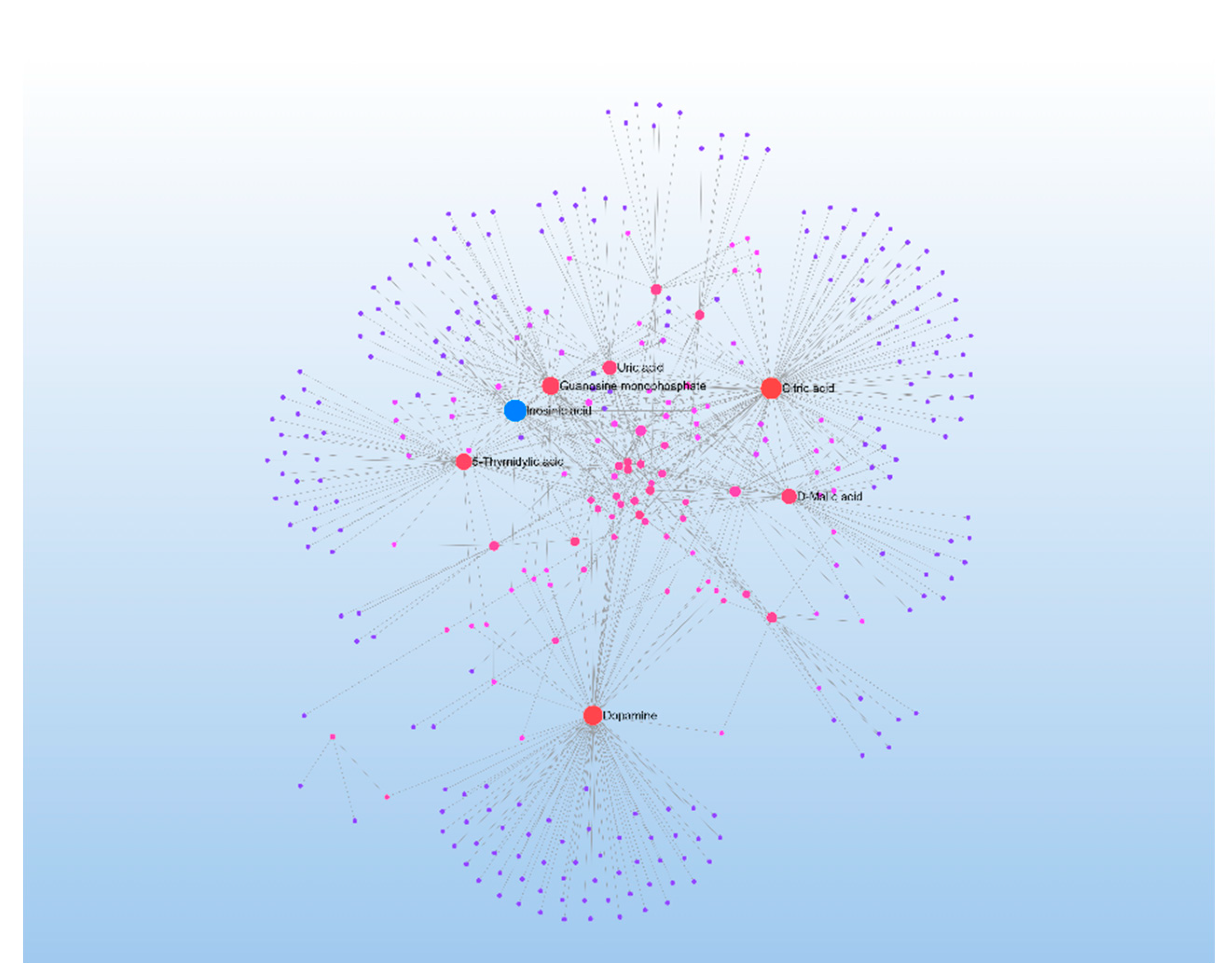
Figure 6). The enriched differential metabolites were then entered into the STRING database (
http://string-db.org/) to obtain a metabolic interaction network, which was subsequently exported to Cytoscape software for the construction of an interaction network (
Figure 7). In the network, each node represents a metabolite, and the connecting lines indicate interactions between metabolites. The size of the nodes reflects the extent of expression differences, with larger nodes representing greater differences. The central metabolites with higher interaction connectivity, such as inosine triphosphate (ITP), are likely key points in the pathway. ITP was selected for further in-depth analysis in this study.
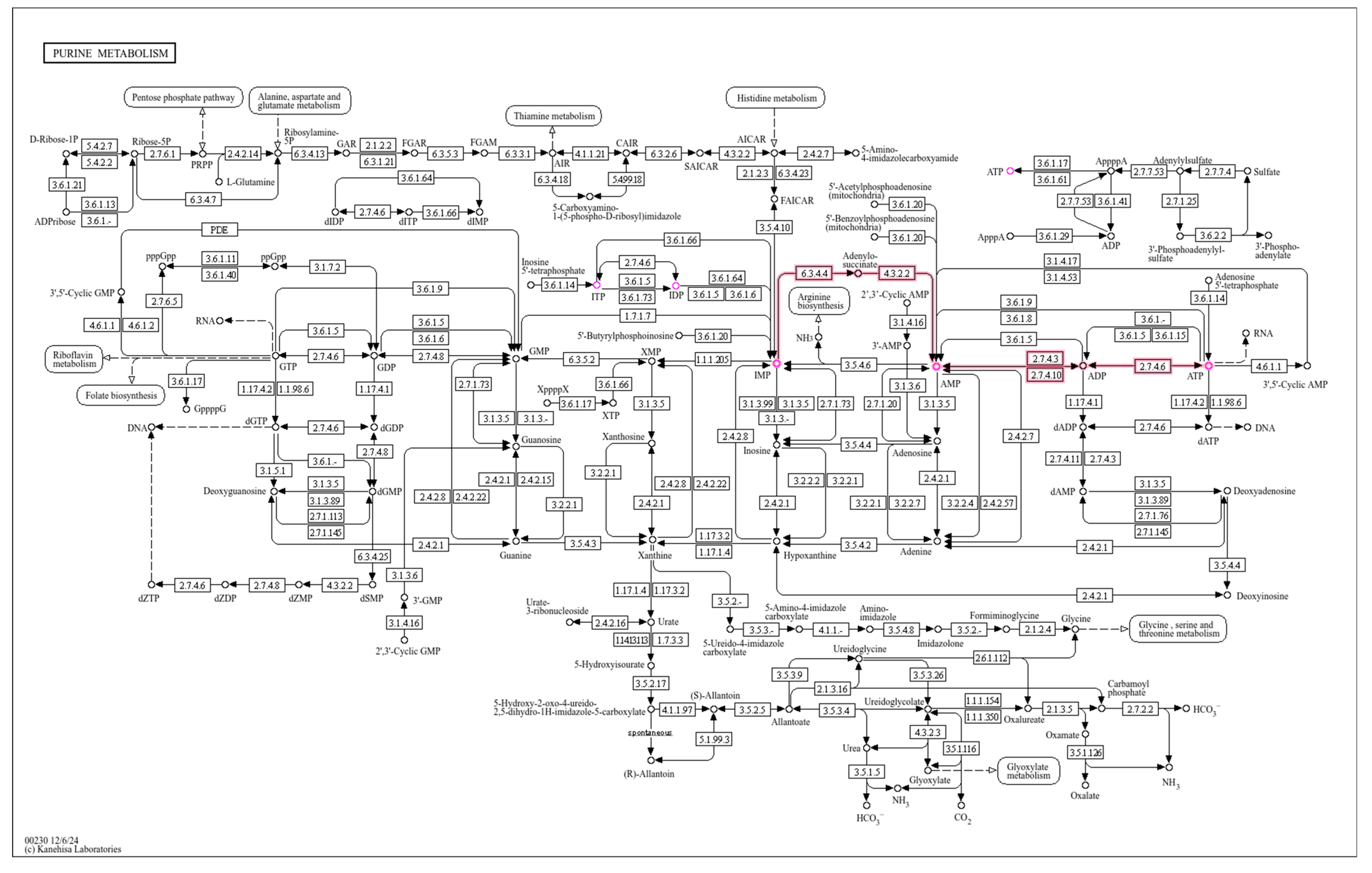
Interaction analysis was performed using MetaboAnalyst 6.0, focusing on the purine metabolism pathway.
Figure 8 shows key compounds in purine metabolism, including ITP-IDP-IMP-AMP-ADP-ATP (ITP-ATP) and their intermediate metabolites. These metabolites play critical roles in energy transfer, nucleic acid synthesis, signal transduction, and uric acid metabolism.
3.4. ITP Intervention Experiment
The results revealed, as shown in
Table 4, that the difference in running ability of rats before and after gavage was statistically significant (male
P < 0.01, female
P < 0.05).
4. Discussion
Running capacity is influenced by complex mechanisms involving multiple factors. Studies have shown significant differences in muscle metabolites and substrate metabolism during endurance running between genders. Women are naturally more suited to moderate-intensity running compared to men, primarily due to differences in substrate metabolism [
19]. Additionally, research comparing professional athletes to individuals with lower physical fitness during running revealed that professional athletes exhibit greater metabolic flexibility, as evidenced by differences in blood lactate levels, fat oxidation, and carbohydrate oxidation [
20]. These findings suggest that variations in metabolic pathways are critical factors influencing the running capacity of rats. In the present study, three repeated experiments (rat exhaustion tests) demonstrated that rats also exhibit natural differences in running capacity. This indicates that both human and animal running capacities inherently differ and are possibly affected by metabolic pathways.
4.1. Key Differential Metabolites and Their Functional Roles
Our metabolomics analysis identified 519 differential metabolites between high and low running capacity groups, including 255 upregulated and 264 downregulated metabolites. Among these, inosine triphosphate (ITP) emerged as a central metabolite in the purine metabolism pathway. ITP, as a nucleotide triphosphate, is directly involved in ATP synthesis through the ITP-IDP-IMP-AMP-ADP-ATP conversion cascade. This pathway not only serves as a critical energy reservoir but also regulates nucleic acid synthesis and cellular signaling [
21,
22]. The upregulation of ITP in high-capacity rats suggests enhanced nucleotide turnover and energy metabolism efficiency, which may directly contribute to prolonged running endurance.
Another notable upregulated metabolite, tryptophan, plays a dual role in running physiology. Tryptophan is a precursor for serotonin synthesis, which modulates central fatigue perception [
23], and its catabolism via the kynurenine pathway generates NAD+, a coenzyme essential for mitochondrial energy production [
24]. Elevated tryptophan levels may thus mitigate fatigue and support sustained aerobic performance. Conversely, uric acid, a downstream product of purine metabolism, showed significant changes. While excessive uric acid accumulation is linked to oxidative stress [
25], its moderate elevation in high-capacity rats may reflect a balance between energy demand and antioxidant defense.
Among the downregulated metabolites, phospholipids such as PE(20:1/22:6) and PC(16:0/22:6) exhibited reduced levels. These glycerophospholipids are key components of cellular membranes and signaling molecules. Their downregulation may indicate membrane remodeling to adapt to running -induced oxidative stress or energy redistribution toward ATP synthesis [
26]. Furthermore, farnesyl triphosphate, involved in protein prenylation and cellular signaling, was significantly reduced. This could reflect a shift in metabolic priorities, favoring immediate energy production over long-term signaling investments during exhaustive running.
4.2. Metabolic Pathway Crosstalk and Network Dynamics
The interaction network analysis highlighted the centrality of the ITP-ATP pathway, with ITP acting as a hub metabolite connecting energy metabolism, redox regulation, and inflammatory responses. The enrichment of glycerophospholipid and taurine metabolism further underscores the systemic nature of running adaptation. For instance, taurine, though not directly measured here, is known to stabilize membranes and enhance calcium handling in muscles, potentially synergizing with purine metabolism to improve contractile efficiency [
27].
4.3. Validation Through ITP Intervention
The exogenous administration of ITP significantly enhanced running capacity in both male and female rats, corroborating the metabolomics findings. This intervention likely amplified the endogenous ITP-ATP flux, thereby increasing ATP availability for muscle contraction and delaying fatigue. Notably, the sex-specific responses (greater improvement in males) align with prior studies on sex-dimorphic substrate utilization [
19], suggesting hormonal or enzymatic modulation of purine metabolism.
5. Limitations
Although this study reveals the impact of the ITP-ATP pathway on Running capacity in rats, there are several limitations. First, the sample size in this study was limited to 20 rats, which may affect the statistical significance and generalizability of the results. Secondly, although three metabolic pathways were identified, only the purine metabolism pathway (ITP-ATP) was validated due to the lack of suitable drugs for glycerophospholipid metabolism and taurine and hypotaurine metabolism pathways. Finally, the study was conducted solely in a rat model, and while it provides valuable biological insights, the applicability of these results to humans remains uncertain. Metabolic differences between species may influence the effectiveness of ITP intervention, and further studies are needed to validate these findings in humans.
6. Conclusion
Rats exhibit natural differences in running capacity. Rats with high running capacities show significantly more active purine metabolism pathways, with key metabolites such as ITP-IDP-IMP-AMP-ADP-ATP exhibiting significantly elevated levels. In this rat model, exogenous supplementation of ITP can promote the activity of the purine metabolism pathway and enhance running capacity. However, due to species-specific metabolic differences, these findings should not be directly extrapolated to humans without further validation. Future studies should explore the translational potential of ITP intervention in preclinical models and clinical trials to assess its applicability in improving human exercise performance.
Funding
This work was supported by the Doctoral Research Start-Up Fund Project under grant X600100102.
References
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise sustains the hallmarks of health. J Sport Health Sci 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ Res 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Miko, H.C.; Zillmann, N.; Ring-Dimitriou, S.; Dorner, T.E.; Titze, S.; Bauer, R. [Effects of Physical Activity on Health]. Gesundheitswesen 2020, 82, S184–S195. [Google Scholar] [CrossRef]
- Raczkowska, E.; Bienkiewicz, M.; Gajda, R. Modulation of the glycaemic index value of shortbread cookies by the use of erythritol and fruit pomace. Sci Rep 2024, 14, 14215. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, II; Egorova, E.S.; Gabdrakhmanova, L.J.; Fedotovskaya, O.N. Genes and Athletic Performance: An Update. Med Sport Sci 2016, 61, 41–54. [Google Scholar] [CrossRef]
- Jang, S.; Drescher, M.J.; Armstrong, T.A.; Neil, E.R.; Eberman, L.E. Exploring Secondary School Athletic Trainers’ Perspectives in Managing Mental Health Situations. Int J Environ Res Public Health 2024, 21. [Google Scholar] [CrossRef]
- Yang, X.; He, M.; Tang, Q.; Cao, J.; Wei, Z.; Li, T.; Sun, M. Metabolomics as a promising technology for investigating external therapy of traditional Chinese medicine: A review. Medicine (Baltimore) 2024, 103, e40719. [Google Scholar] [CrossRef] [PubMed]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med Open 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma Metabolome Profiling of Resistance Exercise and Endurance Exercise in Humans. Cell Rep 2020, 33, 108554. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.G.; Sokolowska, I.; Ngounou Wetie, A.G.; Channaveerappa, D.; Dupree, E.J.; Jayathirtha, M.; Aslebagh, R.; Wormwood, K.L.; Darie, C.C. Mass Spectrometry for Proteomics-Based Investigation. Adv Exp Med Biol 2019, 1140, 1–26. [Google Scholar] [CrossRef]
- Cendali, F.; D’Alessandro, A.; Nemkov, T. Dried blood spot characterization of sex-based metabolic responses to acute running exercise. Anal Sci Adv 2023, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Klinker, J.F.; Seifert, R. Functionally nonequivalent interactions of guanosine 5’-triphosphate, inosine 5’-triphosphate, and xanthosine 5’-triphosphate with the retinal G-protein, transducin, and with Gi-proteins in HL-60 leukemia cell membranes. Biochem Pharmacol 1997, 54, 551–562. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So you think you can PLS-DA? BMC Bioinformatics 2020, 21, 2. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, N.; Hayama, T.; Tomita, R.; Fujioka, T. Accurate LC-MS/MS Analysis of Diacylglycerols in Human Plasma with Eliminating Matrix Effect by Phospholipids Using Fluorous Biphasic Extraction. Anal Chem 2023, 95, 14898–14904. [Google Scholar] [CrossRef] [PubMed]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol Biosyst 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 2021, 49, W388–w396. [Google Scholar] [CrossRef]
- Devries, M.C. Sex-based differences in endurance exercise muscle metabolism: Impact on exercise and nutritional strategies to optimize health and performance in women. Exp Physiol 2016, 101, 243–249. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Sakumi, K.; Abolhassani, N.; Behmanesh, M.; Iyama, T.; Tsuchimoto, D.; Nakabeppu, Y. ITPA protein, an enzyme that eliminates deaminated purine nucleoside triphosphates in cells. Mutat Res 2010, 703, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J Nutr 2006, 136, 544s–547s. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357. [Google Scholar] [CrossRef]
- Wu, D.; Yang, S.; Yuan, C.; Zhang, K.; Tan, J.; Guan, K.; Zeng, H.; Huang, C. Targeting purine metabolism-related enzymes for therapeutic intervention: A review from molecular mechanism to therapeutic breakthrough. Int J Biol Macromol 2024, 282, 136828. [Google Scholar] [CrossRef]
- van der Vusse, G.J.; Glatz, J.F.; Stam, H.C.; Reneman, R.S. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev 1992, 72, 881–940. [Google Scholar] [CrossRef]
- Galloway, S.D.; Talanian, J.L.; Shoveller, A.K.; Heigenhauser, G.J.; Spriet, L.L. Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J Appl Physiol (1985) 2008, 105, 643–651. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).