Submitted:
17 March 2025
Posted:
19 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Viruses and Substances
2.2. Preparation of PLGA NPs
2.3. Laser Light Scattering Techniques
2.4. Transmission Electron Microscopy
2.5. Quantification of 6BIGOE by UV/Vis Spectrophotometric Measurement
2.6. Assessing Drug Impact on Cellular Metabolic Activity and Cytotoxicity (MTT- and LDH Assay)
2.7. Viral Infection
2.8. Plaque Assay
2.9. Western Blot Analysis
3. Results
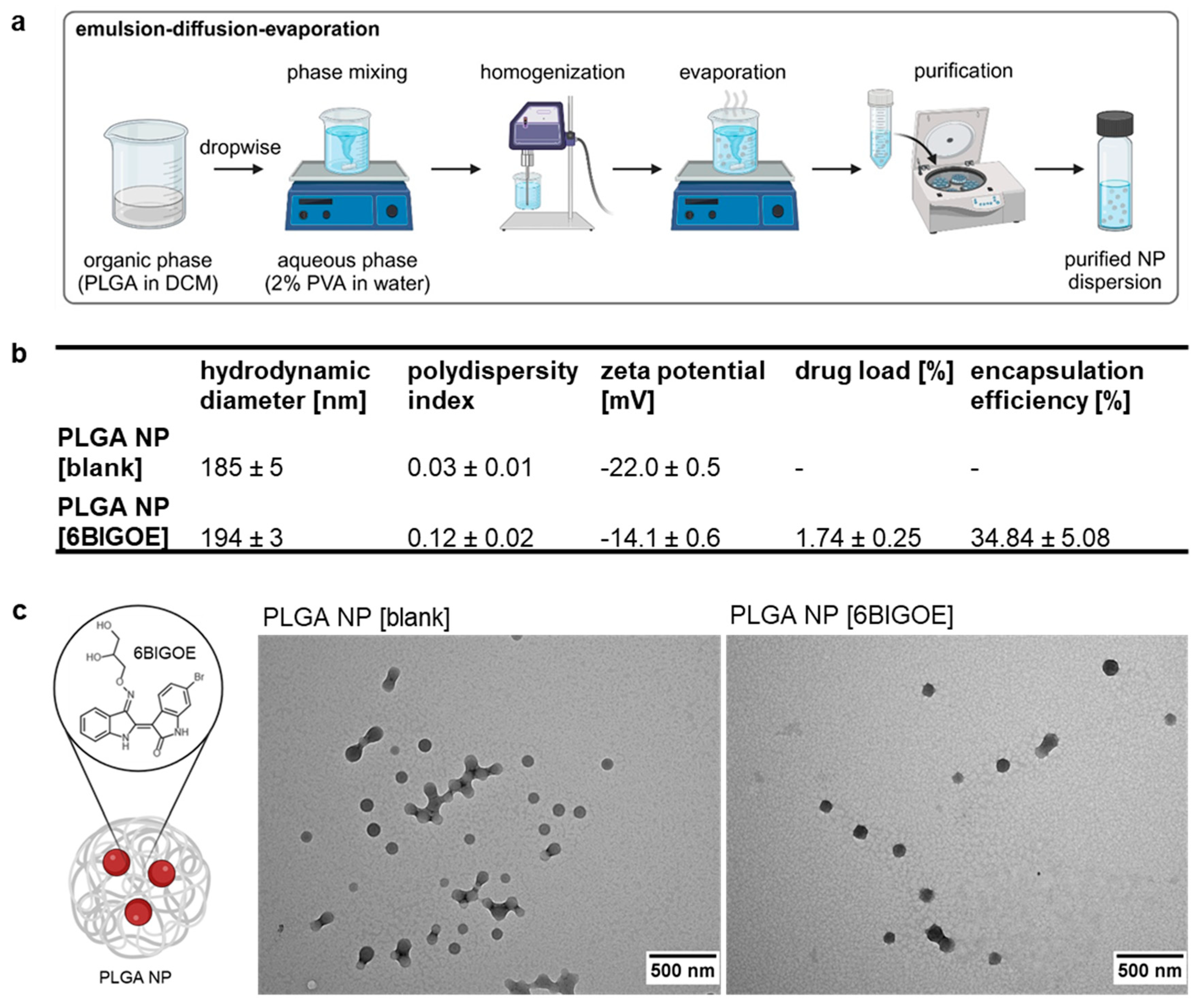
3.1. Preparation and Characterization of PLGA NP [6BIGOE]
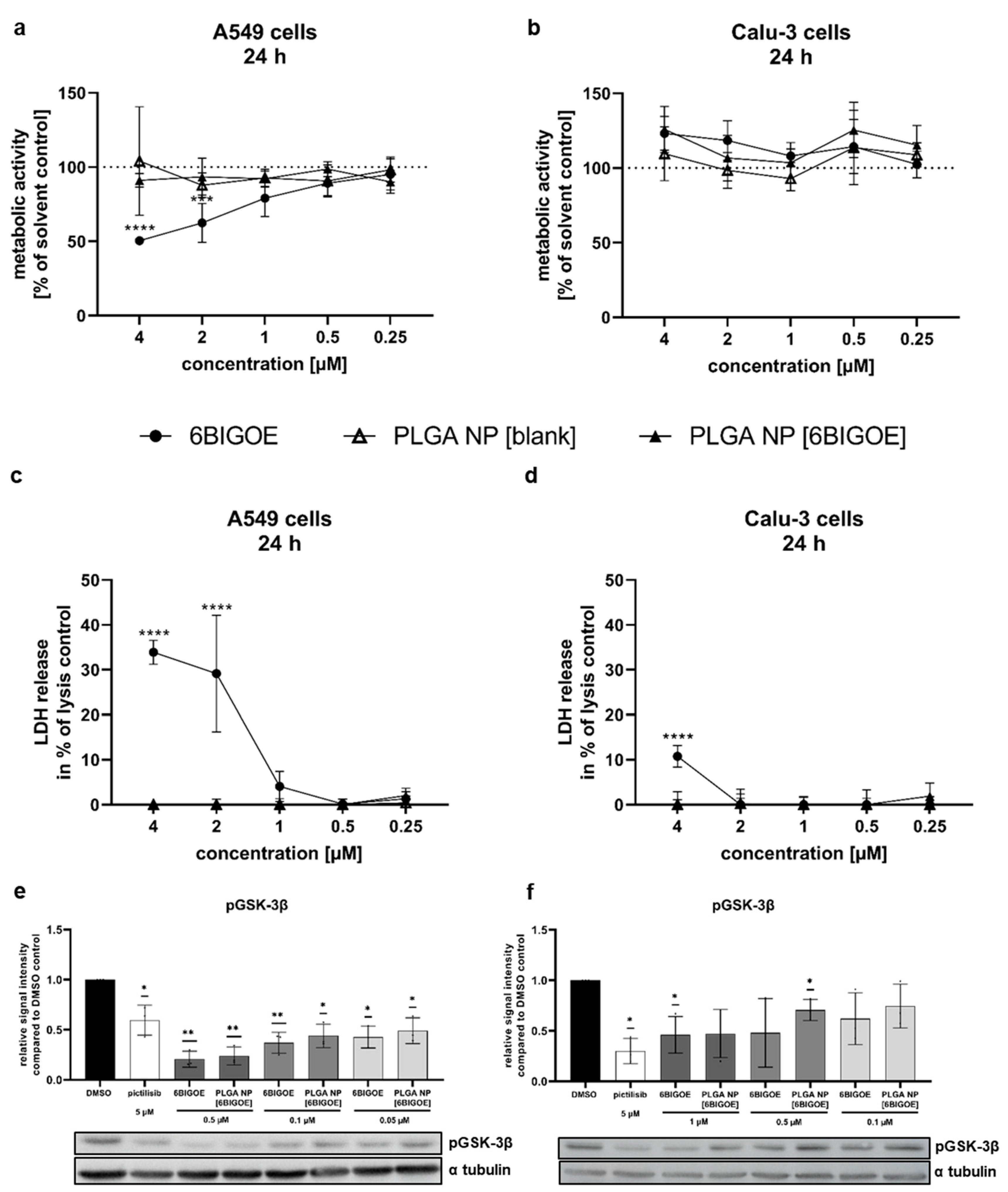
3.2. PLGA [6BIGOE] Has Less Impact on the Cell Metabolic Activity than Free 6BIGOE
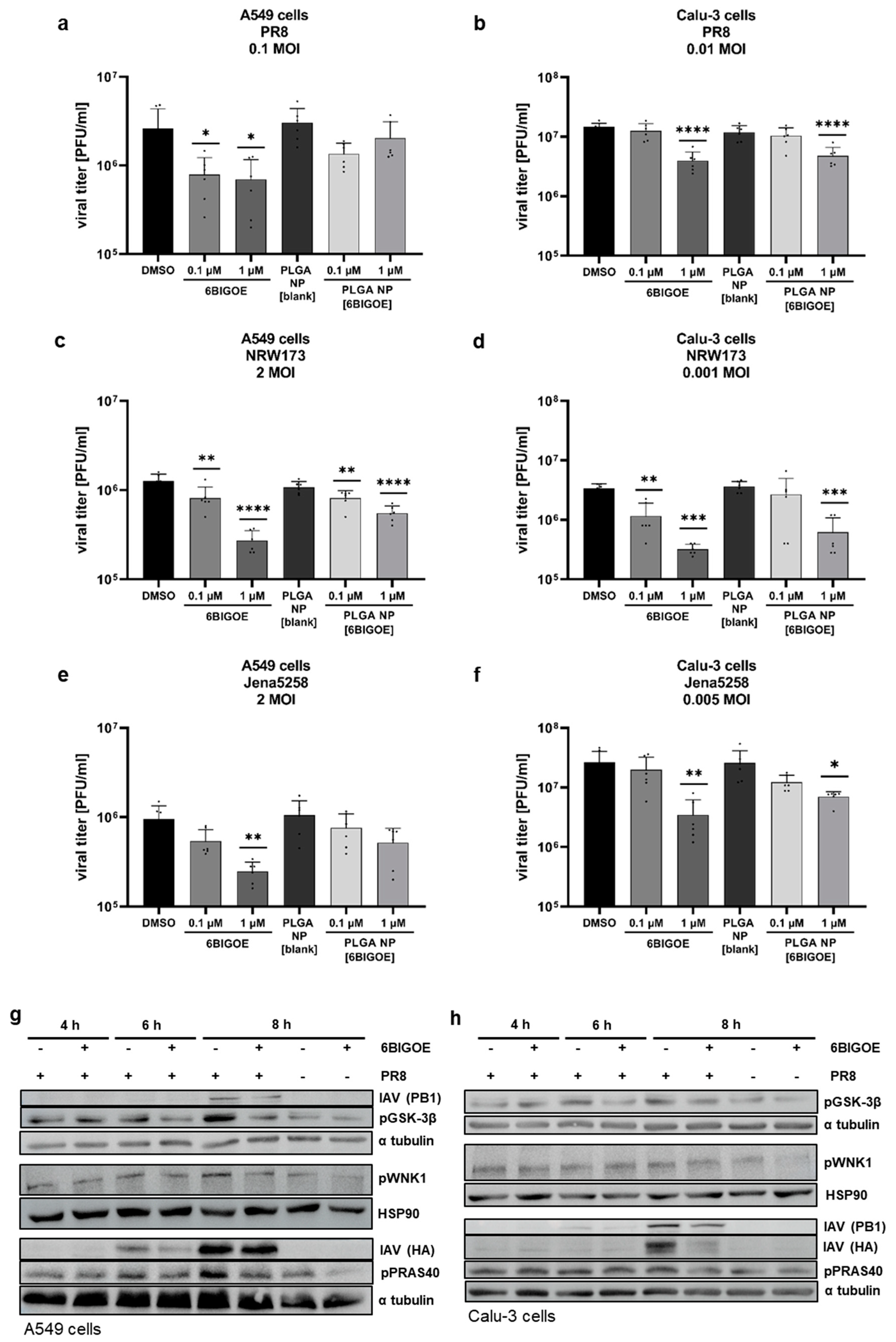
3.3. 6BIGOE Treatment of IAV-Infected Cells Results in Reduced Viral Titers In Vitro and Is Associated with Inhibition of the GSK-3β-Mediated Signaling Processes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clayville, L.R. Influenza update: a review of currently available vaccines. P T 2011, 36, 659-684.
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018, 391, 1285-1300. [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992, 56, 152-179. [CrossRef]
- Fiebach, N.; Beckett, W. Prevention of respiratory infections in adults. Influenza and pneumococcal vaccines. Arch Intern Med 1994, 154, 2545-2557.
- Bonomini, A.; Mercorelli, B.; Loregian, A. Antiviral strategies against influenza virus: an update on approved and innovative therapeutic approaches. Cell Mol Life Sci 2025, 82, 75. [CrossRef]
- Duwe, S.C.; Schmidt, B.; Gartner, B.C.; Timm, J.; Adams, O.; Fickenscher, H.; Schmidtke, M. Prophylaxis and treatment of influenza: options, antiviral susceptibility, and existing recommendations. GMS Infect Dis 2021, 9, Doc02. [CrossRef]
- Hurt, A.C. The epidemiology and spread of drug resistant human influenza viruses. Curr Opin Virol 2014, 8, 22-29. [CrossRef]
- Meineke, R.; Rimmelzwaan, G.F.; Elbahesh, H. Influenza Virus Infections and Cellular Kinases. Viruses 2019, 11. [CrossRef]
- Fichera, E.; Felnerova, D.; Mischler, R.; Viret, J.F.; Glueck, R. New strategies to overcome the drawbacks of currently available flu vaccines. Adv Exp Med Biol 2009, 655, 243-252. [CrossRef]
- Konig, R.; Stertz, S.; Zhou, Y.; Inoue, A.; Hoffmann, H.H.; Bhattacharyya, S.; Alamares, J.G.; Tscherne, D.M.; Ortigoza, M.B.; Liang, Y.; et al. Human host factors required for influenza virus replication. Nature 2010, 463, 813-817. [CrossRef]
- Nagini, S.; Sophia, J.; Mishra, R. Glycogen synthase kinases: Moonlighting proteins with theranostic potential in cancer. Semin Cancer Biol 2019, 56, 25-36. [CrossRef]
- Hermida, M.A.; Dinesh Kumar, J.; Leslie, N.R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul 2017, 65, 5-15. [CrossRef]
- Mancinelli, R.; Carpino, G.; Petrungaro, S.; Mammola, C.L.; Tomaipitinca, L.; Filippini, A.; Facchiano, A.; Ziparo, E.; Giampietri, C. Multifaceted Roles of GSK-3 in Cancer and Autophagy-Related Diseases. Oxid Med Cell Longev 2017, 2017, 4629495. [CrossRef]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3beta in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta Mol Cell Res 2020, 1867, 118659. [CrossRef]
- Terzioglu-Usak, S.; Nalli, A.; Elibol, B.; Ozek, E.; Hatiboglu, M.A. Anvirzel(TM)regulates cell death through inhibiting GSK-3 activity in human U87 glioma cells. Neurol Res 2020, 42, 68-75. [CrossRef]
- Kramer, T.; Schmidt, B.; Lo Monte, F. Small-Molecule Inhibitors of GSK-3: Structural Insights and Their Application to Alzheimer's Disease Models. Int J Alzheimers Dis 2012, 2012, 381029. [CrossRef]
- Mathuram, T.L.; Reece, L.M.; Cherian, K.M. GSK-3 Inhibitors: A Double-Edged Sword? - An Update on Tideglusib. Drug Res (Stuttg) 2018, 68, 436-443. [CrossRef]
- Ring, D.B.; Johnson, K.W.; Henriksen, E.J.; Nuss, J.M.; Goff, D.; Kinnick, T.R.; Ma, S.T.; Reeder, J.W.; Samuels, I.; Slabiak, T.; et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes 2003, 52, 588-595. [CrossRef]
- Hoffmeister, L.; Diekmann, M.; Brand, K.; Huber, R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells 2020, 9. [CrossRef]
- Alfhili, M.A.; Alsughayyir, J.; McCubrey, J.A.; Akula, S.M. GSK-3-associated signaling is crucial to virus infection of cells. Biochim Biophys Acta Mol Cell Res 2020, 1867, 118767. [CrossRef]
- Guendel, I.; Iordanskiy, S.; Van Duyne, R.; Kehn-Hall, K.; Saifuddin, M.; Das, R.; Jaworski, E.; Sampey, G.C.; Senina, S.; Shultz, L.; et al. Novel neuroprotective GSK-3beta inhibitor restricts Tat-mediated HIV-1 replication. J Virol 2014, 88, 1189-1208. [CrossRef]
- Wang, T.; Zhang, J.; Xiao, A.; Liu, W.; Shang, Y.; An, J. Melittin ameliorates CVB3-induced myocarditis via activation of the HDAC2-mediated GSK-3beta/Nrf2/ARE signaling pathway. Biochem Biophys Res Commun 2016, 480, 126-131. [CrossRef]
- Yuan, J.; Zhang, J.; Wong, B.W.; Si, X.; Wong, J.; Yang, D.; Luo, H. Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ 2005, 12, 1097-1106. [CrossRef]
- Rahaus, M.; Desloges, N.; Wolff, M.H. Varicella-zoster virus requires a functional PI3K/Akt/GSK-3alpha/beta signaling cascade for efficient replication. Cell Signal 2007, 19, 312-320. [CrossRef]
- Wu, C.H.; Yeh, S.H.; Tsay, Y.G.; Shieh, Y.H.; Kao, C.L.; Chen, Y.S.; Wang, S.H.; Kuo, T.J.; Chen, D.S.; Chen, P.J. Glycogen synthase kinase-3 regulates the phosphorylation of severe acute respiratory syndrome coronavirus nucleocapsid protein and viral replication. J Biol Chem 2009, 284, 5229-5239. [CrossRef]
- Hirata, N.; Suizu, F.; Matsuda-Lennikov, M.; Edamura, T.; Bala, J.; Noguchi, M. Inhibition of Akt kinase activity suppresses entry and replication of influenza virus. Biochem Biophys Res Commun 2014, 450, 891-898. [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019, 18, 41-58. [CrossRef]
- Gaboriaud-Kolar, N.; Vougogiannopoulou, K.; Skaltsounis, A.L. Indirubin derivatives: a patent review (2010 - present). Expert Opin Ther Pat 2015, 25, 583-593. [CrossRef]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.Z.; Mandelkow, E.M.; et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem 2001, 276, 251-260. [CrossRef]
- Wang, J.; Yang, C.; Liang, Z.; Sun, J.; Zhang, M.; Qiu, S.; Du, X.; He, X.; Pang, X.; Ma, X.; et al. Indirubin-3'-monoxime exhibits potent antiviral and anti-inflammatory effects against human adenoviruses in vitro and in vivo. Biomed Pharmacother 2024, 174, 116558. [CrossRef]
- Mok, C.K.; Kang, S.S.; Chan, R.W.; Yue, P.Y.; Mak, N.K.; Poon, L.L.; Wong, R.N.; Peiris, J.S.; Chan, M.C. Anti-inflammatory and antiviral effects of indirubin derivatives in influenza A (H5N1) virus infected primary human peripheral blood-derived macrophages and alveolar epithelial cells. Antiviral Res 2014, 106, 95-104. [CrossRef]
- Heredia, A.; Davis, C.; Bamba, D.; Le, N.; Gwarzo, M.Y.; Sadowska, M.; Gallo, R.C.; Redfield, R.R. Indirubin-3'-monoxime, a derivative of a Chinese antileukemia medicine, inhibits P-TEFb function and HIV-1 replication. AIDS 2005, 19, 2087-2095. [CrossRef]
- Hsuan, S.L.; Chang, S.C.; Wang, S.Y.; Liao, T.L.; Jong, T.T.; Chien, M.S.; Lee, W.C.; Chen, S.S.; Liao, J.W. The cytotoxicity to leukemia cells and antiviral effects of Isatis indigotica extracts on pseudorabies virus. J Ethnopharmacol 2009, 123, 61-67. [CrossRef]
- Chang, S.J.; Chang, Y.C.; Lu, K.Z.; Tsou, Y.Y.; Lin, C.W. Antiviral Activity of Isatis indigotica Extract and Its Derived Indirubin against Japanese Encephalitis Virus. Evid Based Complement Alternat Med 2012, 2012, 925830. [CrossRef]
- Czapka, A.; Konig, S.; Pergola, C.; Grune, C.; Vougogiannopoulou, K.; Skaltsounis, A.L.; Fischer, D.; Werz, O. The indirubin derivative 6-bromoindirubin-3'-glycerol-oxime ether (6BIGOE) potently modulates inflammatory cytokine and prostaglandin release from human monocytes through GSK-3 interference. Biochem Pharmacol 2020, 180, 114170. [CrossRef]
- Dan, N.T.; Quang, H.D.; Van Truong, V.; Huu Nghi, D.; Cuong, N.M.; Cuong, T.D.; Toan, T.Q.; Bach, L.G.; Anh, N.H.T.; Mai, N.T.; et al. Design, synthesis, structure, in vitro cytotoxic activity evaluation and docking studies on target enzyme GSK-3beta of new indirubin-3'-oxime derivatives. Sci Rep 2020, 10, 11429. [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm 2012, 2012, 195727. [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv 2021, 28, 1397-1418. [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [CrossRef]
- Kwok, P.C.; Chan, H.K. Nanotechnology versus other techniques in improving drug dissolution. Curr Pharm Des 2014, 20, 474-482. [CrossRef]
- Dobrucki, L.W.; Pan, D.; Smith, A.M. Multiscale Imaging of Nanoparticle Drug Delivery. Curr Drug Targets 2015, 16, 560-570. [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377-1397. [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 2012, 161, 505-522. [CrossRef]
- Czapka, A.; Grune, C.; Schadel, P.; Bachmann, V.; Scheuer, K.; Dirauf, M.; Weber, C.; Skaltsounis, A.L.; Jandt, K.D.; Schubert, U.S.; et al. Drug delivery of 6-bromoindirubin-3'-glycerol-oxime ether employing poly(D,L-lactide-co-glycolide)-based nanoencapsulation techniques with sustainable solvents. J Nanobiotechnology 2022, 20, 5. [CrossRef]
- Bachmann, V.; Schadel, P.; Westhoff, J.; Peric, M.; Schomberg, F.; Skaltsounis, A.L.; Hoppener, S.; Pantsar, T.; Fischer, D.; Vilotijevic, I.; Werz, O. Bromo-substituted indirubins for inhibition of protein kinase-mediated signalling involved in inflammatory mediator release in human monocytes. Bioorg Chem 2024, 149, 107470. [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012, 9, 671-675. [CrossRef]
- Vougogiannopoulou, K.; Ferandin, Y.; Bettayeb, K.; Myrianthopoulos, V.; Lozach, O.; Fan, Y.; Johnson, C.H.; Magiatis, P.; Skaltsounis, A.L.; Mikros, E.; Meijer, L. Soluble 3',6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase -3 alter circadian period. J Med Chem 2008, 51, 6421-6431. [CrossRef]
- Heshmati, N.; Wagner, B.; Cheng, X.; Scholz, T.; Kansy, M.; Eisenbrand, G.; Fricker, G. Physicochemical characterization and in vitro permeation of an indirubin derivative. Eur J Pharm Sci 2013, 50, 467-475. [CrossRef]
- Tchoumtchoua, J.; Halabalaki, M.; Gikas, E.; Tsarbopoulos, A.; Fotaki, N.; Liu, L.; Nam, S.; Jove, R.; Skaltsounis, L.A. Preliminary pharmacokinetic study of the anticancer 6BIO in mice using an UHPLC-MS/MS approach. J Pharm Biomed Anal 2019, 164, 317-325. [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int J Mol Sci 2022, 23. [CrossRef]
- Feng, S.S.; Mu, L.; Win, K.Y.; Huang, G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem 2004, 11, 413-424. [CrossRef]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials 2009, 30, 2790-2798. [CrossRef]
- Heshmati, N.; Cheng, X.; Eisenbrand, G.; Fricker, G. Enhancement of oral bioavailability of E804 by self-nanoemulsifying drug delivery system (SNEDDS) in rats. J Pharm Sci 2013, 102, 3792-3799. [CrossRef]
- Wiese-Rischke, C.; Murkar, R.S.; Walles, H. Biological Models of the Lower Human Airways-Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research. Pharmaceutics 2021, 13. [CrossRef]
- Shen, B.Q.; Finkbeiner, W.E.; Wine, J.J.; Mrsny, R.J.; Widdicombe, J.H. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am J Physiol 1994, 266, L493-501. [CrossRef]
- Lieber, M.; Smith, B.; Szakal, A.; Nelson-Rees, W.; Todaro, G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 1976, 17, 62-70. [CrossRef]
- Kwok, H.H.; Poon, P.Y.; Fok, S.P.; Ying-Kit Yue, P.; Mak, N.K.; Chan, M.C.; Peiris, J.S.; Wong, R.N. Anti-inflammatory effects of indirubin derivatives on influenza A virus-infected human pulmonary microvascular endothelial cells. Sci Rep 2016, 6, 18941. [CrossRef]
- Ehrhardt, C.; Ludwig, S. A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol 2009, 11, 863-871. [CrossRef]
- Ehrhardt, C.; Wolff, T.; Pleschka, S.; Planz, O.; Beermann, W.; Bode, J.G.; Schmolke, M.; Ludwig, S. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J Virol 2007, 81, 3058-3067. [CrossRef]
- Srinivasan, S.; Burckhardt, C.J.; Bhave, M.; Chen, Z.; Chen, P.H.; Wang, X.; Danuser, G.; Schmid, S.L. A noncanonical role for dynamin-1 in regulating early stages of clathrin-mediated endocytosis in non-neuronal cells. PLoS Biol 2018, 16, e2005377. [CrossRef]
- Clayton, E.L.; Sue, N.; Smillie, K.J.; O'Leary, T.; Bache, N.; Cheung, G.; Cole, A.R.; Wyllie, D.J.; Sutherland, C.; Robinson, P.J.; Cousin, M.A. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nat Neurosci 2010, 13, 845-851. [CrossRef]
- Reis, C.R.; Chen, P.H.; Srinivasan, S.; Aguet, F.; Mettlen, M.; Schmid, S.L. Crosstalk between Akt/GSK3beta signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J 2015, 34, 2132-2146. [CrossRef]
- Denisova, O.V.; Soderholm, S.; Virtanen, S.; Von Schantz, C.; Bychkov, D.; Vashchinkina, E.; Desloovere, J.; Tynell, J.; Ikonen, N.; Theisen, L.L.; et al. Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrob Agents Chemother 2014, 58, 3689-3696. [CrossRef]
- Wu, M.S.; Yen, H.R.; Chang, C.W.; Peng, T.Y.; Hsieh, C.F.; Chen, C.J.; Lin, T.Y.; Horng, J.T. Mechanism of action of the suppression of influenza virus replication by Ko-Ken Tang through inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway and viral RNP nuclear export. J Ethnopharmacol 2011, 134, 614-623. [CrossRef]
- Kovacina, K.S.; Park, G.Y.; Bae, S.S.; Guzzetta, A.W.; Schaefer, E.; Birnbaum, M.J.; Roth, R.A. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem 2003, 278, 10189-10194. [CrossRef]
- Nascimento, E.B.; Snel, M.; Guigas, B.; van der Zon, G.C.; Kriek, J.; Maassen, J.A.; Jazet, I.M.; Diamant, M.; Ouwens, D.M. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal 2010, 22, 961-967. [CrossRef]
- Lin, J.; Fang, Y.; Zhang, M.; Wang, X.; Li, L.; He, M.; Xue, A.; Zhu, K.; Shen, Y.; Li, B. Phosphorylation of PRAS40 contributes to the activation of the PI3K/AKT/mTOR signaling pathway and the inhibition of autophagy following status epilepticus in rats. Exp Ther Med 2020, 20, 3625-3632. [CrossRef]
- Wiza, C.; Nascimento, E.B.; Ouwens, D.M. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab 2012, 302, E1453-1460. [CrossRef]
- Lv, D.; Guo, L.; Zhang, T.; Huang, L. PRAS40 signaling in tumor. Oncotarget 2017, 8, 69076-69085. [CrossRef]
- Zhou, Q.; Tang, S.; Zhang, X.; Chen, L. Targeting PRAS40: a novel therapeutic strategy for human diseases. J Drug Target 2021, 29, 703-715. [CrossRef]
- Mata, M.A.; Satterly, N.; Versteeg, G.A.; Frantz, D.; Wei, S.; Williams, N.; Schmolke, M.; Pena-Llopis, S.; Brugarolas, J.; Forst, C.V.; et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat Chem Biol 2011, 7, 712-719. [CrossRef]
- Kuss-Duerkop, S.K.; Wang, J.; Mena, I.; White, K.; Metreveli, G.; Sakthivel, R.; Mata, M.A.; Munoz-Moreno, R.; Chen, X.; Krammer, F.; et al. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog 2017, 13, e1006635. [CrossRef]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 2014, 46, 372-383. [CrossRef]
- Morgensztern, D.; McLeod, H.L. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 2005, 16, 797-803. [CrossRef]
- Gallolu Kankanamalage, S.; Karra, A.S.; Cobb, M.H. WNK pathways in cancer signaling networks. Cell Commun Signal 2018, 16, 72. [CrossRef]
- Nishida, H.; Sohara, E.; Nomura, N.; Chiga, M.; Alessi, D.R.; Rai, T.; Sasaki, S.; Uchida, S. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension 2012, 60, 981-990. [CrossRef]
| Target | Manufacturer |
|---|---|
| IAV (HA) | GeneTex (GTX127357) |
| IAV (PB1) | GeneTex (GTX125923) |
| α-tubulin | Cell signaling Technology (2125) |
| HSP90 | Cell signaling Technology (4877) |
| pGSK-3β (Ser9) | Cell signaling Technology (5558) |
| pPRAS40 (Thr246) | Cell signaling Technology (13175) |
| pWNK1 (Thr60) | Cell signaling Technology (4946) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





