Submitted:
09 March 2025
Posted:
10 March 2025
You are already at the latest version
Abstract
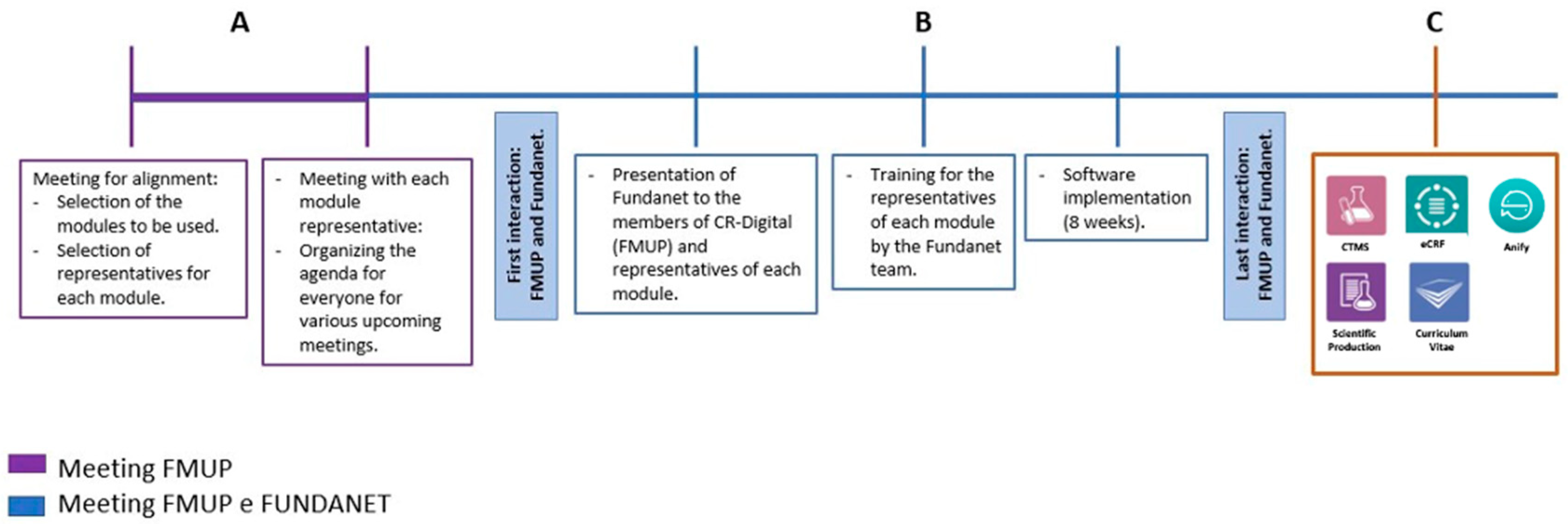
Clinical research is a cornerstone of medical advancement, requiring efficient data management and coordination across multiple stakeholders. This study explores the implementation of a digital research management system at the Faculty of Medicine of the University of Porto (FMUP) to address the challenges posed by its extensive and diverse clinical research portfolio. Given the institution's need to centralize research activities, improve efficiency, and ensure regulatory compliance, the CR-Digital project was launched to integrate a comprehensive database management system. Over a two-year period, FUNDANET was selected and implemented due to its adaptability to FMUP’s research framework, its ability to enhance collaboration, and its alignment with legal and financial requirements. The implementation process was structured into seven key phases, with the primary challenge being the alignment of diverse departmental objectives and preferences. These ranged from ensuring seamless interoperability with electronic health records to meeting the usability needs of researchers and administrators. Despite these complexities, FUNDANET successfully provided a robust platform encompassing digital research management, clinical study tools, and advanced data analytics, streamlining research workflows and optimizing decision-making processes. This study highlights the lessons learned during the system’s deployment, demonstrating the importance of selecting adaptable technological solutions, fostering stakeholder engagement, and implementing structured change management strategies. The insights gained from this project can inform other institutions seeking to modernize their clinical research infrastructure, ultimately contributing to more efficient, transparent, and data-driven research environments.
Keywords:
Introduction
Methods
- Digital platform management – Centralized oversight and administration of research projects.
- Digital tools for clinical studies – Support for study design, execution, and data management.
- Advanced data analytics – Tools for real-time research performance analysis and reporting.
- Task 1: Conducting a comprehensive review of clinical research frameworks and regulations.
- Task 2: Selecting a suitable digital platform for research management.
- Task 3: Integrating the platform with electronic health records (EHRs).
- Task 4: Developing advanced data analytics capabilities.
- Task 5: Conducting usability testing and stakeholder validation.
- Task 6: Communicating and disseminating project results.
- Task 7: Managing technical and financial aspects of the project.
Results
- Project management – A centralized platform for trial documentation, stakeholder coordination, and integrated data handling.
- Recruitment and follow-up – Tools for patient monitoring, automated scheduling, version control, and anonymized data tracking.
- Laboratory management – Oversight of sample collection kits, test records, kit reception, consumption control, and linkage to patient consultations.
- Financial management – Contract monitoring, clinical trial budget tracking, unbilled activity management, and automated billing proposals.
- Performance analytics – Real-time data visualization through Power BI, generating research performance indicators such as study typology, scope, patient recruitment rates, and institutional participation levels.
- Task 1: Identifying research infrastructure models aligned with FMUP’s goals while ensuring compliance with EU and national regulatory frameworks.
- Tasks 2-4: Addressing diverging stakeholder preferences, balancing clinician requirements with institutional data governance policies.
- Task 5: Securing stakeholder engagement and ensuring usability tests provided meaningful insights for system optimization.
- Task 6: Achieving consensus among research departments on communication and dissemination strategies.
- Task 7: Navigating bureaucratic complexities related to EU funding regulations and extensive reporting obligations.
Discussion
- The role of digital transformation in research efficiency: The introduction of a centralized digital platform significantly enhanced efficiency by streamlining project management, reducing administrative burdens, and ensuring compliance with regulatory requirements. Prior to implementation, research teams operated in silos, leading to inefficiencies in data access and duplication of effort. FUNDANET addressed these issues by providing a unified and structured workflow for all stakeholders.
- Challenges in aligning stakeholder interests: One of the most significant barriers to implementation was the need to reconcile the differing objectives of various departments. While clinical researchers prioritized ease of data entry and trial setup, administrative teams were more concerned with data security, compliance, and financial oversight. By involving all key stakeholders early in the decision-making process, FMUP was able to facilitate adoption and minimize resistance to change.
- Interoperability and data integration: Seamless integration with electronic health records was a critical factor in the platform's success. Ensuring that patient data from multiple sources could be harmonized and securely managed was essential for enhancing research accuracy and reliability. The interoperability of FUNDANET with existing hospital and research databases improved data accessibility while maintaining patient confidentiality.
- Regulatory and financial challenges: As a publicly funded initiative, the project had to comply with stringent EU funding regulations, requiring rigorous financial tracking and transparent reporting. The experience gained in managing these compliance challenges has provided a roadmap for future large-scale research digitization initiatives.
- Impact on clinical research scalability and innovation: By providing real-time analytics and performance tracking, FUNDANET has not only optimized ongoing research projects but has also laid the groundwork for scaling future research efforts. The ability to generate comprehensive data reports has improved decision-making and resource allocation, fostering a culture of data-driven research.
Conclusion
Author Contributions
Funding
Acknowledgments
Conflict of Interest Statement
References
- Röhrig B, Du Prel JB, Wachtlin D, Blettner M. Types of study in medical research. Dtsch Arztebl Int. 2009;106(15):262-268. [CrossRef]
- Azeka E, Fregni F, Auler JOC. The past, present, and future of clinical research. Clinics. 2011;66(6):931-932.
- Ferreira JP, et al. Investigator-led clinical research in Portugal. Acta Med Port. 2023; [CrossRef]
- Rahman MM, et al. Big data in healthcare: Challenges and potential applications. J Med Syst. 2020;44(5):102.
- Tariq HR, Hackett N. Interoperability in healthcare systems. J Health Inform Res. 2022;6(3):121-136.
- Gupta AK, et al. Regulatory frameworks for clinical research. Int J Clin Res. 2021;58(2):89-101.
- Park JH, et al. Financial challenges in clinical research. Clin Trials Rev. 2020;10(4):187-200.
- Wang LS, et al. Real-time analytics and AI in clinical research. Med Inform. 2023;34(5):145-159.
- Smith DK, et al. Future trends in digital health and clinical trials. J Med Tech. 2023;29(7):331-347.
- European Medicines Agency. Digital transformation in clinical trials. 2022. Available at: www.ema.europa.eu.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




