Submitted:
04 March 2025
Posted:
05 March 2025
You are already at the latest version
Abstract
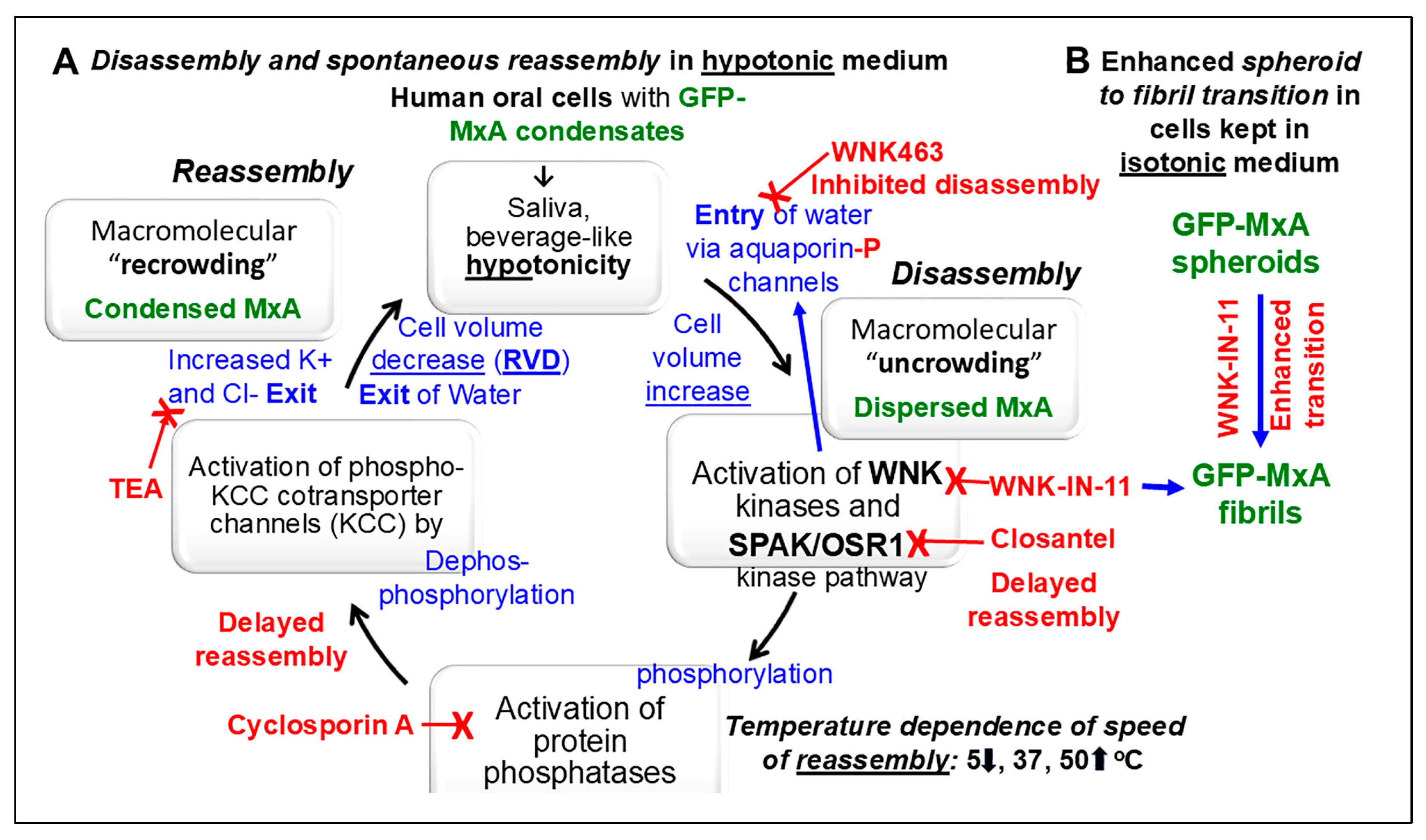
Phase-separated membraneless biomolecular condensates in the cytoplasm or nucleus are now recognized to play a major role in modulating diverse functions in mammalian cells, and contribute to cancer pathogenesis. Mechanisms that regulate the partitioning of components into condensed vs dispersed phases in intact cells, and those that trigger spheroid to fibril transition of condensate structure have attracted attention. We selected a common circumstance for our investigations - all of us episodically imbibe cold and warm drinks such as water, tea, or coffee subjecting our oral epithelial cells to stresses of hypotonicity and temperature. Moreover, oral cancer, in the absence of overt causes such as tobacco or alcohol, most frequently occurs in a U-shaped zone (floor of mouth, side of tongue, anterior fauces and retromolar region) reflecting the path of liquid transit through the mouth. In previous studies, we investigated the partitioning of the broad-spectrum antiviral human MxA protein (also called Mx1) between condensate (storage granule) and dispersed (antivirally active) phases in human oral cancer cells. We had observed that at 37oC, in OECM1 oral carcinoma cells, GFP-MxA condensates were exquisitely sensitive to hypotonicity – these disassembled within 1-2 min of exposure of cells to saliva-like one-third hypotonicity, and underwent spontaneous reassembly in the next 5-7 min even when continued in hypotonic medium. In the present studies we investigated whether this process was temperature sensitive representative of cold vs warm drinks. It was slowed at 5oC, and speeded up at 50oC. The involvement in this disassembly/reassembly process of WNK-SPAK/OSR1 serine-threonine kinase pathway, which regulates water and Na, K and Cl influx and efflux, was evaluated using pathway inhibitors WNK463, WNK-IN-11 and closantel. The pan-WNK inhibitor WNK463 inhibited disassembly, while the SPAK/OSR1 inhibitor closantel markedly slowed reassembly. Unexpectedly, the WNK1-selective inhibitor (WNK-IN-11), triggered dramatic and rapid (within 1 hr) spheroid to fibril transition of GFP-MxA condensates in live cells. The latter cells retained their antiviral phenotype against vesicular stomatitis virus. Thus, overall, cellular water fluxes and temperature changes dramatically but reversibly affected the GFP-MxA condensate landscape in oral epithelial cells. The WNK-SPAK/OSR1 kinases appear to be part of a mechanism for condensate recovery and restoration. The data raise a novel condensate-dysregulation hypothesis for understanding the occurrence of oral cancer along the liquid transit pathway in the mouth.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Plasmids and Transient Transfection
2.3. Live-Cell Fluorescence Imaging
2.4. Phase Transition Experiments and Fluorescence Imaging
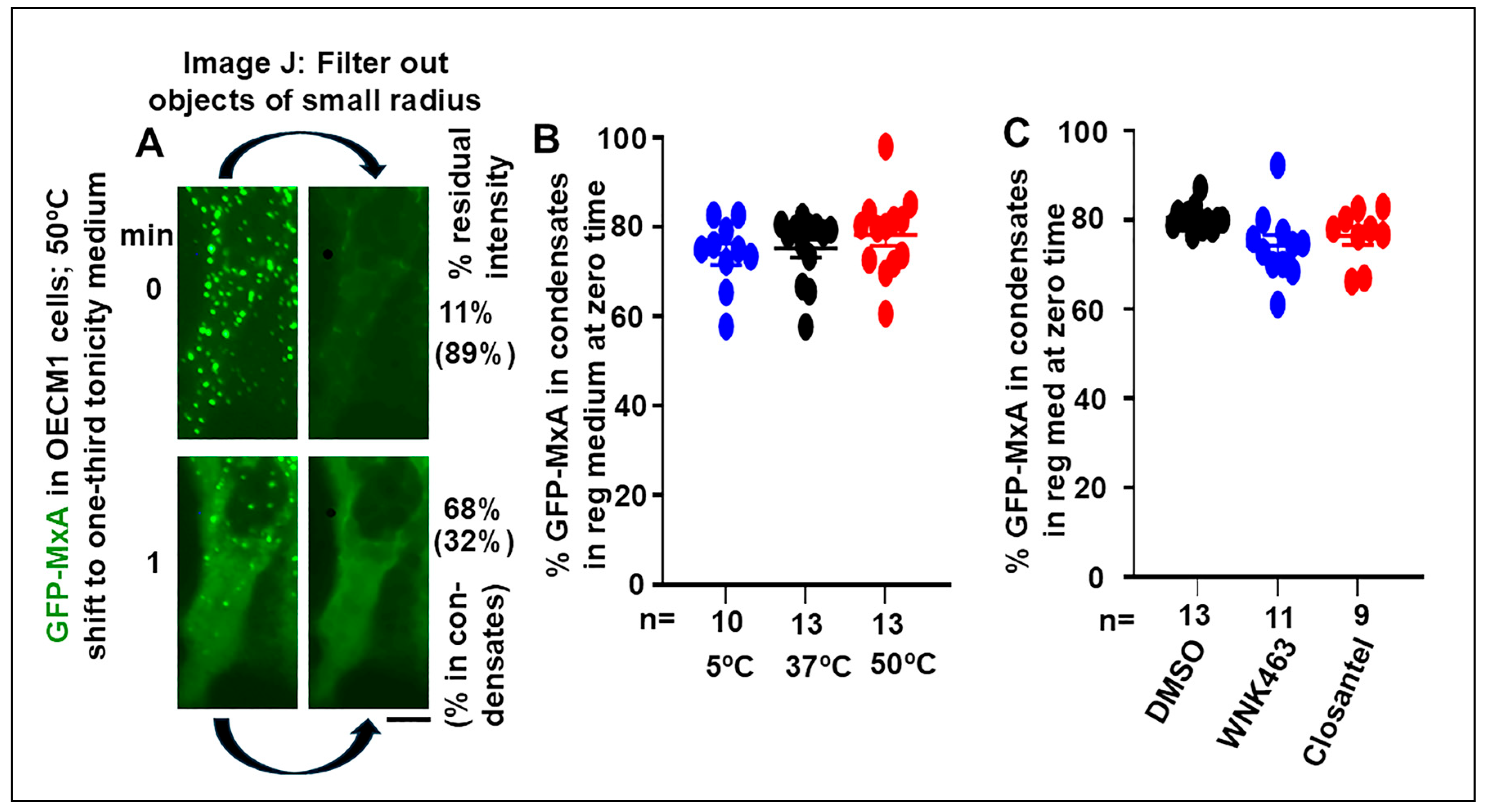
2.5. Quantitation of Relative Amounts of GFP-MxA in Condensates vs Dispersed State in a Cell
2.6. VSV Stock and Virus Infection
2.7. Antibody Reagents and Chemicals
2.8. Statistical Testing
3. Results
3.1. Quantitation of GFP-MxA in Condensed vs Dispersed Phases at the Single-Cell Level
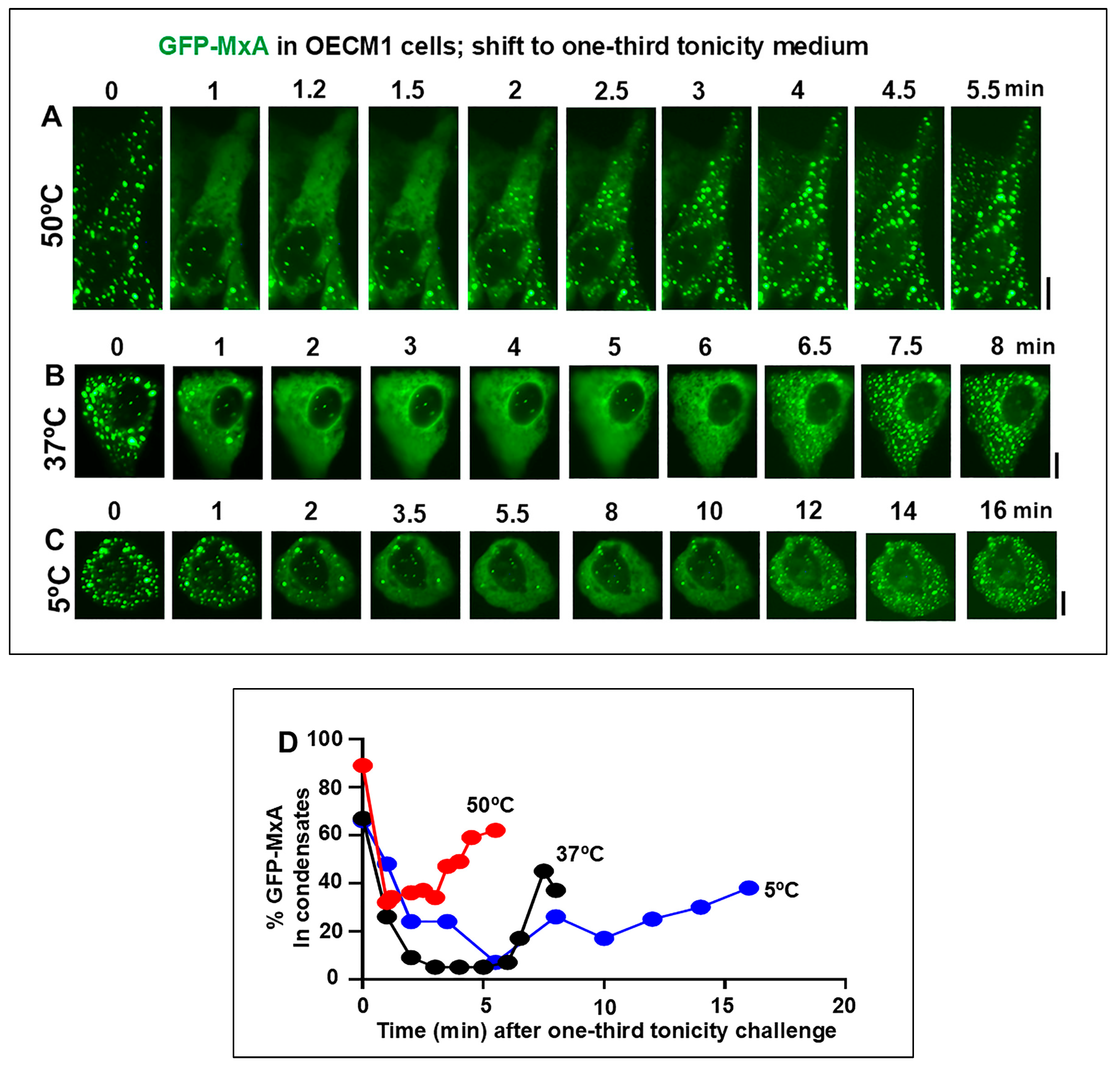
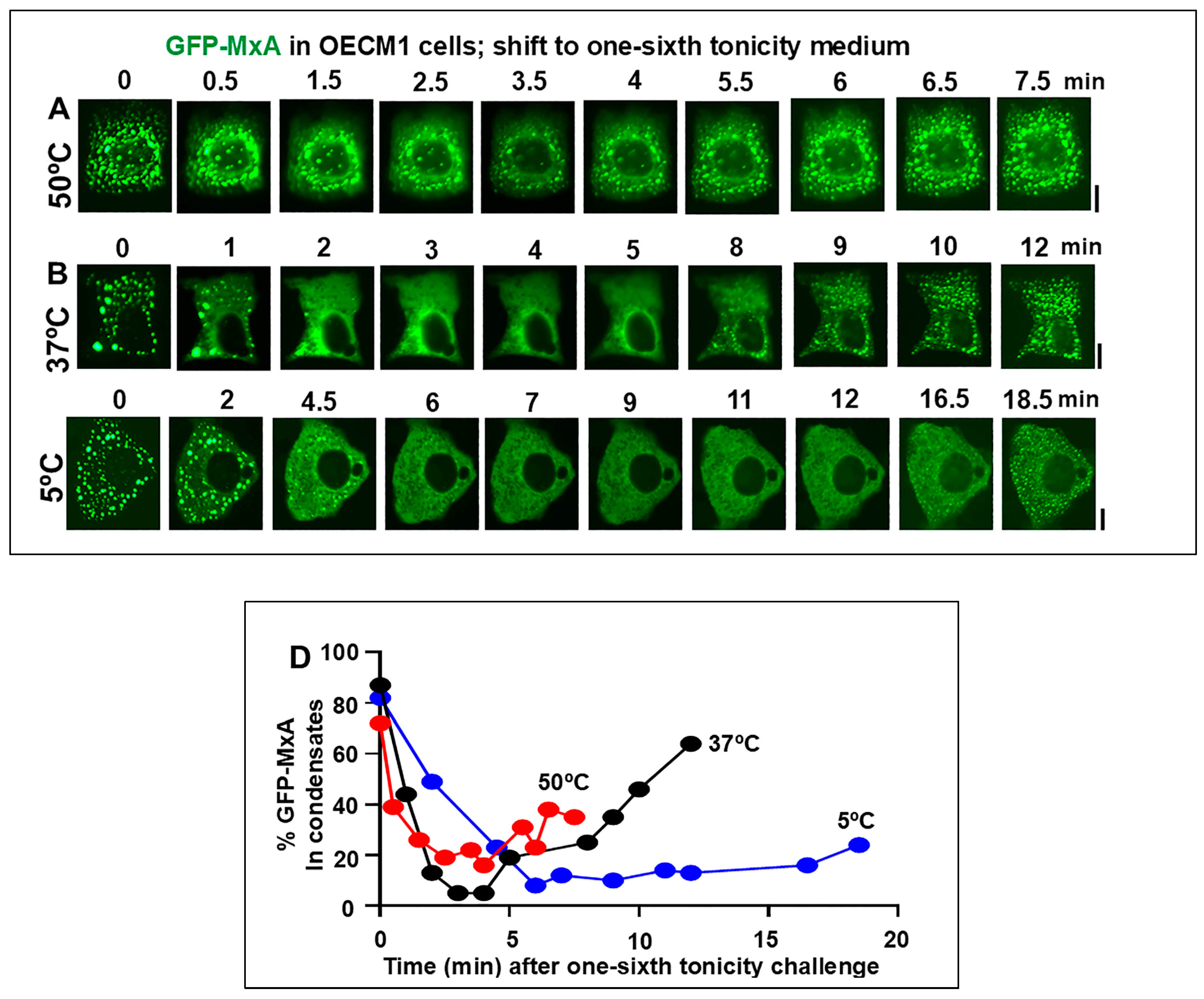
3.2. Temperature sensitivity of the Spontaneous Reassembly of GFP-MxA Condensates Dispersed by an Hypotonic Challenge
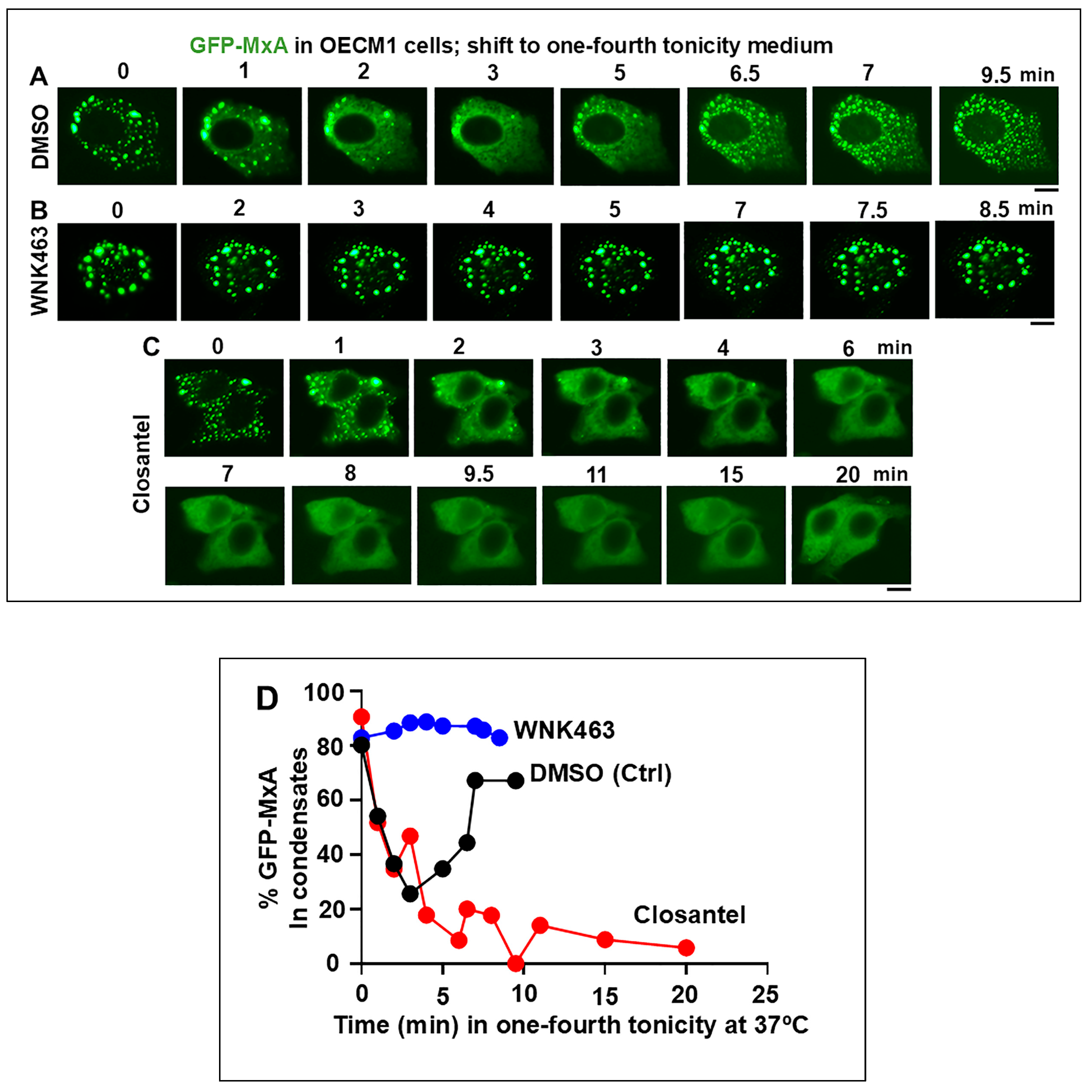
3.3. Involvement of the WNK-SPAK/OSR1 Kinase Pathway in the Dynamic Response of GFP-MxA Condensates to Hypotonicity
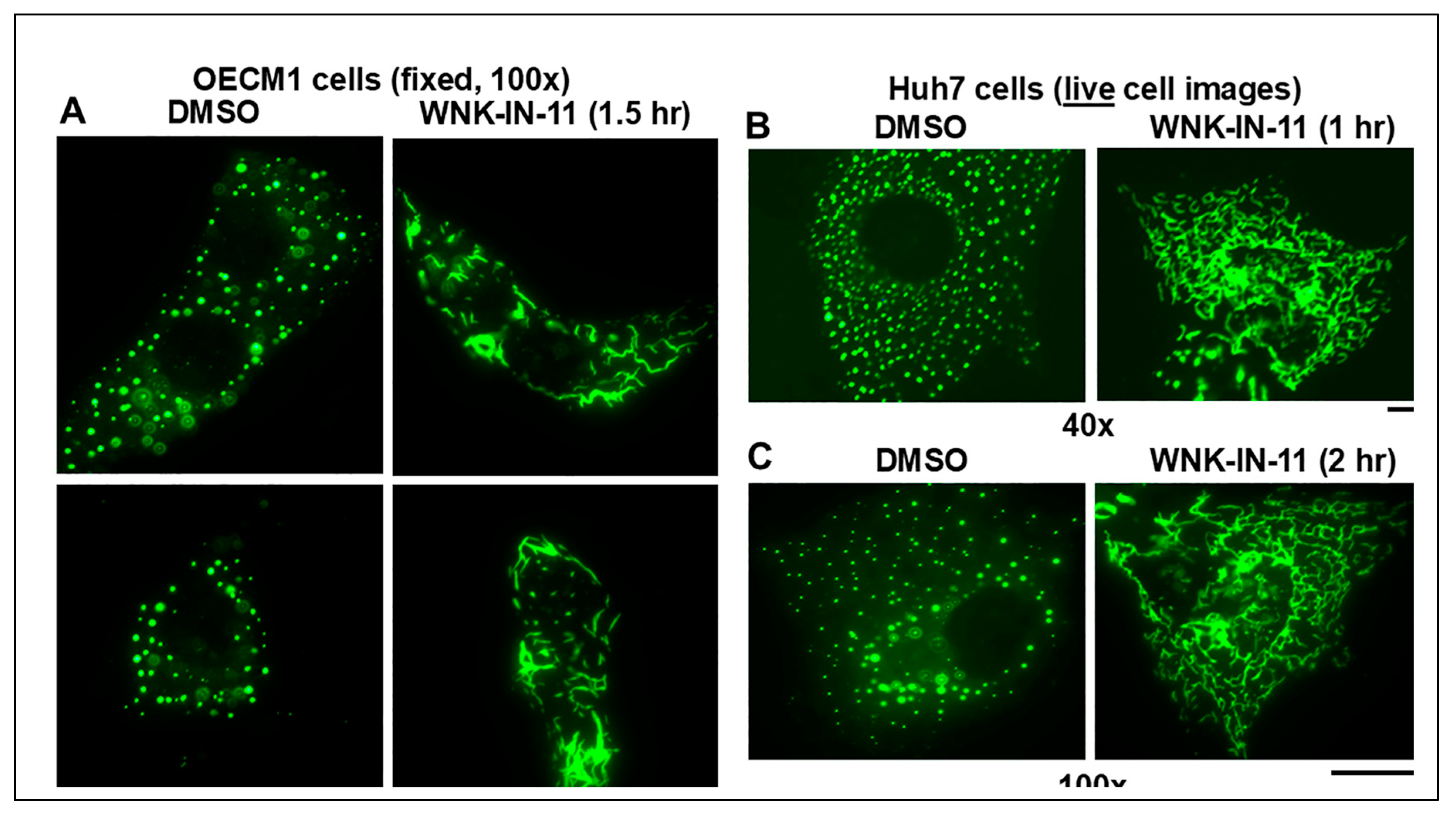
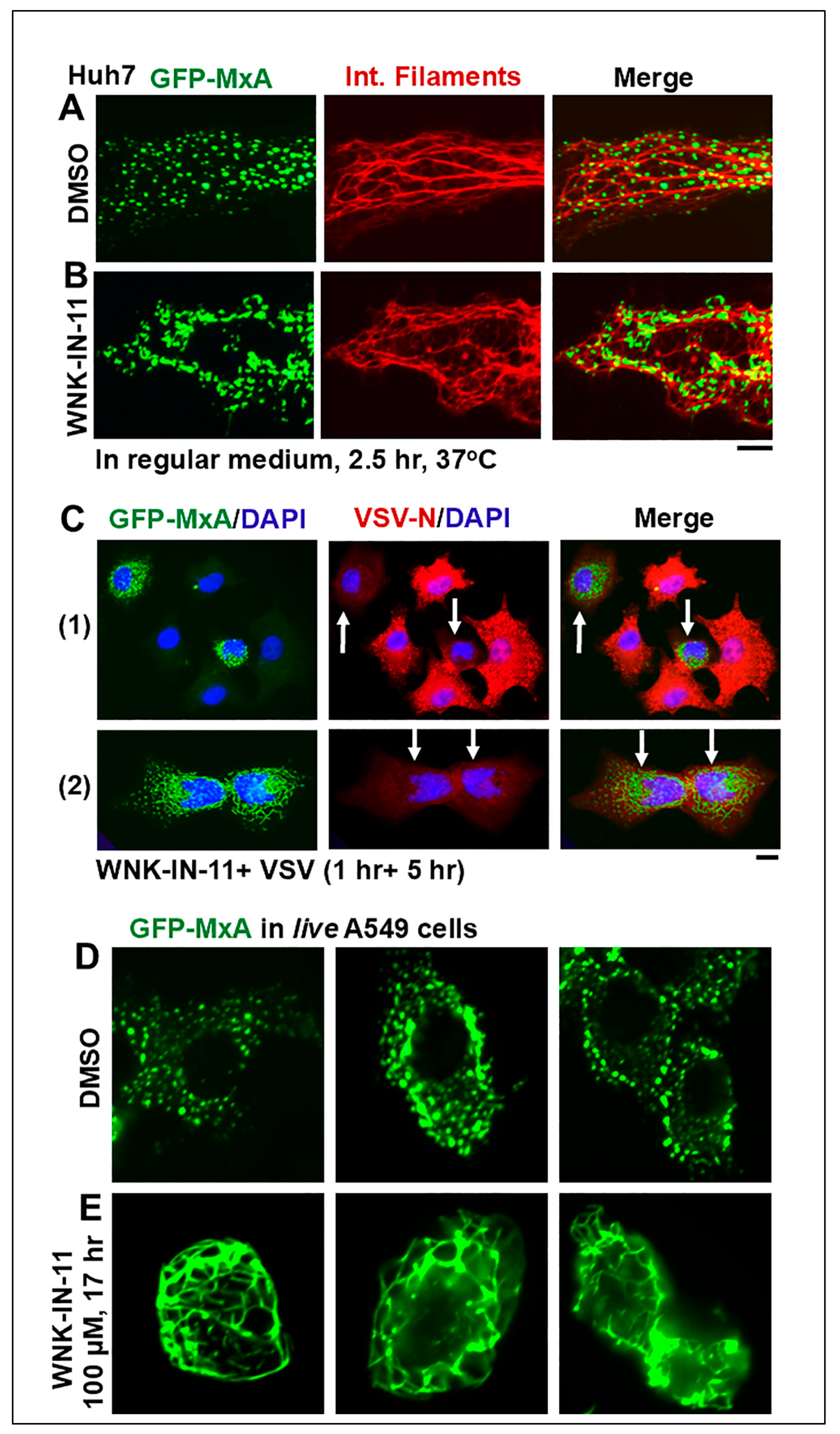
3.4. Dramatic and Rapid Spheroid to Fibril Transition of GFP-MxA Condensates in Live Cells Triggered by the WNK1-Kinase-Selective Inhibitor WNK-IN-11
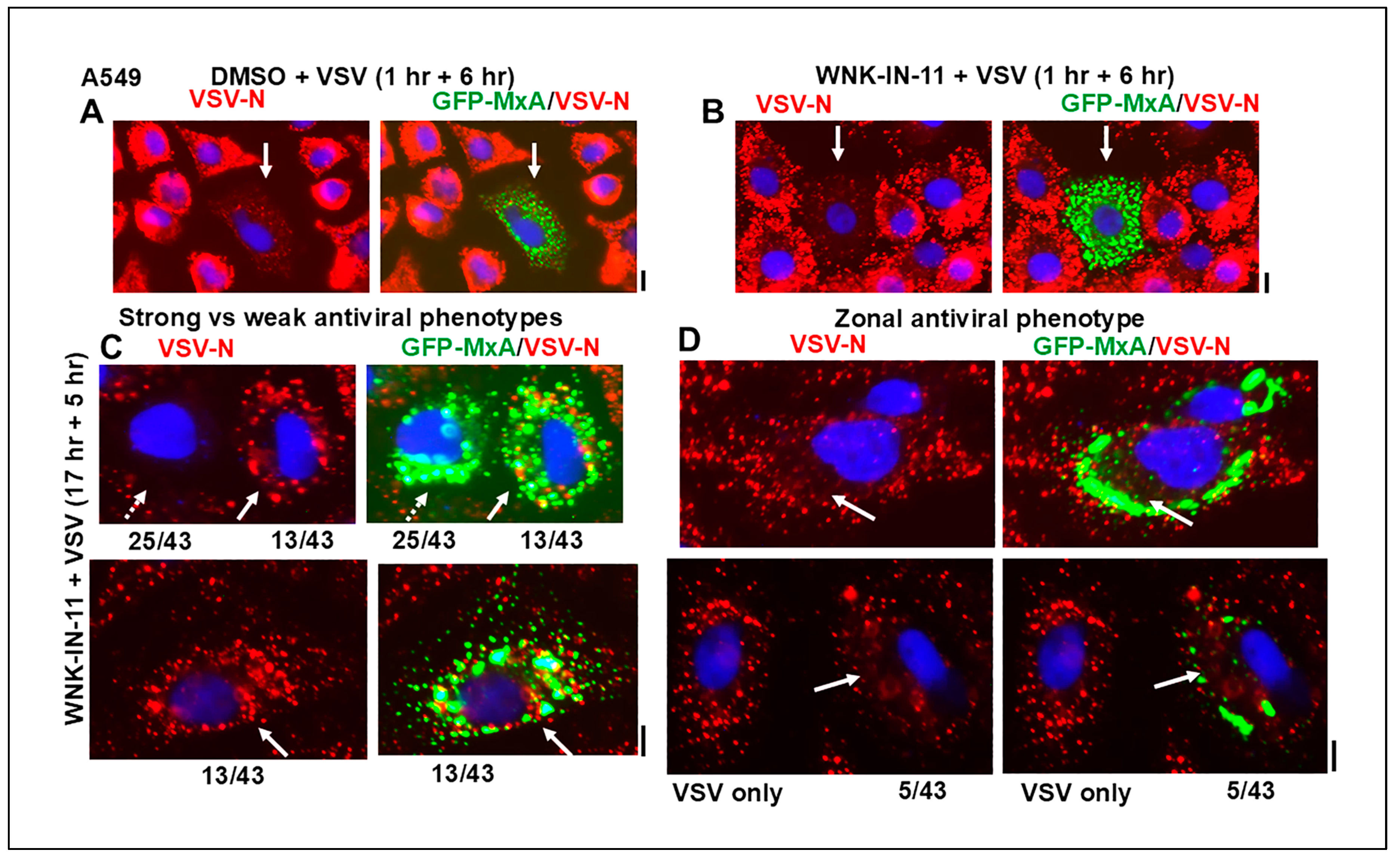
3.5. Relationship(s) Between GFP-MxA Condensates and the Antiviral Phenotype Against VSV at the Single-Cell Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflict of interest
References
- Cawson, R.A. Premalignant lesions in the mouth. Br Med Bull 1975, 31, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mashberg, A. , Meyers, H. Anatomical site and size of 222 early asymptomatic oral squamous cell carcinomas. Cancer 1976, 37, 2149–2157. [Google Scholar] [PubMed]
- Rivera, C. Essentials of oral cancer. Int J Clin Exp Pathol 2015, 8, 11884–11894. [Google Scholar] [PubMed]
- Squier, C.A. The permeability of keratinized and non-keratinized oral epithelium to horse radish peroxidase. J Ultrastruct Res 1973, 43, 160–177. [Google Scholar]
- Squier, C.A. , Rooney, L. The permeability of keratinized and nonkeratinized oral epithelium to lanthanum in vivo. J Ultrastruct Res 1976, 286–295. [Google Scholar]
- Squier, C.A. , Hall, B.K. The permeability of skin and oral mucosa to water and horseradish peroxidase as related to the thickness of permeability barrier. J Invest Dermatol 1985, 84, 176–179. [Google Scholar]
- Lesch, C.A. , Squier, C.A., Cruchley, A., Williams, D.M., Speight, P. The permeability of human oral mucosa and skin to water. J Dent Res 1989, 68, 1345–1349. [Google Scholar]
- Gholizadeh, P. , Eslami, H., Yousefi, M., Asgharzadeh, M., Aghazadeh, M., Kafil, H.S. Role of oral microbiome on oral cancers: a review. Biomed Pharmacotherapy 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Galvin, S. , Moran, G.P., Healy, C.M. Influence of site and smoking on malignant transformation in the oral cavity: is microbiome the missing link? Front Oral Health 2023, 4, 1166037. [Google Scholar]
- Lim, Y.X. , D’Silva, N.J. HPV-associated oropharyngeal cancer: in search of surrogate biomarkers for early lesions. Oncogene 2024, 43, 543–554. [Google Scholar]
- Mitrea, D.M.; Kriwacki, R.W. Phase separation in biology, functional organization of a higher order. Cell Commun. Signal 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Banani, S.F. , Lee, H.O., Hyman, A.A. and Rosen, M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Brangwynne, C.P. Liquid phase condensation in cell physiology and disease. Science 2017, 357, eaaf4382. [Google Scholar] [CrossRef]
- Alberti, S. The wisdom of crowds: regulating cell function through condensed states of living matter. J Cell Sci. 2017, 130, 2789–2796. [Google Scholar] [CrossRef]
- Gomes, E.; Shorter. J. The molecular language of membraneless organelles. J Biol Chem 2019, 294, 7115–7127. [Google Scholar] [CrossRef]
- Alberti, S. , Gladfelter, A. and Mittag, T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar]
- Sehgal, P.B. , Westley, J., Lerea, K.M. DiSenso-Browne, S. and Etlinger, J.D. (2020) Biomolecular condensates in cell biology and virology: phase-separated membraneless organelles (MLOs). Analytical Biochem 2020, 597, 113691. [Google Scholar] [CrossRef]
- Jiang, L., Kang, Y. Biomolecular condensates: a new lens on cancer biology. BBA Reviews on Cancer 1880, 189245.
- Boija, A. , Klein, I.A., Young, R.A. Biomolecular condensates and cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef]
- Han, T.W. , Portz, B., Young, R.A., Boija, A., Klein, I.A. RNA and condensates: disease implications and therapeutic opportunities. Cell Chem Biol 2020, 31, 1593–1609. [Google Scholar] [CrossRef]
- Tripathi, S. , Shimekhi, H.K., Gorman, S.D., Chandra, B., Baggett, D.W. et al. Defining the condensate landscape of fusion oncoproteins. Nat Commun 2023, 14, 6008. [Google Scholar]
- Chen, R. , Stainier, W., Dufourt, J., Lagha, M., Lehmann, R. Direct observation of translational activation by a ribionucleoprotein granule. Nat Cell Biol 2024, 26, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Davis, D. , Yuan, H., Liang, F.X., Yang, Y.M., Westley, J., Petzold, C., Dancel-Manning, K., Deng, Y., Sall, J. and Sehgal, P.B. Human antiviral protein MxA forms novel metastable membraneless cytoplasmic condensates exhibiting rapid reversible tonicity- driven phase transitions. J Virol. 2019, 93, e01014–19. [Google Scholar] [PubMed]
- Heinrich, B.S. , Maliga, Z., Stein, D.A., Hyman, A.A. and Whelan, S.P.J. Phase transitions drive the formation of vesicular stomatitis virus replication compartments. MBio 2018, 9, e02290–17. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.X. , Beurs, L.K., Das, P.B., Panda, D., Das, A. and Pattnaik, A.K. Induction of stress granule-like structures in vesicular stomatitis virus-infected cells. J Virol 2013, 87, 372–383. [Google Scholar] [CrossRef]
- Nikolic, J. , Bars, R.L., Lama, Z., Scrima, N., Lagaudriere-Gesbet, C., Gaudin, Y. and Blondel, D. Negri bodies are viral factories with properties of liquid organelles. Nat Commun 2017, 8, 58. [Google Scholar] [CrossRef]
- Hoenen, T. , Shabman, R.S., Groseth, A., Herwig, A., Weber, M., Schudt, G., Dolnik, O., Basler, C.F., Becker, S. and Feldmann, H. Inclusion bodies are a site of ebolavirus replication. J. Virol 2012, 86, 11779–11788. [Google Scholar]
- Alenquer, M.; Vale-Costa, S.; Sousa, A.L.; Etibor, T.A.; Ferreira, F.; Amorim, M.J. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nature Comm 2019, 10, 1629. [Google Scholar] [CrossRef]
- Peng, Q., Wang, L., Qin, Z., Wang, J., Zheng, X., Wei, L., Zhang, X., Zhang, X.; Liu, C., Li, Z., et al. Phase separation of Epstein-Barr virus EBNA2 and its coactivator EBNALP controls gene expression. J. Virol. 2020. [CrossRef]
- Cubuk, J. , Alston, J. J., Incicco, J.J., Singh, S., Stuchell-Brereton, M.D., Ward, M.D., Zimmerman, M.I., Vithani, N., Griffith, D., Wagoner, J.A., et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA, Nature Commun 2021, 12, 1936. [Google Scholar]
- Perdikari, T.M. , Murthy, A. C., Ryan, V.H., Watters, S., Naik, M.T. and Fawzi, N.L. SARSCoV-2 nucleocapsid protein undergoes liquid-liquid phase separation stimulated by RNA and partitions into phases of human ribonucleoproteins, EMBO J 2020, 39, e106478. [Google Scholar]
- Davis, D.; Yuan, H.; Yang, Y.M.; Liang, F.X.; Sehgal. P.B. Interferon-alpha- induced cytoplasmic MxA structures in hepatoma Huh7 and primary endothelial cells. Contemp Oncol (Pozn) 2018, 22, 86–94. [Google Scholar]
- Haller, O. and Kochs, G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 2002, 3, 710–717. [Google Scholar] [PubMed]
- Haller, O. , Staeheli, P., Kochs, G. Interferon-induced Mx proteins in antiviral host defense. Biochimie 2007, 89, 812–818. [Google Scholar] [PubMed]
- Haller, O., Staeheli, P., Schwemmle, M. and Kochs, G. Mx GTPases: dynamin- like antiviral machines of innate immunity. Trends Microbiol 215, 23, 154–163.
- Verhelst, J., Hulpiau, P. and Saelens, X. Mx proteins: antiviral gatekeepers that restrain the uninvited. Microbiol. Mol. Biol. Rev. 213, 77, 551–566.
- Staeheli, P. , Pavlovic, J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol 1991, 65, 4498–4501. [Google Scholar] [CrossRef]
- Schwemmle, M. ,Weining, K.C., Richter, M.F., Shumacher, B., Staeheli, P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 1996, 206, 545–554. [Google Scholar] [CrossRef]
- Steiner, F. , Pavlovic, J. Subcellular localization of MxB determines its antiviral potential against influenza virus. J Virol 2020, 94, e00125-e220. [Google Scholar]
- Kochs, G.; Haener, M.; Aebi, U.; Haller, O. Self-assembly of human MxA GTPase into highly ordered dynamin-like oligomers. J Biol Chem 2002, 277, 14172–14176. [Google Scholar]
- Kochs, G.; Janzen, C.; Hohenberg, H.; Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc Natl Acad Sci USA 2002, 99, 3153–3158. [Google Scholar]
- Haller, O.; Gao, S.; von der Malsburg, A.; Daumke, O.; Kochs, G. Dynamin-like MxA GTPase: structural insights into oligomerization and implications for antiviral activity. J Biol Chem 2010, 285, 28419–28424. [Google Scholar]
- Nigg, P.E.; Pavlovic, J. ; Pavlovic, J. Oligomerization and GTP-binding requirements of MxA for viral target recognition and antiviral activity against influenza A virus. J Biol Chem 2015, 290, 29893–29906. [Google Scholar] [CrossRef]
- Dick, A.; Graf, L.; Olal, D.; von der Malsburg, A.; Gao, S.; Kochs, G.; Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J Biol Chem 2015, 290, 12779–12792. [Google Scholar] [CrossRef]
- Graf, L.; Dick, A.; Sendker, F.; Barth, E.; Marz, M.; Daumke, O.; Kochs, G. Effects of allelic variations in the human myxovirus resistance protein A on its antiviral activity. J Biol Chem 2018, 293, 3056–3072. [Google Scholar] [PubMed]
- Janzen, C. , Kochs, G., Haller, O. A monomeric GTPase-negative MxA mutant with antiviral activity. J Virol 2000, 74, 8202–8206. [Google Scholar] [CrossRef] [PubMed]
- DiPaolo, C. , Hefti, H.P., Meli, M., Landis, H., and Pavlovic, J. Intramolecular backfolding of the carboxyl-terminal end of MxA protein is a prerequisite for its oligomerization. J Biol Chem 1999, 274, 32071–32078. [Google Scholar]
- Sehgal, P.B., Yuan, H., Centone, A., DiSenso-Browne, S.V. Oral antiviral defesnse: saliva- and beverage-like hypotonicity dynamically regulate formation of membraneless biomolecular condensates of antiviral human MxA in oral epithelial cells. Cells 2024, 13, 590.
- Sehgal, P.B. , Yuan, H., Jin, Y. Rapid reversible osmoregulation of cytoplasmic biomolecular condensates of human interferon-α-induced antiviral MxA GTPase. Int. J. Mol. Sci. 2022, 23, 12739. [Google Scholar]
- Mahanonda, R. , Sa-Ard-Iam, N., Rerkyen, P., et al. MxA expression induced by α-defensin in healthy human periodontal tissue. Eur J Immunol 2012, 42, 946–956. [Google Scholar]
- Imangulli, M.M. , Swaim, W.D., League, S.C., Gress, R.E., Pavletic, S.Z.; Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 2009, 113, 3620–3630. [Google Scholar]
- Rodriquez-Hernandez, C.J. , Sokolski, K.J., Stocke, K.S. et al. Microbiome-mediated incapacitation of interferon lambda production in the oral mucosa. Proc Natl Acad Sci USA 2021, 118, e2105170118. [Google Scholar]
- Santos, M.T.B.R. , Ferreira, M.C.D., Guare, R.O. et al. Gingivitis and salivary osmolality in children with cerebral palsy. Int J Paediatr Dent 2016, 26, 463–470. [Google Scholar]
- Ruiz, L.A. , Diniz, M.B, Loyola-Rodriguez, J.P., et al. A controlled study comparing salivary osmolality, caries experience and caries risk in patients with cerebral palsy. Med Oral Patol Oral Cir Bucal 2018, 23, e211–5. [Google Scholar]
- Feldman, M. , Barnett, C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gasteroenterology 1995, 108, 125–131. [Google Scholar]
- Gresz, V., Kwon, T.H., Hurley, P.T., Varga, G., Zelles, T., Nielsen, S., Case, R.M., Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol Gastroint Liver Physiol 2001, 281: G247-G254.
- Shen, M.R. , Chou, C.Y., Ellory, J.C. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflugers Archiv 2000, 440, 751–760. [Google Scholar] [PubMed]
- Bize, I. , Guvenc, B., Robb, A., Buchbinder, G., Brugnara, C. Serine/threonine protein phosphatases a2d regulation of K-Cl cotransport in human erythrocytes. Am J Physiol 1999, 277, C926–C936. [Google Scholar] [PubMed]
- Akella, R., Humphreys, J.M., Sekulski, K., He, H., Durbacz, M. et al. Osmosensing by WNK kinases. Mol Biol Cell 2021, 32 ; 1614-1623.
- Boyd-Shiwarski, C.R. , Shiwarski, D.J., Griffths, S.E., Beacham, R.T., Norrell, L. et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell 2022, 185, 4488–4506. [Google Scholar] [PubMed]
- Xiu, M. , Li, Y., Gao, Y. An update regarding the role of WNK kinases in cancer. Cell Death and Disease 2022, 13, 795. [Google Scholar]
- Teixeira, L.R. , Akella, R., Humphreys, J.M., He, H., Goldsmith, E.J. Water and chloride as allosteric inhibitors in WNK kinase osmosensing. eLife 2024, 12, RP88224. [Google Scholar]
- Carey, B.L., Ahmed, M., Puckett, S., Lyles, D.S. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol 2008, 82:12104-12115.
- Sehgal, P.B.; Yuan, H.; Scott, M.F.; Deng, Y.; Liang, F.-X.; Mackiewicz, A. Murine GFP-Mx1 forms phase-separated nuclear condensates and associates with cytoplasmic intermediate filaments: novel antiviral activity against vesicular stomatitis virus. J Biol Chem 2020, 294, 15218–15234. [Google Scholar]
- Thomson, MN, Cuevas, C.A., Bewarder, T.M., Dittmayer, C., Miller, L.N. et al. WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am J Physiol Renal Physion 318, F216-F228.
- Zhu, W., Begum, G., Pointer, K., Clark, P.A., Yang, S.S., Lin, S.H., Kahle, K.T., Kuo, J.S., Sun, D. WMK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Molecular Cancer 2014, 13, 31.
- Lewis, JL, III. Water and sodium balance. Merck Manual Professional Version, 2024, Merck & Co., Inc, Rahway, NJ, USA.
- Sehgal, P.B. Biomolecular condensates in cancer cell biology: interleukin-6-induced cytoplasmic and nuclear STAT3/PY-STAT3 condensates in hepatoma cells. Contemp Oncol (Pozn) 2019, 23, 16–22. [Google Scholar]
- Sehgal, P.B. Interleukin-6 at the host-tumor interface: STAT3 in biomolecular condensates in cancer cells. Cells 2022, 11, 1164. [Google Scholar] [CrossRef]
- Haas, B.R., Cuddapah, V.A., Watkins, S., Rohn, K.J.; Dy, T.E., Sontheimer, H. With-No-Lysine kinase 3 (WNK3 stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol 2011, 301, C1150-C1160.
- Li, L. , Xie, D., Yu, S., Ma, M., Fan, K., Chen, J., Xiu, M., Xie, K., Li, Y., Gao, Y. WNK1 interaction with KEAP1 promotes NRF2 stabilization to enhance the oxidative stress response in hepatocellular carcinoma. Cancer Res 2024, 84, 2776–2791. [Google Scholar]
- Kim, S. , Kehri, J.H. Inhibition of WNK kinases in NK cells disrupts cellular osmoregulation and control of tumor metastasis. J Innate Immunol 2024, 16, 451–469. [Google Scholar]
- Brandtzaeg, P. Immunobiology of the tonsils and adenoids. Mucosal Immunol 2015. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




