1. Introduction
Cancer is one of the significant factors affecting life expectancy. According to a 2019 report by the World Health Organization (WHO), cancer is the leading cause of death in individuals under the age of 70 in 112 out of 183 countries and ranks third or fourth in an additional 23 countries. The standard primary cancer therapeutic options include surgery, chemotherapy, and radiotherapy. In the 21st century, the use of radionuclides in the combined diagnosis and treatment of cancer is becoming increasingly common. Alpha and beta emitters are current choices for targeted radionuclide therapy. β−- emitters are suitable for larger and solid cancers because of the relatively long-range emissions. However, part of the released energy is deposited in the surrounding normal tissues relative to the tumor. α-particles have a much shorter range and greater linear energy transfer (LET), depositing more energy into smaller volumes, ideal for small tumors and metastasis, but with some limitations. The first limitation is the small number of radionuclides with suitable properties for nuclear medicine purposes and their availability. The second one is that most of the α-emitters suitable for therapy have a decay chain with multiple radioactive daughters, leading to unnecessary exposure of healthy tissues to the daughters if they are not well controlled.
Targeted Auger electron therapy can successfully replace α therapy. Radionuclides that emit Auger electrons (AE) are much cheaper and more readily available than α-emitters. Targeted Auger electron therapy is also more accurate and causes less damage to adjacent healthy cells. The linear energy transfer (LET) of Auger electrons ranges from 1 to 23 keV/µm [
1], therefore, Auger electrons are similar to α particles and produce highly damaging effects in cells. The energy of Auger electrons is deposited over nanometer distances, resulting in high LET that is potent for causing lethal damage in cancer cells by inducing double-stranded DNA breaks [
2]. It is possible to inject approximately tenfold the radioactivity of Auger emitters compared to β
- particle emitters without toxic side effects, particularly affecting the bone marrow. Therefore, it might be assumed that radiopharmaceuticals labeled with Auger emitters will gain widespread application in radionuclide therapy in the near future. This assumption is grounded on the significant cytotoxicity and therapeutic efficacy reported, as well as the availability of several low-energy electron-emitting radionuclides in non-carrier-added forms, characterized by variable physical half-lives and established chemical properties [
2].
In therapy using AE, one of the key factors limiting the broader application of this method is the need to precisely deliver the radionuclide, which is the emitter of Auger electrons, to the interior of the cell nucleus. This requires the development of a radiopharmaceutical that will recognize cancer cells and also allow the isotope to be placed within the cell nucleus in the immediate vicinity of the DNA strand. However, recent studies postulated that since the cell and nucleus membranes have a critical function for cell survival, the effects of AE emitted by membrane-bound radiolabeled biomolecules also need to be considered [
3]. One approach to targeting the cell membrane using AE emitters involves attaching a radioactive isotope to a molecule, such as an antibody, peptide, or small molecule, that specifically binds to cancer cell receptors. When the isotope decays, it emits Auger electrons that can damage cell membranes and associated proteins if the isotope is in close proximity. If the Auger electrons interact with the lipid bilayer or membrane proteins, they could cause localized damage that may disrupt membrane integrity. This could lead to the loss of membrane function and ion imbalances and lead to the death of cancer cells, which opens up new perspectives in the design of effective radiotherapeutic methods [
3].
Neuroendocrine tumors (NETs) are a group of tumors that are characterized by the overexpression of somatostatin receptors (SSTR), particularly SSTR type 2. This overexpression allows for the use of molecular imaging and radionuclide therapy for NET tumors. Radiolabeled somatostatin analogs, such as TOC, TATE, and NOC, which are conjugated with the chelator DOTA, are commonly applied for this purpose. Mentioned conjugates act as SSTR agonists and are internalized into the cytoplasm through the receptor. Several reviews have detailed the use of these radiolabeled agents in the management of NETs [
4].
On the other hand, LM3 (p-Cl-Phe- cyclo(D-Cys-Tyr-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys)D-Tyr- NH
2) is a SSTR antagonist. The conjugate of DOTA with LM3 is often labeled with
68Ga for NETs diagnosis and
177Lu for therapeutic purposes [
5]. As demonstrated with
68Ga-DOTA-LM3, LM3 radioconjugates demonstrate favorable biodistribution, high tumor uptake and retention, and also minimal safety concerns. In a study involving 40 patients,
68Ga-DOTA-LM3 showed greater diagnostic efficacy than the agonist
68Ga-DOTATATE [
6]. The first human study of the SSTR antagonist,
177Lu-DOTA-LM3, for radionuclide therapy has demonstrated significant efficacy in treating advanced metastatic NETs. This new SSTR antagonist shows favorable biodistribution and higher tumor uptake compared to the SSTR agonist,
177Lu-DOTATOC, and has even resulted in complete remission for some patients.
The advantages of the LM3 antagonist became more evident when comparing the antagonist
161Tb-DOTA-LM3 with the agonist
161Tb-DOTATATE. Similar to
177Lu,
161Tb emits soft beta radiation and additionally releases 2.27 Auger and IC electrons (with energies exceeding 3 keV) per decay [
7]. The observed higher dose-response may stem from the significantly higher membrane-bound fraction of
161Tb-DOTA-LM3, along with the emission of Auger electrons from
161Tb, which causes additional damage to the cell membrane. Consequently, the SSTR2 antagonist
161Tb-DOTA-LM3 demonstrated greater therapeutic efficacy for labeling with
161Tb compared to the agonist
161Tb-DOTATATE, making
161Tb-DOTA-LM3 the preferred choice for clinical translation. Studies involving
161Tb have clearly demonstrated that receptor antagonists labeled with an Auger electron emitter, which remains on the cell membrane, cause membrane damage, unlike agonists that are internalized into the cytoplasm.
In this study, we explored the potential of using the widely applied imaging radionuclide 99mTc as an Auger electron emitter for the treatment of NETs. We used the 99mTc-labeled LM3 peptide, which acts as an antagonist to the SSTR receptor, targeting the cell membrane as the site of action.
While
99mTc is commonly used for diagnostic imaging, particularly in Single Photon Emission Computed Tomography (SPECT), the potential for Auger electron emission could offer therapeutic benefits. According to Eckerman and Endo [
8], the electrons emitted from the core shells of
99mTc can be categorized as follows: Auger electrons, which have a yield of 4.4 per decay and internal conversion electrons of 1.1 per decay. On average, approximately 5.5 electrons are emitted from the atomic shells.
99mTc is not an ideal AE source for targeted radiotherapy because it has a relatively low yield of emitted electrons. However, this is higher than the number of shell electrons emitted by the
161Tb (0.9 Auger + 1.4 IC) [
7,
9]. Additionally, the absence of emitted β
- radiation enables us to better verify the hypothesis that the cell membrane serves as a more effective therapeutic target than the cytoplasm. It also permits the use of high therapeutic doses..
2. Results and Discussion
Current nuclear medicine practice primarily utilizes SSTR2-targeting radiopharmaceuticals that function as receptor agonists, such as
99mTc-EDDA/HYNIC-TOC,
177Lu-DOTATATE, and
68Ga-DOTATOC. However, both preclinical and clinical development are increasingly concentrating on SSTR2 antagonists. [
10,
11]. This is due to the ability of antagonists to recognize more binding sites on the receptor [
12]. This led to increased tumor uptake and better tumor-to-background contrast, resulting in enhanced image sensitivity and higher radiation doses to the tumor.
As mentioned in the introduction, a significant increase in cytotoxicity was observed for the SSTR2 receptor antagonist
161Tb-DOTA-LM3 compared to the agonist
161Tb-DOTATOC. This phenomenon can be attributed to the cytotoxic effects of Auger electrons on the cell membrane, where
161Tb-DOTA-LM3 accumulates. Since the radionuclide
161Tb emits both β
- particles and Auger electrons, these findings suggest that the cell membrane is a more sensitive target than the cytoplasm for the dense ionization produced by Auger electrons. Previous studies have shown that when targeting tumor clusters,
161Tb delivered 2- to 6-fold higher absorbed doses to cell membranes than
177Lu [
13]. Up to now, no cytotoxicity studies have been conducted for SSTR2 antagonists labeled with Auger emitters that do not emit β
- particles. The commercial availability of a bioconjugate of the SSTR2 receptor agonist -TEKTROTYD and the antagonist - TECANT-1 ready for labeling with
99mTc allows for easy performing of these studies and evaluation of the possibility of therapeutic application of
99mTc in the treatment of metastatic neuroendocrine tumors.
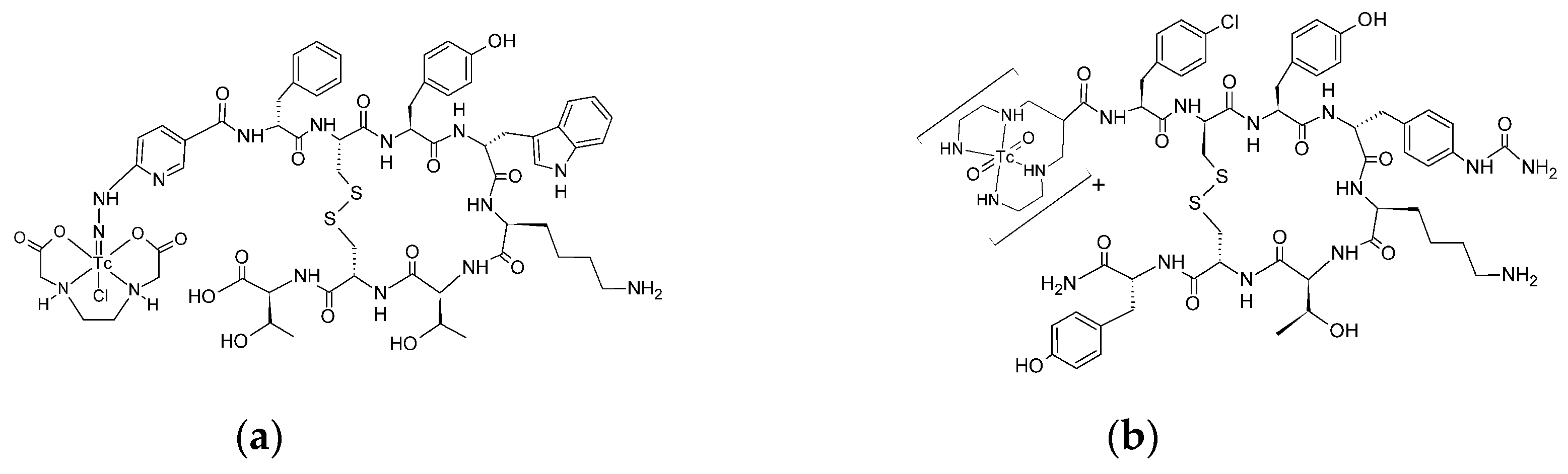
The structure of radiobioconjugates is presented in
Figure 1. As shown in the figure, TEKTROTYD is a conjugate of octreotide molecule with HYNIC ligand, and TECANT-1 is a conjugate of LM3 (p-Cl-Phe-cyclo(DCys-Tyr-DAph(Cbm)-Lys-Thr-Cys)-D-Tyr-NH
2) molecule with tetraamine linear ligand.
As expected HYNIC and tetraamine were very effective ligands for
99mTc. After reducing
99mTcO
4 with SnCl, TECANT-1 and TEKTROTYD were efficiently labeled with
99mTc, achieving yields of 98.9±0.4% and 94.4±3.8% respectively. After purification using a Sep-Pak C18 column, the radiochemical purity increased to 99.6±0.4%. As reported in multiple studies,
99mTc-TECANT-1 and
99mTc-TEKTROTYD exhibited high stability in biological fluids, including human serum [
14].
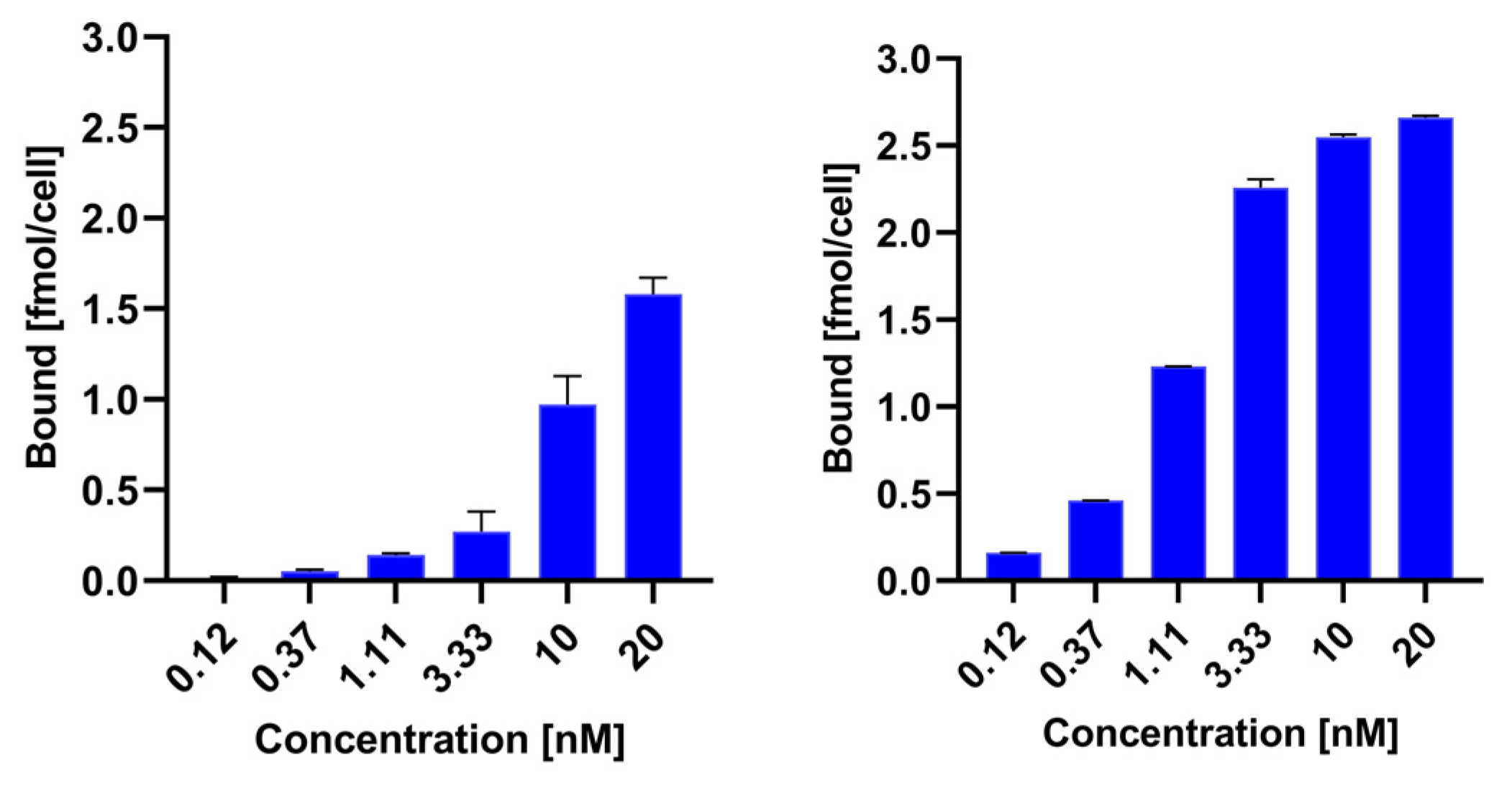
To compare the receptor binding of
99mTc-TEKTROTYD and
99mTc-TECANT-1, we calculated the specific binding of both radioconjugates by subtracting nonspecific binding from total binding.
Figure 2 illustrates the relationship between specific binding and the concentration of the radioconjugates. Specific binding for the
99mTc-labeled antagonist TECANT-1 is significantly greater than for the agonist TEKTROTYD. These results are consistent with previous preclinical studies demonstrated much higher tumor accumulation of these non-internalizing SST analogues than for SSTR agonists. A possible explanation may be that antagonist radioligands may label a higher number of receptor-binding sites than agonist radioligands [
15]. Scatchard analysis in SSTR2–transfected HEK293 cells showed more than 10 times the number of binding sites for the SST antagonist
111In-DOTA-BASS than for the SST agonist [
111In-DTPA
0,Tyr
3,Thr
8]-octreotide [
10].
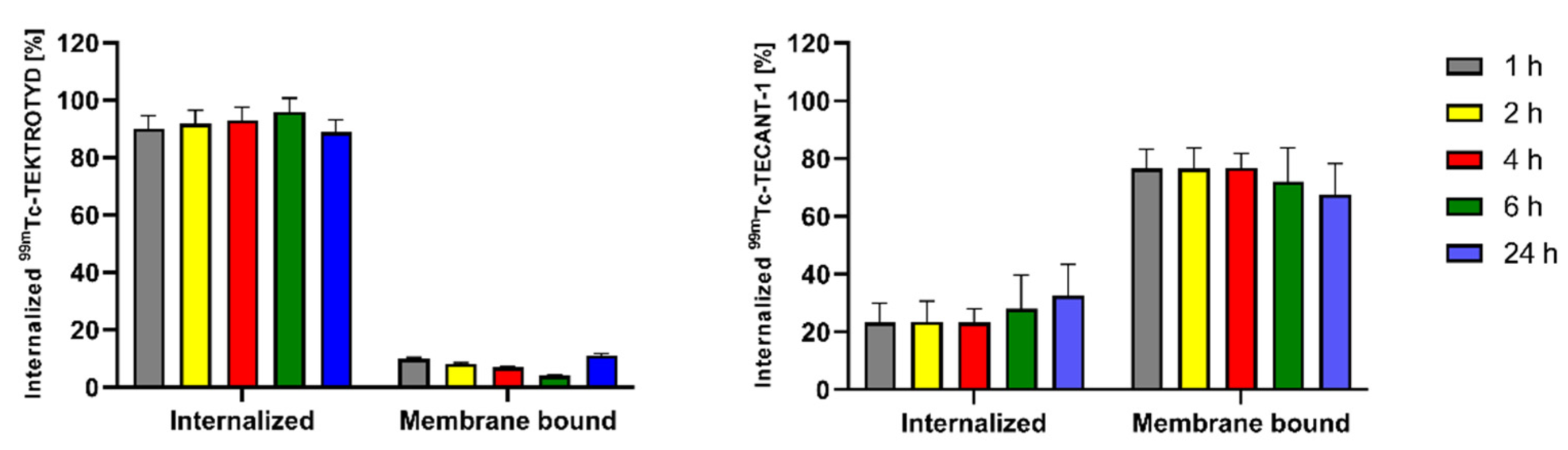
Due to the limited range of Auger electrons, internalization of radiobioconjugates is crucial for achieving optimal therapeutic effects. As shown in
Figure 3, approximately 90% of
99mTc-TEKTROTYD is internalized into AR-42-J cells through SSTR2 receptors while in the case of
99mTc-TECANT-1 most of the radioconjugate is accumulated in the cell membrane. The situation is similar to the previously described analogous conjugates of octreotide (TOC) with DOTA, an agonist of the SSTR2 receptor, and the conjugate of DOTA with the LM3 peptide, an antagonist of this receptor. About 9% of the cellular uptake of radiolabeled DOTA-LM3 was internalized, whereas in the case of DOTATOC, the internalized fraction was much higher, about 81% [
16]. The internalized fraction is increasing with time, from 1 h to 4 h. The differences in receptor internalization can be understood by recognizing that an agonist binds to a receptor, inducing internalization. This process involves the receptor being taken into the cell. In contrast, an antagonist binds to a cell membrane receptor but does not activate it, remaining attached to the cell membrane instead. The almost identical internalization of
161Tb-labeled DOTATOC and DOTA-LM3 conjugates and those of the peptides conjugated with
99mTc-labeled HYNIC and N4 chelators shows that their properties do not depend on the type of attached chelator.
In the cytotoxicity studies of the short range Auger electrons, considerable attention has been focused on delivering Auger electron emitting radionuclides to the cell nucleus, preferably close to DNA. This is the best way to achieve a therapeutic effect. Most publications on AE therapy have been devoted to this issue. However, further work has shown that internalization into cancer cells and delivery of Auger electron emitters to the cell nucleus is not necessary to kill the cancer cell, and the therapeutic effect can also be achieved indirectly through free radicals generated in the cytoplasm. It was found also that targeting the cell membrane has also been shown to be an effective strategy for killing cancer cells using AE [
3,
17,
18].
In our studies, we decided to apply the differential levels of
99mTc-TEKTROTYD and
99mTc-TECANT-1 accumulation in the cell membrane and cytoplasm to evaluate which target is more effective for cell destruction.
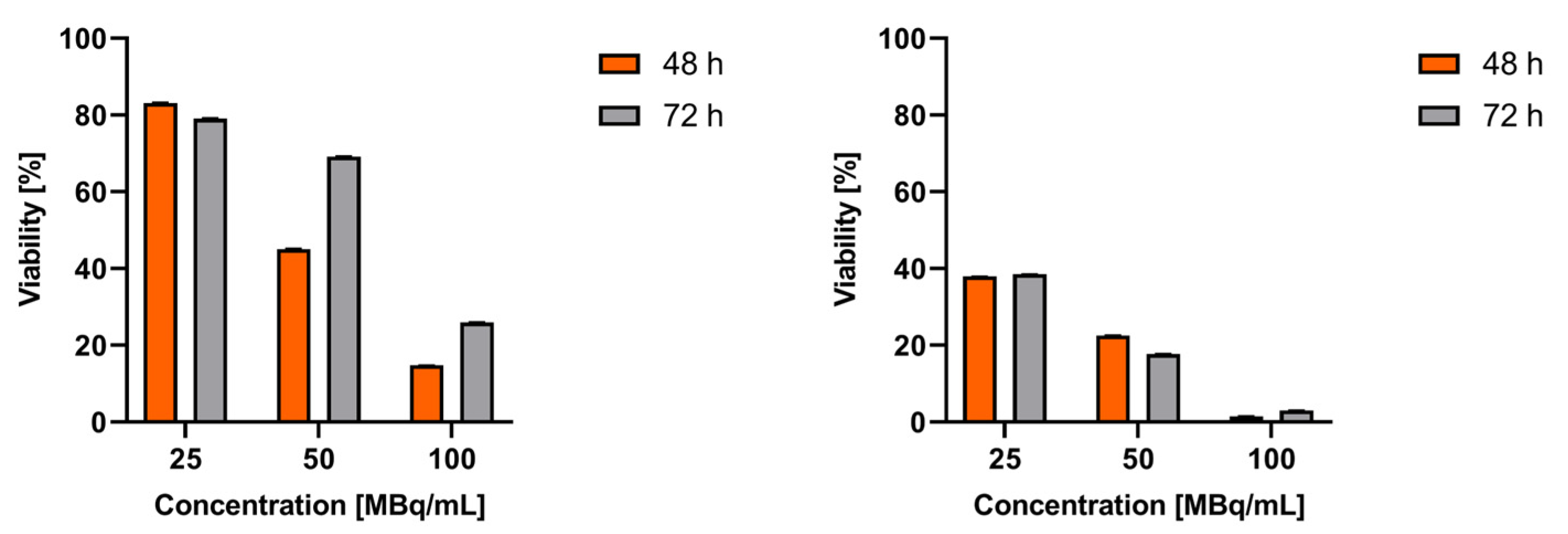
Figure 4 shows the results of cytotoxicity studies of
99mTc-labeled SSTR2 receptor agonist and antagonist performed on AR42J cells. As shown in
Figure 4, the toxicity of the
99mTc-labeled receptor antagonist is 2-3 times higher than that of the agonist. These results clearly indicated that the cell membrane is a much better therapeutic target than the cytoplasm. This is confirmed by numerous results of studies comparing the cytotoxicity of DOTATATE (SSTR2 receptor agonist) radiobioconjugates and DOTA-LM3 (SSTR2 receptor agonist) labeled with
177Lu and
161Tb. Lu
3+ and Tb
3+ cations have almost identical chemical properties, but the radionuclide
161Tb, unlike
177Lu, is an emitter of β
- radiation and Auger electrons, while
177Lu basically emits only β
- radiation. It was observed that the non-internalizing conjugate
161Tb-DOTA-LM3 showed much more significant cytotoxicity than
177Lu-DOTA-LM3, while in the case of radiobioconjugates
177Lu-DOTATATE and
161Tb-DOTATATE internalizing to the cytoplasm, no significant differences were observed.
When comparing the cytotoxicity of the peptides octreotide and LM3 labeled with 99mTc and 161Tb, we observe a significantly more significant effect with the 161Tb radioconjugates. This difference is largely attributed to 161Tb being an emitter of low-energy β- radiation, which has a much longer range than that of Auger and conversion electrons. Whether 161Tb accumulates in the cell membrane or cytoplasm, the emitted β- particles can interact with DNA in a toxic manner. So, for the 161Tb-DOTA-LM3 radioconjugate, the observed toxicity is likely due to the cumulative effect of β- particles damaging the DNA within the cell nucleus, along with the interaction of Auger electrons affecting the cell membrane. In contrast, for the 99mTc-N4-LM3 radioconjugate (TECANT-1), we see only cytotoxicity effect associated with the destruction of the cell membrane.
Also, it’s important to consider the conversion electrons (CE) emitted by 161Tb and 99mTc as well. Both radionuclides emit CE with similar yield: 1.1 per decay for 99mTc and 1.4 for 161Tb. These CE have a micrometer range, allowing them to interact with cell DNA. Therefore, the slight cytotoxicity associated with the agonist 99mTc-TEKTROTYD may be attributed to its EC emitted by 99mTc in the cytoplasm.
3. Materials and Methods
3.1. Chemical Reagents
The following chemical reagents: TEKTROTYD (20 micrograms kits) (HYNIC-(D-Phe1,Tyr3-Octreotide) and TECANT-1 (N4-p-Cl-Phe-cyclo(D-Cys-Tyr-DAph(Cbm)-Lys-Thr-Cys)-D-Tyr-NH2, where D-Aph(Cbm): D-4-amino-carbamoyl-phenylalanine) were purchased from (Polatom, Otwock, Poland). Sodium hydroxide (NaOH, VWR CHEMICA, EC), Aceton (VWR CHEMICA, EC), Ethyl alcohol Absolut 99.8% (POCH, Poland), Methanol (VWR CHEMICA, EC), Ammonium acetate (VWR CHEMICALS, EC), ITLC-SG (chromatography paper, Serial/Lot No: 6446280-03, Agilent Technology), N-(3-dimethylaminopropyl)-N-0-ethylcarbo-diimide hydrochloride (EDC, >99%), and Acetonitril, > 99.9% for HPLC (Sigma-Aldrich, France). The aqueous solutions were prepared using ultrapure deionized water 18.2 MW_cm (Hydrolab, Straszyn, Poland), Sep-Pak-18 classic cartrige (Waters corporation, Milford Massachusetts, USA), and Glycine (Sigma, Life science, Product of Switzerland).
3.2. Cell Lines and Reagents for Biological Studies
AR-42-J(Epithelial-like cell isolated from pancreas of a rat with tumor) was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured according to the ATCC protocol (humidified atmosphere of 5% CO2 at 37 oC). Cells were cultured in F-12K (Kaighn’s Modification of Ham’s F-12 with L-glutamine) enriched with 10% heat-inactivated fetal bovine serum and antibiotics: penicillin and streptomycin (100 IU/mL). Trypsin EDTA solution C (0.25%) was used to detach the cells. All chemicals mentioned above were purchased from Capricorn Scientific GmbH (Ebsdorfergrund, Germany) and ATCC collection, USA. CellTiter96® Aqueous One Solution Reagent (MTS compound) from Promega (Mannheim, Germany) and phosphate-buffered saline (PBS) from Capricorn Scientific GmbH (Ebsdorfergrund, Germany) were used for cell studies.
3.3. Radionuclide (Na+[99mTc]TcO4̶)
No-carrier-added Na+99mTcO4- was obtained from a commercial 99Mo/99mTc-generator (Polatom, Otwock, Poland). POLTECHNET – kit for elution was purchased from Polatom. The kit was composed of 16 vials with an eluent of 10 mL volume containing 9 mg/mL (0.9%) NaCl solution and 16 evacuated vials.
3.4. Instruments
The radioactivity of 99mTc was counted by BIODEX dose calibrator (Model: ATOMLAB™ 500) and Wizard2 Detector Gamma Counter. The radiolabeling yield was determined by using TLC and a Storage Phosphor System Cyclone Plus (PerkinElmer, Waltham, MA, USA). The samples of in vitro studies, including affinity and internalization, were measured by Wizard2 Detector gamma counter. The MTS assay was performed by BioTek (SYNERGY H1, Winooski, Vermont, USA) and absorbance at 490 nm was measured for determination of cell metabolic activity percentage. The analysis was performed by BioTek Gen5 (Microplate Reader and Imager Software, Winooski, Vermont, USA). Heater Grant (QBD2, Royston, Cambridgeshire, Great Britain) was used in the incubation of the TEKTROTYD radiolabeling process.
3.5. Radiolabeling and Purification of TEKTROTYD
The radiolabeling process was performed according to the kit protocols, with minor modifications. The labeling kit consisted of two vials containing components necessary for the preparation of 99mTc- TEKTROTYD. Vial I contains the active substance: HYNIC-[D-Phe1, Tyr3-Octreotide] trifluoroacetate, along with excipients including stannous chloride dihydrate, N-[Tris(hydroxymethyl)methyl]glycine (tricine), and mannitol. Vial II contains excipients such as: ethylenediamine-N,N’-diacetic acid (EDDA), disodium phosphate dodecahydrate, sodium hydroxide, and either sodium hydroxide or hydrochloric acid for pH adjustment. A total of 4 mL of Na+[99mTc]TcO4- was eluted from the 99Mo/99mTc generator using a 0.9% NaCl solution, which was then placed in an evacuated vial. The eluted activity was measured using a dose calibrator. The solution was then added to vial II and gently mixed for 15 seconds. Subsequently, the contents of vial II were transferred to vial I and mixed for an additional 30 s. The sample was then heated at 100 °C for 10 min. to facilitate ethanol evaporation, followed by cooling to room temperature.
For purification, the obtained sample was passed through a Sep-Pak C18 column, and the column was washed with 1 mL of water. Finally, 99mTc- TEKTROTYD was eluted with 1 mL of 90% EtOH and collected in separate glass vials.
For quality control, the radiopeptide solution was heated to evaporate EtOH. After evaporation, the radiocompound was dissolved in 500 µL of 0.01M PBS.
3.6. Radiolabeling of TECANT-1
The radiolabeling process was performed according to the modified kit protocol. Briefly, the Na+[99mTc]TcO4- was eluted from the 99Mo/99mTc generator using a 0.9% NaCl solution and added to a mixture of 25 μL of 0.5 M phosphate buffer, and 5 μL of 0.1M trisodium citrate., This was followed by the addition of TECANT-1 (20 μg, in H2O) and 5 μL SnCl2 in EtOH. The radiolabeling solution was incubated at RT for 10 minutes. After this time, the vial was opened, and 10 µL of the solution was taken to determine the pH of the radiopeptide solution. The sample was then heated at 100 °C for 10 min to facilitate ethanol evaporation, and cooled to room temperature. The purification of 99mTc-TECANT-1 was performed using the same procedure as for the 99mTc- TEKTROTYD.
3.7. Biding Affinity Assay
The SSTR2 binding affinity of 99mTc-TEKTROTYD (99mTc-EDDA/HYNIC-3Tyr-octreotide and 99mTc-TECANT-1 (99mTc-N4-p-Cl-Phe-cyclo(D-Cys-Tyr-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys)D-Tyr- NH2) was determined by saturation binding affinity assay. AR-42-J cells were seeded in 6-well plates (7 × 105 cells/well for octreotide) and 12-well plates (4 × 105 cells/well for LM-3) and incubated for 48 hours. After the incubation the cells were washed twice with PBS and incubated for 2 h with increasing concentrations of radiopeptides (0.004 −20 nM). To determine non-specific binding, wells were incubated with an additional 2000-fold excess of unlabeled octreotide acetate. Following incubation, the cells were washed with PBS, collected in tubes and then lysed twice using 800 μL of 1 M NaOH (for 12-well plate) and 1.5 mL of 1 M NaOH (for 6-well plates). The lysates were collected in separate tubes and radioactivity was measured using Wizard2 gamma counter.
3.8. Internalization Assay
The intracellular retention of 99mTc-TEKTROTYD and 99mTc-TECANT-1 in SKOV-3 cells was assessed at various time intervals. Briefly, AR-42-J cells (7 × 105 cells/well) were seeded in 6-well plates and incubated for 2 days. Following this incubation, the cells were washed twice with cold PBS and then treated with 5 nM of tested compounds. To avoid internalization, the cells were incubated at 4 °C for 1 hour. Non-specific binding was assessed by adding a 1000-fold molar excess of unlabeled octreotide acetate. After incubation, unbound fraction was collected in tubes, and the cells were washed twice with 1 mL of cold PBS. Fresh medium was then added, and the cells were incubated at 37 ºC for up to 24 hours. At each time interval, the second medium portion was collected, and cells were washed twice with 1 mL of PBS. Next, the membrane-bound fraction was collected using 0.05 M glycine−HCl buffer (pH 2.8). Finally, the internalized fraction was obtained by lysing the cells with 1.5 mL of 1 M NaOH.
3.9. Cytotoxicity Studies
A cytotoxicity assay was performed using the AR-42-J cell line. 104 cells were seeded in 96-well plate and incubated overnight at 37 ºC in a 5% CO2 atmosphere. The following day, the medium was removed and the cells were treated with radiocompounds at concentrations of 100, 50, 25, 12.5 MBq/mL The cells were then incubated for 24, 48, and 72 h. At each specified time point, 20 µL of MTS reagent was added to cells and incubated for 2 h. Absorbance at 490 nm was measured to estimate the percentage of cell metabolic activity.
3.10. Statistical Analysis
GraphPad Prism version 8.4.3 software (GraphPad Software Inc., San Diego, CA, USA) was used for the statistical analysis of the experimental data. Data points and SD are from at least two or more measurements.
4. Conclusions
The therapeutic application of 99mTc offers several potentially advantageous features, including an optimal half-life, stable daughter nuclides, and Auger electron energies that are suitable for effective radiotherapy of targeted tumors. Additionally, 99mTc is readily available and inexpensive. However, its limited efficiency in the emission of Auger electrons per decay - when compared to isotopes like 111In and 125I - may restrict its use in targeted Auger electron therapy.
Based on the cellular studies presented in this work, it can be concluded that the SSTR2 receptor antagonist 99mTc-TECANT-1 demonstrates a significantly enhanced cytotoxic effect compared to the agonist 99mTc-TEKTROTYD. This difference in cytotoxicity is even more pronounced than when using the same peptides labeled with 161Tb, where an additional effect from beta particle emission also contributes.
It’s important to note that 99mTc does not emit beta radiation or high-energy gamma quanta, which allows for very high radiation doses to be administered without causing significant side effects. Further dosimetry calculations and in vivo studies are necessary to determine the potential effectiveness of Auger electrons emitted from 99mTc-TECANT-1 in targeted radiotherapy for neuroendocrine tumors.
Author Contributions
Conceptualization, AM-P. and AB; methodology, SNS, AM-P, PK, MŁ, RW, EM; formal analysis, AM-P.; investigation, SNS, EM, MŁ, RW; resources, WW; writing—original draft preparation, AB, AM-P, MŁ; writing—review and editing, MŁ, RW; JN; visualization, MŁ, AM-P.; supervision, AM-P; project administration, AM-P, AB; funding acquisition, AM-P, AB. All authors have read and agreed to the published version of the manuscript.