1. Introduction
Right ventricular thrombosis (RVT) is a rare and frequently underdiagnosed life-threatening condition [
1]. It can be detected in 2.6–18% of patients with pulmonary embolism (PE) [
2,
3,
4].
The main risk factors for RVT are: younger age, previous bleeding events, congestive heart failure, intracardiac procedures, cancer, episodes of syncope, transient systolic blood pressure <100 mmHg, and arterial oxyhaemoglobin saturation <90% [
2]. Hypercoagulability, secondary to Factor 5 Leiden or antithrombin 3 mutations, is another factor that has been associated with RVT occurrence [
5]. In certain cases, hypercoagulability may be secondary to occult malignancy [
6].
Most patients with RVT have significant right ventricular (RV) dilatation and dysfunction. Cases of RVT have also been reported in patients with acute inferior-wall myocardial infarction complicated by RV infarction [
7]. From a pathophysiological point of view, mechanisms for RVT formation in situ are related to the so-called “Virchow’s triad”, which consists of blood stasis, endothelial dysfunction and concomitant hypercoagulability.
Based on its etiology, RVT is classified in types A, B and C. The type A is a highly mobile serpiginous thrombus, entrapped within the Chiari’s network and/or in right heart cavities, originating from embolization of a deep venous thrombosis (DVT) and commonly associated with PE. The type B is nonmobile, originates in situ and is associated with cardiac abnormalities. Type C has intermediate characteristics between type A and B [
8,
9].
RVT is generally asymptomatic before the occurrence of complications such as PE or paradoxical stroke [
10]. However, the mortality rate associated with RVT is high, ranging between 27% and 100% [
1,
2], especially if it is complicated by PE. Indeed, the prognosis of these patients is primarily related to the hemodynamic consequences of RVT rather than its characteristics.
Due to the severity of RVT occurrence and complications, immediate diagnosis and rapid prognostic risk stratification of RVT patients are mandatory.
Two-dimensional transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) are the first-line imaging modalities for detecting and monitoring RVT [
9]. It is noteworthy that both TTE and TEE have a higher sensitivity and specificity for detecting left ventricular thrombosis rather than RVT. The first assessment of RVT by TTE may be difficult in the emergency setting and in patients with tachy-arrhythmias, haemodynamic instability or poor acoustic windows [
11]. Additionally, approximately 50% of RV thrombi are identified by using off-axis echocardiographic sections [
9]. Therefore, RVT presence may be considerably underestimated in clinical practice.
Given that the echogenicity of thrombotic formation may be indistinguishable from that of surrounding myocardium, TTE may provide limited information concerning the differential diagnosis of intracardiac masses [
12]. In the setting of poor acoustic windows and/or suboptimal TTE imaging, contrast echocardiography may considerably improve the endocardial definition by enhancing the blood pool–myocardial interface, thus facilitating RVT detection and characterization [
13].
Due to its unique capability in providing an accurate and reproducible assessment of RV structure, function and tissue characterization, cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) has shown higher sensitivity and specificity than TTE and/or TEE for detecting RVT [
14,
15].
Finally, contrast-enhanced chest computed tomography (CT) represents another useful technique for evaluating RVT, due to its rapid acquisition and high spatial and temporal resolution [
16,
17,
18].
Herein, we present a challenging case of type C RVT, originated in situ and complicated by subsegmental PE, diagnosed by a multi-modality imaging approach in a 46-year-old man with acute-on-chronic respiratory failure secondary to acute exacerbation of interstitial lung disease.
2. Clinical Course
A 46-year-old man (BSA 1.92 m2, BMI 24.5 Kg/m2), without previous cardiovascular events, affected by anti-Mi2 dermatomyositis and lymphocytic interstitial pneumonia (LIP) with chronic respiratory failure, treated with azathioprine 50 mg daily, prednisone 25 mg daily, intravenous (IV) rituximab 700 mg once weekly and oxygen therapy (1 to 6 liter per minute), was admitted to the Emergency Department (ED) of our Institution due to ongoing dyspnea, chest pain and general malaise. At the hospital admission, arterial oxygen saturation (SaO2) in ambient air was 66%, blood pressure was 110/70 mmHg, heart rate was 102 b.p.m., body temperature was 36.3°C. Blood gas analysis showed hypoxemia (PaO2 = 39.6 mmHg) and hypocapnia (PaCO2 = 27.2 mmHg), pH = 7.3, lactates 7.7 mmol/L (normal range 0.36-1.25 mmol/L). Blood tests revealed serum hemoglobin 12.8 g/dl, serum white blood cells of 13900 x10^6/L (normal range 4000-11000 x10^6/L), serum Neutrophil-Lymphocyte Ratio (NLR) of 18.6, serum creatinine of 1.57 mg/dl, serum troponin I of 0.42 ng/ml (normal range 0.00-0.04 ng/ml), serum C-reactive protein (CRP) of 58 mg/L (normal range 0-5 mg/L), serum D-dimer of 4215 microg/L (normal range 1-500 microg/L) and serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) of 6474 pg/ml (normal range <125 pg/ml).
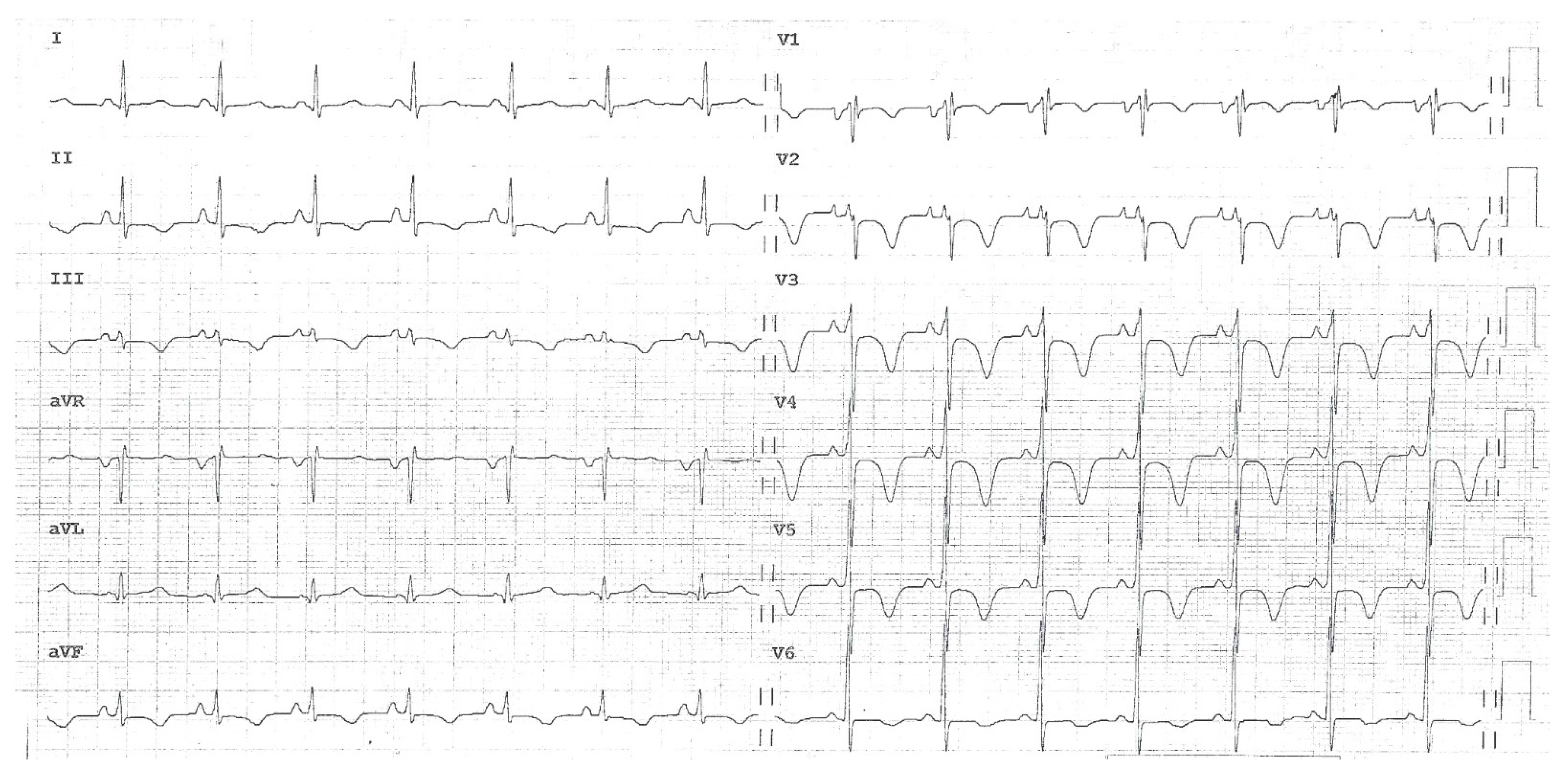
The ECG recorded in the ED showed sinus rhythm with normal atrio-ventricular conduction, mild RV conduction delay and deep T-wave inversion in right precordial leads and inferior leads, suggesting RV overload (
Figure 1).
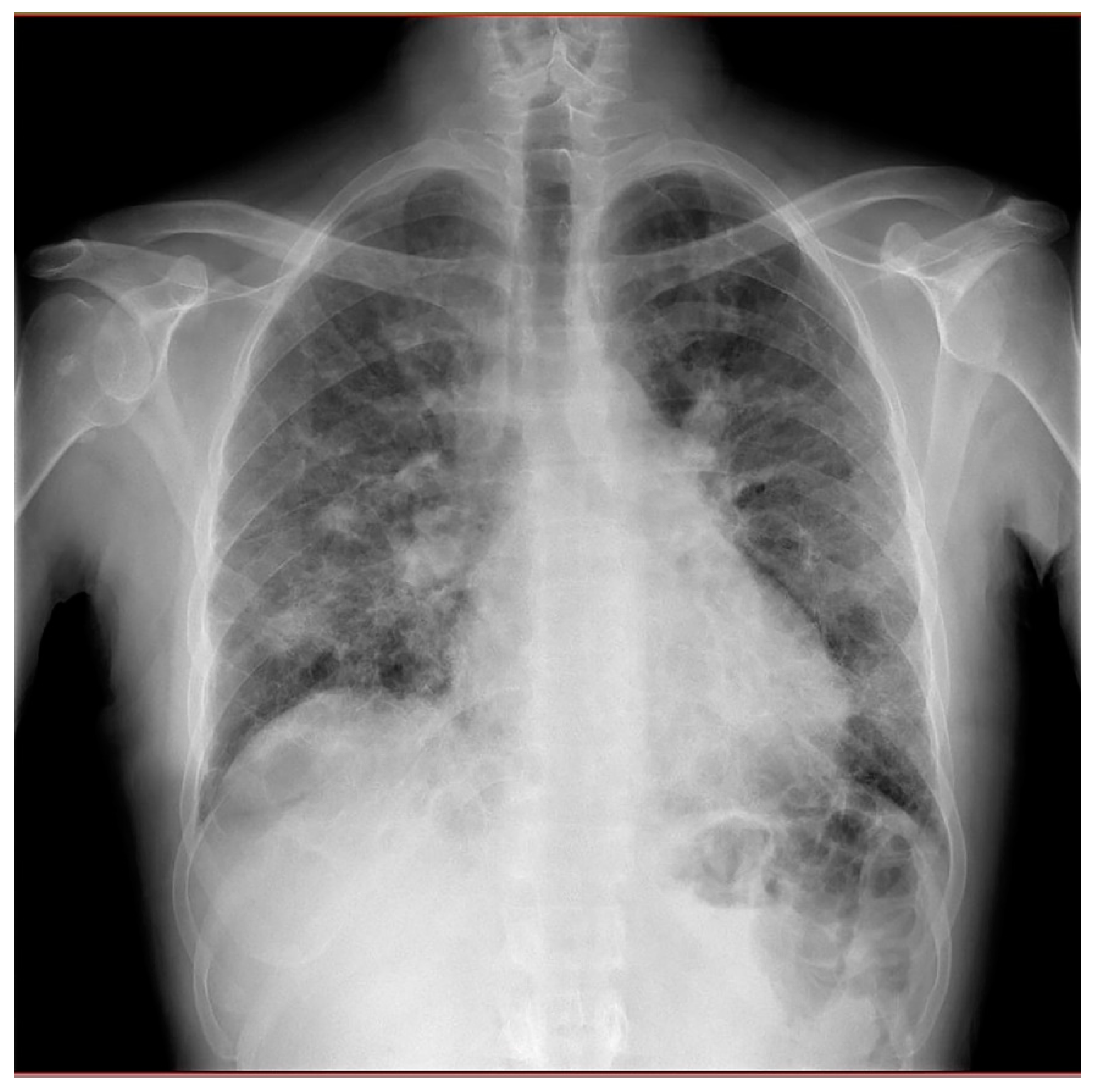
Chest X-rays showed diffuse fibrosing interstitial lung disease with multiple, bilateral parenchymal opacities, with no clear evidence of inflammatory foci (
Figure 2).
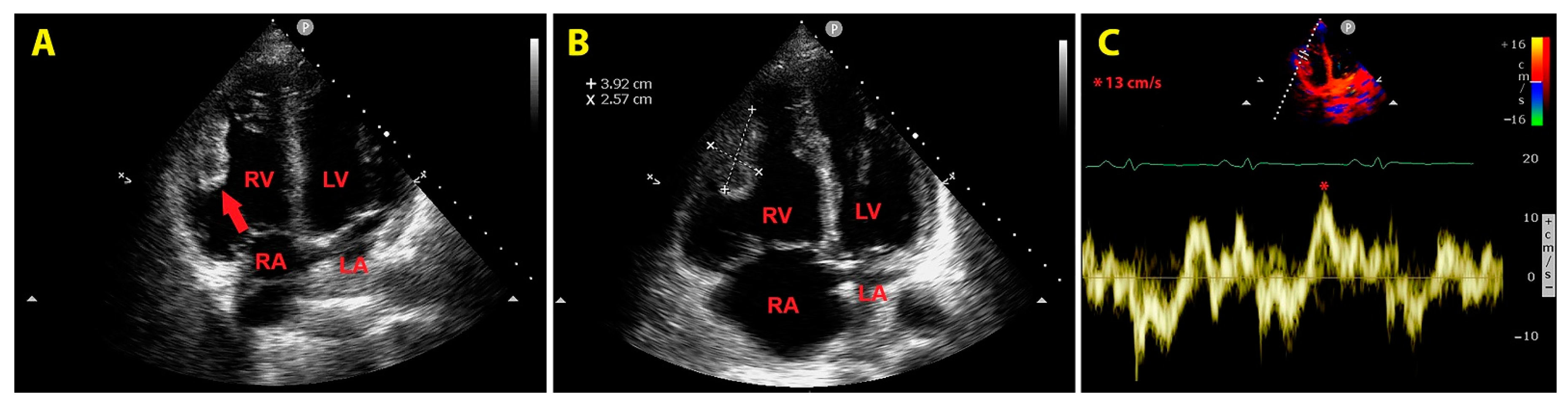
An urgent bedside echocardiogram highlighted a significant dilatation of right-sided cardiac chambers (RV to left ventricular basal diameter ratio = 2.4; RV inflow tract diameter = 60 mm), RV hypertrophy (RV free wall thickness = 10 mm) and mild hypokinesia of the RV lateral wall, as assessed by tricuspid annular plane systolic excursion (TAPSE) magnitude (17 mm); a moderate tricuspid regurgitation was present. The peak tricuspid regurgitation velocity (TRV) was 3.4 m/s, indicating high probability of pulmonary hypertension (PH); the inferior vena cava was significantly dilated (transverse diameter = 2.8 cm), with inspiratory excursions <50%; accordingly, the estimated systolic pulmonary artery pressure (sPAP) was 60 mmHg. On RV focused apical four-chamber view, a large sessile echogenic formation with hyperechoic edges (size 3.9 cm x 2.6 cm), attached to the mid-apical portion of the RV free wall, protruding into the RV cavity, was detected (
Figure 3, A and B). By placing a 5 mm sample volume of pulsed wave (PW)-tissue Doppler imaging (TDI) at the level of the mobile portion of RV mass, a RV mass peak antegrade velocity (Va) of 13 cm/s was obtained; moreover, on PW-TDI, the RV mass showed a pattern of incoherent motion, totally discordant and independent from the surrounding myocardial tissue (
Figure 3C).
Careful observation allowed to detect akinesia of the RV mid-apical wall. Therefore, McConnell's sign was excluded. Compared to right-sided cardiac chambers, the left-sided cavity chambers size was reduced; the left ventricle showed a D-shaped left ventricular configuration due to the flattening of the interventricular septum caused by the significant RV overload. Left ventricular ejection fraction (assessed by the modified Simpson’s biplane method) was preserved (estimated value = 60%). A first degree of diastolic dysfunction (E/A ratio <1 on transmitral PW-Doppler) was diagnosed, in the presence of normal left ventricular filling pressure, as measured by the E/average e’ ratio (estimated value = 5.2). The mitral and aortic valves were normal, whereas a mid-systolic notch of the RV outflow tract PW-Doppler envelope, compatible with PH, was detected. The urgent TTE was compared with a previous one, performed electively three months earlier, that showed normal biventricular cavity sizes, normal biventricular systolic function and normal sPAP (estimated value 27 mmHg). Accordingly, the actual TTE findings were considered to be suggestive of acute RV overload due to PH, complicated by RV mass of nonunivocal interpretation.
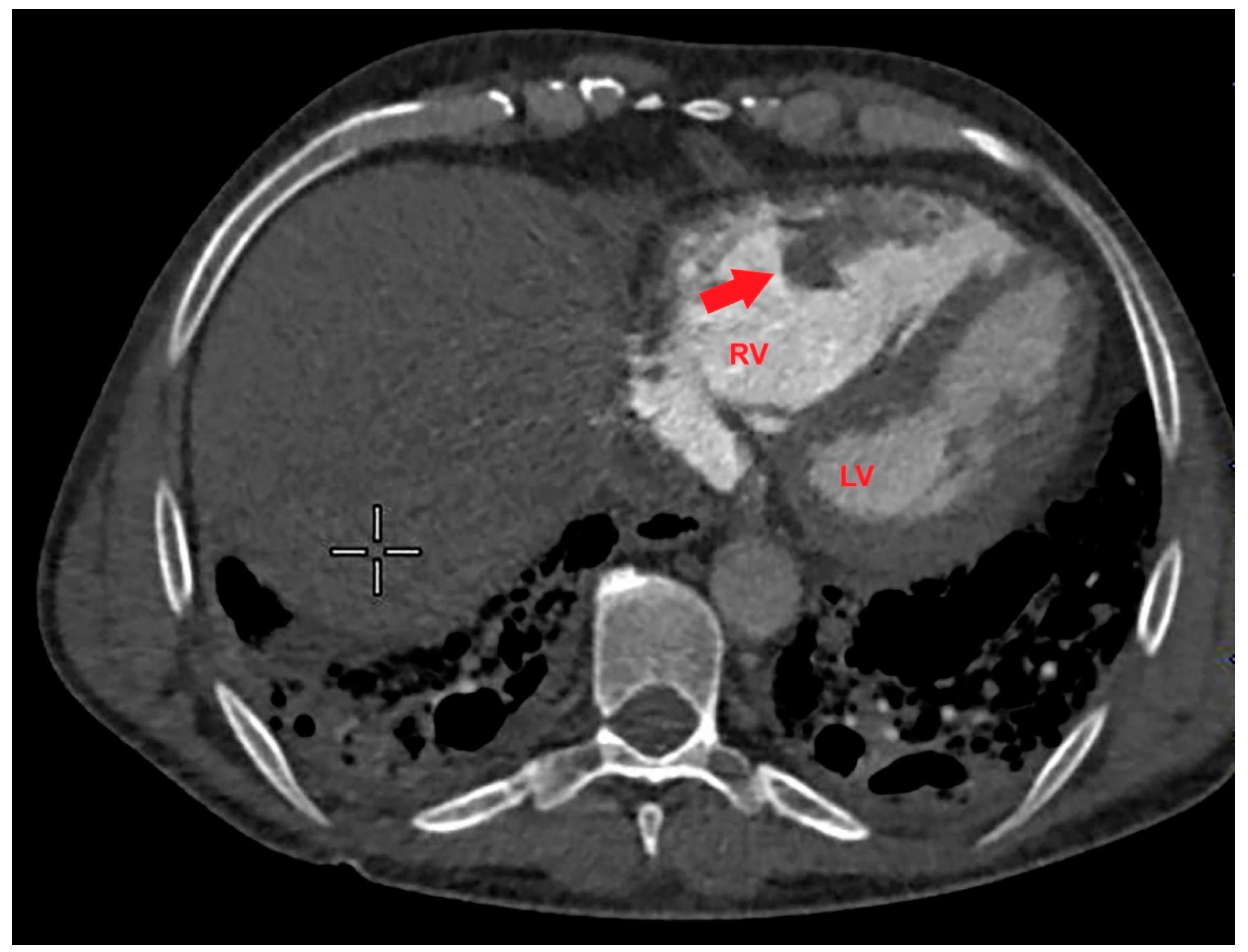
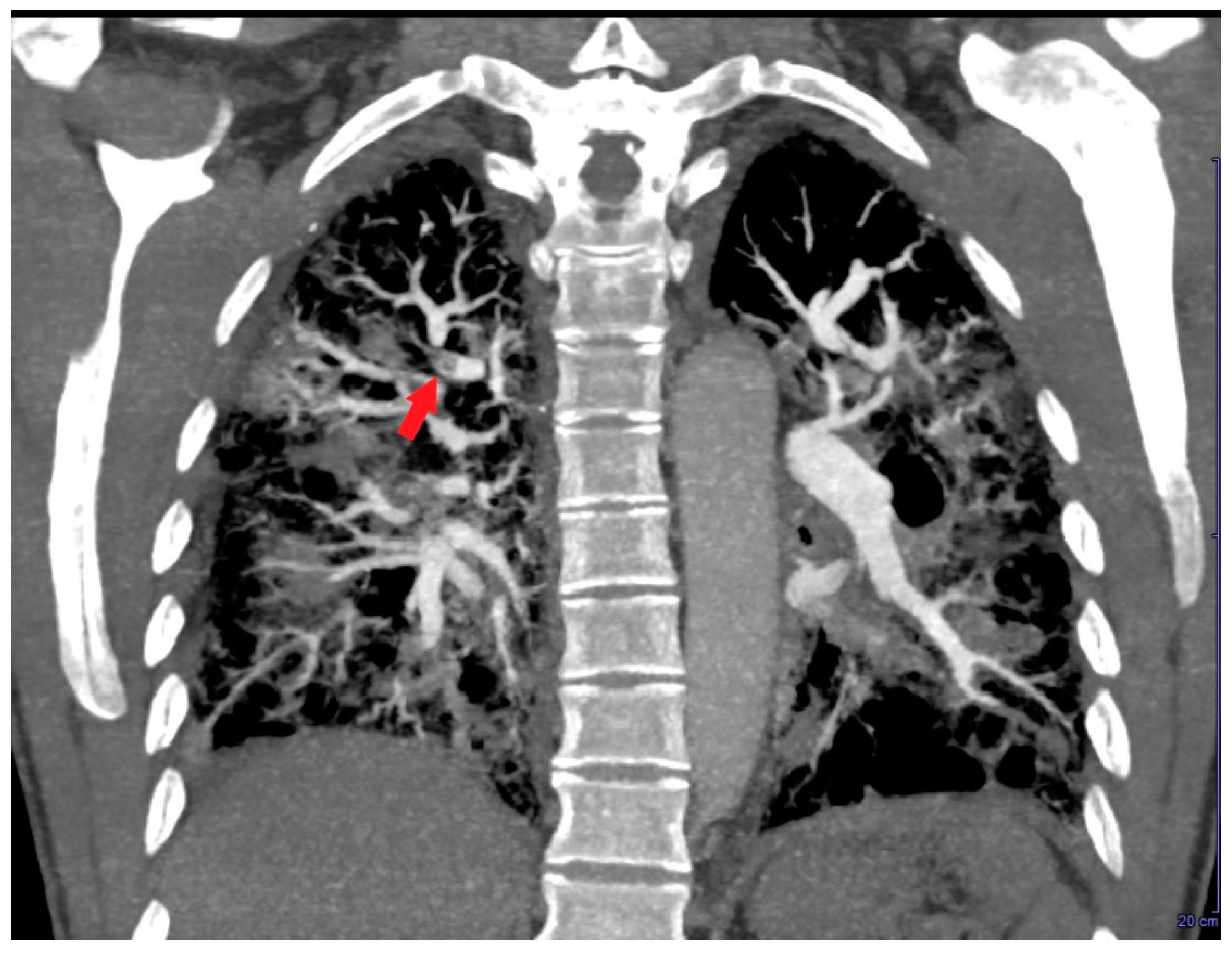
Based on the ECG and echocardiographic findings and the complex patient’s history, the pulmonologist suggested a diagnostic study with contrast-enhanced chest CT. The examination confirmed severe RV dilatation with a large filling defect involving the mid-apical region of the right ventricle (
Figure 4).
CT scan also confirmed severe interstitial pulmonary fibrosis with diffuse ground glass opacities and thin-walled cysts and excluded PE.
A venous Doppler ultrasound of the lower extremities excluded concomitant DVT.
The patient was admitted to the Intensive Care Unit (ICU) and underwent high-flow oxygen therapy (reservoir mask with 10-15 L/min) and medical treatment with anticoagulants (enoxaparin sodium 6000 I.U. twice daily by subcutaneous injection), IV antibiotics (Piperacillin/Tazobactam 4 g/0.5 g three times daily), IV corticosteroids (methylprednisolone 40 mg three times daily), IV antibiotics (ceftriaxone 2 g daily) and IV diuretics (furosemide 40 mg daily).
Even if the patient did not have fever during hospitalization, aerobic and anaerobic blood cultures were performed to exclude the infectious origin of the RV mass. However, blood cultures yielded negative results.
A repeated TTE during the stay in the ICU demonstrated a slight reduction in RV mass size (3 cm x 2 cm), that changed its echogenicity, being characterized by an anaechoic central space and a hyperechoic border (
Figure 5A). The same echocardiographic findings of the RV mass were confirmed from a RV focused mid-esophageal section obtained during TEE (
Figure 5B).
A further contrast-enhanced chest CT scan revealed segmental and subsegmental filling defects in the right upper lobe (
Figure 6) and a concomitant mild reduction in RV mid-apical filling defect.
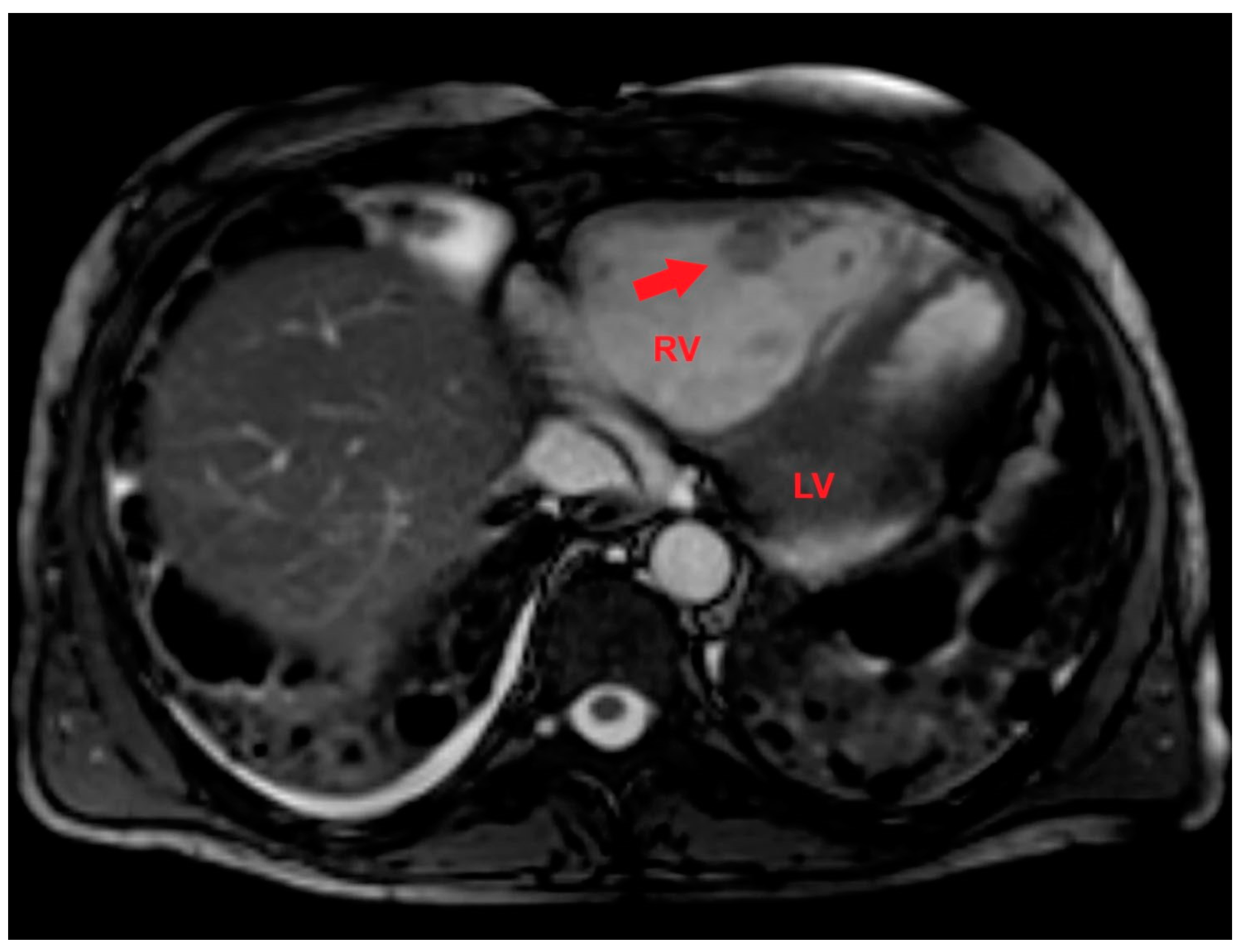
After multidisciplinary discussion, a late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) was performed. LGE-CMR allowed to detect a RV mass with a central area of colliquative necrosis and inhomogenous peripheral enhancement (
Figure 7).
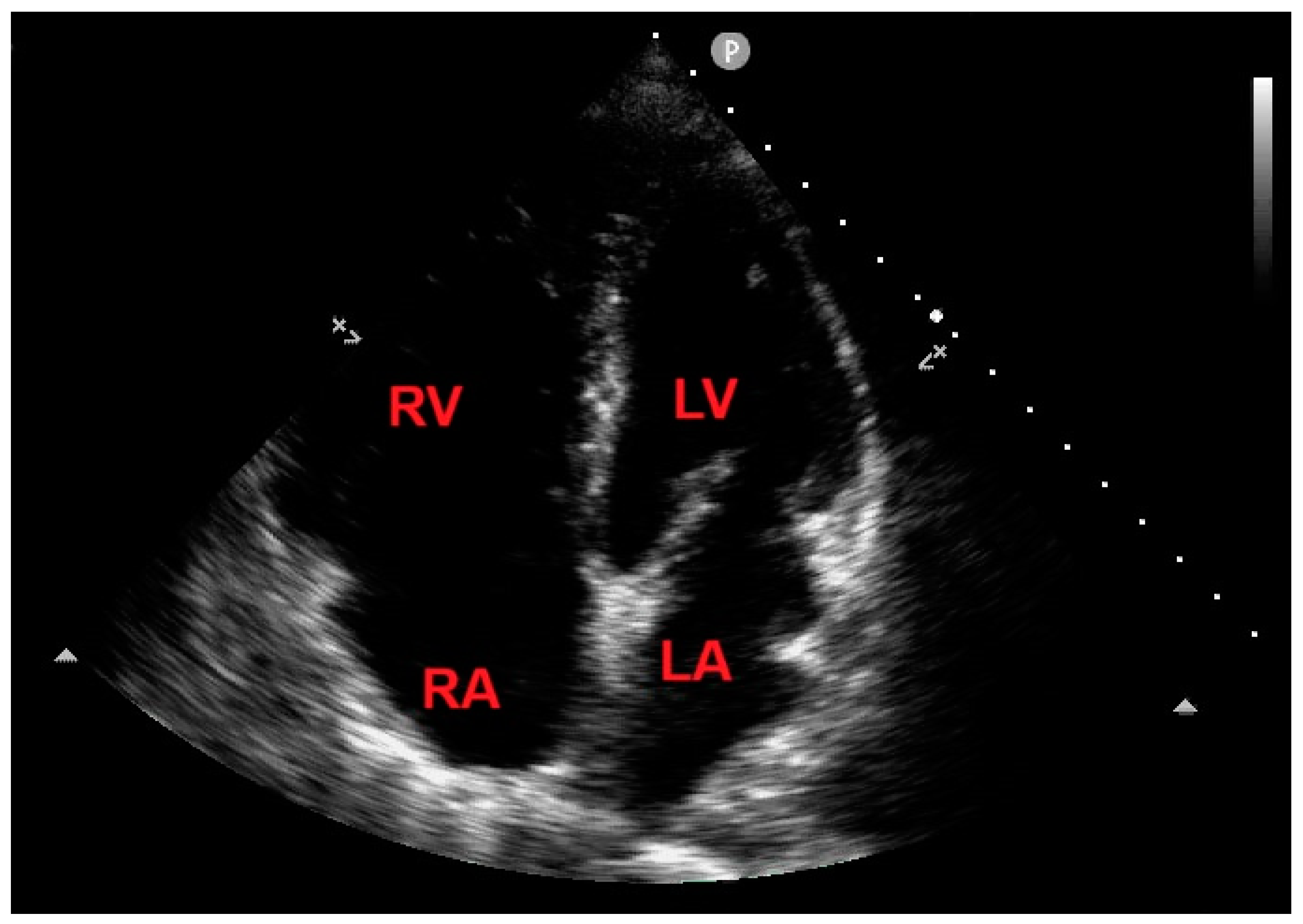
After seven days of ICU monitoring, the patient’s clinical conditions gradually improved and he was transferred to the Division of Pneumology. On the 10
th day of hospitalization, an echocardiographic control showed the total disappearance of the RV mass (
Figure 8) and concomitant normalization of TRV (estimated value 2.5 m/s).
In light of the favourable echocardiographic evolution, the RV mass was more appropriately considered as a thrombotic formation, with peripheral organization and liquefactive central necrosis, originating in situ, at the level of a significantly dilated and dysfunctional right ventricle, causing subsegmental PE and completely resolved after anti-coagulant treatment.
Repeated blood tests demonstrated the normalization of serum levels of CRP (2.8 mg/L) and troponin I (0.02 ng/ml) and significant reduction of serum levels of both D-dimer (430 microg/L) and NT-proBNP (69 pg/ml). On repeated blood gas analysis in ambient air, SaO2 was 91.4%, PaO2 was 59 mmHg, PaCO2 was 43 mmHg, pH was 7.41 and lactates were 1.01 mmol/L. During the hospital stay, the patient underwent also a thrombophilia screening that resulted negative. The hematology consultant recommended anticoagulant therapy at discharge with apixaban 5 mg twice daily lifelong.
On the 14th day of hospitalization, the patient was discharged with the diagnosis of acute-on-chronic respiratory failure secondary to acute exacerbation of interstitial lung disease, leading to acute RV overload with akinesia of the RV mid-apical segments, complicated by RV thrombosis in situ, causing subsegmental PE. The suggested discharge medical treatment included apixaban 5 mg twice daily, oxygen therapy via nasal cannula 1 L/min at rest and 6 L/min on effort, azathioprine 50 mg daily and prednisone 25 mg daily.
3. Discussion
This challenging clinical case highlights the complexity of the differential diagnosis of RV masses, involving tumors, vegetations and thrombi.
3.1. Right Ventricular Tumors
RV neoplastic lesions may be benign or malignant. The most common benign cardiac tumors are myxomas, but those originating from the RV free wall are extremely rare, representing only 5% of cases [
19,
20]. RV myxomas may have obstructive features, potentially leading to right heart failure with systemic congestion or causing arrhythmias, syncope and even sudden death [
21]. Depending on their mobility, RV myxomas may be complicated by PE [
22,
23]. Rhabdomyomas represent another type of benign RV neoplastic lesion, predominantly detected in children and commonly associated with tuberous sclerosis complex; they are multiple lesions, that generally regress spontaneously [
24].
Primary malignant cardiac tumors are generally right atrial masses, rapidly infiltrating valves or chamber walls and destroying the primary structure of the hearts [
25]. Other characteristics of malignant tumors are the wide point of attachment, the size >5 cm, the pericardial effusion and the extracardiac extension [
26]. Among the malignant cardiac tumors, the most frequent ones are cardiac sarcomas [
27], particularly angiosarcomas, representing from 30% to 45% of sarcomas [
28]. Lymphomas are rare, accounting for 1% to 2% of primary malignant cardiac tumors, involving the right atrium or the right ventricle. Lymphomas are more frequently non-Hodgkin type, associated with immunodeficiency syndromes [
26].
Distinctive features of primary malignant cardiac tumors assessed by CMR are heterogeneous enhancement, tumor necrosis and multiple foci of calcification [
29]. Compared with benign tumors, malignant masses have generally non-left localization, have a greater diameter, are sessile, polylobate, have inhomogeneous appearance, are infiltrating and are accompanied by pericardial effusion. Differently from the avascular nature of thrombi, cardiac tumors have a vascular supply. At LGE, malignant masses are more frequently hyperintense compared with the benign ones [
30].
Due to its high spatial and temporal resolution, fast acquisition times, and multiplanar image reconstruction capabilities, cardiac CT with ECG gating represents a valid alternative to CMR in many patients, particularly in those with contraindications to CMR. CT may precisely assess the lesion margins, may define the cardiovascular extent of the mass and exclude concomitant obstructive coronary artery disease [
31]. Additionally, when combined with 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET), cardiac CT is also useful to detect primary malignant cardiac tumors and metastates, that generally show significantly higher glucose uptake than primary benign cardiac tumors [
32].
3.2. Right Ventricular Endocarditis
Right-sided infective endocarditis (IE), accounting for 5% to 10% of all IE cases, are generally associated with intravenous drug use, intracardiac devices and central venous catheters, involving primarily the tricuspid valve and rarely the pulmonary valve [
33,
34]. On the other hand, isolated RV mural endocarditis is very rare and generally arise from the RV moderator band, where trabeculations could act as a facilitating location for infection [
35,
36,
37]. RV mural endocarditis may be suspected in patients with prolonged fever and RV endocardial masses, particularly attached to the RV moderator band, even when blood cultures are persistently negative. Cases of RV mural endocarditis involving the RV free wall have been reported in individuals who did regular use of intravenous drugs. These cases have been ascribed to the coarse trabeculae of the RV acting as a nidus for infection, similarly to the RV moderator band [
38,
39].
The differential diagnosis between right-sided vegetations and thrombi may be difficult, because both are generally masses without gadolinium contrast enhancement [
40]. However, a number of CMR findings may be suggestive of IE, particularly delayed enhancement involving the cardiovascular structures indicating endothelial inflammation, irreversible myocardial damage or fibrosis [
41,
42], LGE of the endothelial lining or perivalvular abscess [
43]. Multislice CT may contribute to the diagnosis of right-sided IE, by providing high-resolution anatomical assessment of vegetations, valvular and peri-valvular lesions and also extra-cardiac lesions [
44,
45].
3.3. Right Ventricular Thrombosis
Given its higher sensitivity and specificity over echocardiographic techniques, CMR may facilitate the differential diagnosis between intracardiac thrombi and tumors, as demonstrated in various case reports and case series [
11,
12,
46]. On CMR, RVT is generally visualized as a homogeneously dark mass with no contrast uptake, characteristics consistent with a homogeneous, avascular mass [
47]. However, peripheral enhancement may be occasionally observed in chronic organic thrombotic formations due to fibrotic components [
48].
Another imaging technique commonly employed for the diagnostic study of RV masses is the chest CT scan, where RVT generally appears as a hypodense mass within enhanced RV cavity [
49].
In the present case, a multidisciplinary evaluation and a dynamic multimodality imaging approach allowed the correct differential diagnosis between RVT and both RV tumors or vegetation. Notably, a RV neoplastic lesion was excluded due to the absence of pericardial effusion and extracardiac extension, and the rapid response to the anticoagulant treatment. Moreover, the infectious or inflammatory origin of the RV mass was not considered as a plausible hypothesis, for the absence of a septic syndrome, for the negativity of blood cultures and for the RV mass location, without any relation with the RV coarse trabeculae and/or the moderator band, generally considered as possible sites for infection.
In our findings, the serial echocardiographic monitoring performed during the hospitalization appeared to be more effective than contrast-enhanced chest CT and CMR for clarifying the real nature of the RV mass. During the acute phase, the bedside TTE arised the suspicion of severe PH complicated by mid-apical RV akinesia and superimposed RV mass. The implementation of conventional TTE with PW-TDI provided a precise measurement of the RV mass mobility and embolic potential. In the sub-acute phase, TTE showed a slight reduction of RV mass size and identified a central anechoic area, confirmed by TEE examination. The subsequent contrast-enhanced chest CT made diagnosis of segmental and subsegmental PE, indicating that the reduction of RV mass size and central echogenicity was likely related to its fragmentation with consequent pulmonary embolization. The increased RV mass peak Va, assessed by PW-TDI in the acute phase, early predicted its subsequent embolization. Finally, after 10 days of hospitalization, TTE demonstrated the total disappearance of the RV mass, thus confirming its thrombotic nature.
In the present case, the thrombotic nature of the RV mass might be suspected by the echocardiographic evidence of the underlying RV mid-apical free wall akinesia (similarly to what observed for the left ventricular apical aneurysms complicated by thrombosis) and by its gradual regression after initiation of anticoagulant treatment. In light of our findings, the RVT we detected was a type C RVT, with intermediate characteristics between type B (originating in situ and associated with significant RV dilatation and dysfunction) and type A (highly mobile and usually complicated by PE).
For improving the RV mass detection, we used color PW-TDI rather than contrast echocardiography. As previously demonstrated [
50], color-TDI may improve the visualization of intracardiac masses, characterized by a pattern of motion totally different from that of surrounding myocardial structures. Indeed, these intracardiac masses are codified with different colours compared to the adjacent myocardium. Additionally, as recently demonstrated by our study group [
51], PW-TDI sampling of the free mobile portion of intracardiac pathological masses allows to identify their typical pattern of incoherent motion (totally asynchronous with respect to the cardiac walls) and to precisely measure the mass peak Va. The higher is the mass peak Va, the higher appears the risk of embolic complications [
52]. In the present case, the patient was diagnosed with a peak mass Va >10 cm/s, thus confirming that this simple PW-TDI-derived parameter may represent an innovative important predictor of the embolic risk associated with mobile intracardiac masses. A potential implication for clinical practice is that the echocardiographic detection of a RV mass peak Va >10 cm/sec, as assessed by PW-TDI, may strengthen the indication for prompt anticoagulant treatment.
4. Conclusions
The differential diagnosis of RV masses is complex, involving thrombi, tumors and vegetations.
RVT should always be suspected in young patients with dilated and dysfunctional right ventricles and with systemic disorders associated with hypercoagulability.
A dynamic multi-modality imaging approach, comprensive of color- and PW-TDI, may improve the visualization of RV masses, allowing the clinicians to identify, among the RV masses, those with increased embolic potential.
Author Contributions
Conceptualization, A.S., A.L., F.L. and A.C.; methodology, A.S., G.A.R. and M.Z.; software, A.S.; validation, G.L.N., M.Z., M.L., S.H..; formal analysis, A.S.; investigation, A.S., G.A.R. and M.Z.; resources, S.H.; data curation, A.S., G.A.R. and M.Z.; writing—original draft preparation, A.S.; writing—review and editing, G.L.N. and S.H.; visualization, G.L.N., M.Z., M.L. and S.H.; supervision, M.L. and S.H.; project administration, S.H.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Ricerca Corrente IRCCS MultiMedica.
Institutional Review Board Statement
In accordance with the guidelines by the Comitato Etico. Territoriale Lombardia 5, ethical review and approval were not required for this case report.
Informed Consent Statement
Informed consent was obtained from the individual included in the present case report.
Data Availability Statement
Data extracted from the present case report will be publicly available on Zenodo (
https://zenodo.org).
Acknowledgments
The authors wish to thank Monica Fumagalli for her graphical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chartier, L.; Béra, J.; Delomez, M.; Asseman, P.; Beregi, J.P.; Bauchart, J.J.; Warembourg, H.; Théry, C. Free-floating thrombi in the right heart: Diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999, 99, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Barrios, D.; Rosa-Salazar, V.; Jiménez, D.; Morillo, R.; Muriel, A.; Del Toro, J.; López-Jiménez, L.; Farge-Bancel, D.; Yusen, R.; Monreal, M.; et al. Right heart thrombi in pulmonary embolism. Eur. Respir. J. 2016, 48, 1377–1385. [Google Scholar] [CrossRef]
- Nkoke, C.; Faucher, O.; Camus, L.; Flork, L. Free Floating Right Heart Thrombus Associated with Acute Pulmonary Embolism: An Unsettled Therapeutic Difficulty. Case Rep. Cardiol. 2015, 2015, 364780. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Benhamou, M.; Berthier, F.; Baudouy, M. Mobile thrombi of the right heart in pulmonary embolism: Delayed disappearance after thrombolytic treatment. Chest 2005, 127, 1051–1053. [Google Scholar] [CrossRef]
- Dinesh Kumar, U.S.; Nareppa, U.; Shetty, S.P.; Wali, M. Right ventricular thrombus in case of atrial septal defect with massive pulmonary embolism: A diagnostic dilemma. Ann. Card. Anaesth. 2016, 19, 173–176. [Google Scholar] [CrossRef]
- Varki, A. Trousseau's syndrome: Multiple definitions and multiple mechanisms. Blood 2007, 110, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Iga, K.; Konishi, T.; Kusukawa, R. Intracardiac thrombi in both the right atrium and right ventricle after acute inferior-wall myocardial infarction. Int. J. Cardiol. 1994, 46, 169–171. [Google Scholar] [CrossRef]
- Charif, F.; Mansour, M.J.; Hamdan, R.; Najjar, C.; Nassar, P.; Issa, M.; Chammas, E.; Saab, M. Free-Floating Right Heart Thrombus with Acute Massive Pulmonary Embolism: A Case Report and Review of the Literature. J. Cardiovasc. Echogr. 2018, 28, 146–149. [Google Scholar] [CrossRef]
- Roy, R.; Guile, B.; Sun, D.; Szasz, T.; Singulane, C.C.; Nguyen, D.; Abutaleb, A.; Lang, R.M.; Addetia, K. Right Ventricular Thrombus on Echocardiography. Am. J. Cardiol. 2024, 211, 64–68. [Google Scholar] [CrossRef]
- Goh, F.Q.; Leow, A.S.; Ho, J.S.; Ho, A.F.; Tan, B.Y.; Yeo, L.L.; Li, T.Y.; Galupo, M.J.; Chan, M.Y.; Yeo, T.C.; et al. Clinical characteristics, treatment and long-term outcomes of patients with right-sided cardiac thrombus. Hellenic J. Cardiol. 2022, 68, 1–8. [Google Scholar] [CrossRef]
- Tsang, B.K.; Platts, D.G.; Javorsky, G.; Brown, M.R. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ. 2012, 21, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Naef, D.; Köpfli, P.; Hufschmid, U.; Niemann, T.; Gebker, R.; Beer, J.H.; Hireche-Chiakoui, H. Right ventricular thrombus, a challenge in imaging diagnostics: A case series. Eur. Heart J. Case Rep. 2021, 5, ytab340. [Google Scholar] [CrossRef]
- Kurt, M.; Shaikh, K.A.; Peterson, L.; Kurrelmeyer, K.M.; Shah, G.; Nagueh, S.F.; Fromm, R.; Quinones, M.A.; Zoghbi, W.A. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J. Am. Coll. Cardiol. 2009, 53, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Marcu, C.B.; Beek, A.M.; Van Rossum, A.C. Cardiovascular magnetic resonance imaging for the assessment of right heart involvement in cardiac and pulmonary disease. Heart Lung Circ. 2006, 15, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Galea, N.; Carbone, I.; Cannata, D.; Cannavale, G.; Conti, B.; Galea, R.; Frustaci, A.; Catalano, C.; Francone, M. Right ventricular cardiovascular magnetic resonance imaging: Normal anatomy and spectrum of pathological findings. Insights Imaging 2013, 4, 213–223. [Google Scholar] [CrossRef]
- Syed, I.S.; Motiei, A.; Connolly, H.M.; Dearani, J.A. Pulmonary embolism, right ventricular strain, and intracardiac thrombus-in-transit: Evaluation using comprehensive cardiothoracic computed tomography. J. Cardiovasc. Comput. Tomogr. 2009, 3, 184–186. [Google Scholar] [CrossRef]
- Kusume, T.; Kubokawa, S.; Kaname, N.; Nakaoka, Y.; Kotani, T.; Imai, R.; Nishida, K.; Seki, S.; Kawai, K.; Hamashige, N.; et al. Right ventricular mobile thrombus in end-stage hypertrophic cardiomyopathy. J. Cardiol. Cases. 2017, 15, 173–175. [Google Scholar] [CrossRef]
- Artico, J.; Belgrano, M.; Bussani, R.; Sinagra, G. The curious case of a massive right heart thrombosis: A case report. Eur Heart J. Case Rep. 2021, 5, ytab156. [Google Scholar] [CrossRef]
- Hirota, J.; Akiyama, K.; Taniyasu, N.; Maisawa, K.; Kobayashi, Y.; Sakamoto, N.; Komatsu, N. Injury to the tricuspid valve and membranous atrioventricular septum caused by huge calcified right ventricular myxoma: Report of a case. Circ. J. 2004, 68, 799–801. [Google Scholar] [CrossRef]
- Karagöz, A.; Keskin, B.; Karaduman, A.; Tanyeri, S.; Adademir, T. Multidisciplinary Approach to Right Ventricular Myxoma. Braz. J. Cardiovasc. Surg. 2021, 36, 257–260. [Google Scholar] [CrossRef]
- Singh, V.; Singh, S.K.; Devenraj, V.; Kumar, S. Giant right ventricular myxoma obstructing both inflow and outflow tract. Indian J. Thorac. Cardiovasc. Surg. 2019, 35, 499–501. [Google Scholar] [CrossRef]
- Singhal, P.; Luk, A.; Rao, V.; Butany, J. Molecular basis of cardiac myxomas. Int. J. Mol. Sci. 2014, 15, 1315–1337. [Google Scholar] [CrossRef]
- Lu, C.; Yang, P.; Hu, J. Giant right ventricular myxoma presenting as right heart failure with systemic congestion: A rare case report. BMC Surg. 2021, 21, 64. [Google Scholar] [CrossRef]
- Sciacca, P.; Giacchi, V.; Mattia, C.; Greco, F.; Smilari, P.; Betta, P.; Distefano, G. Rhabdomyomas and tuberous sclerosis complex: Our experience in 33 cases. BMC Cardiovasc. Disord. 2014, 14, 66. [Google Scholar] [CrossRef]
- Mondal, S.; Jubar, J.; Kostibas, M.P. Near Total Occlusion of Right Ventricle by Cardiac Mass. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2085–2090. [Google Scholar] [CrossRef]
- Burazor, I.; Aviel-Ronen, S.; Imazio, M.; Markel, G.; Grossman, Y.; Yosepovich, A.; Adler, Y. Primary malignancies of the heart and pericardium. Clin. Cardiol. 2014, 37, 582–588. [Google Scholar] [CrossRef]
- Leja, M.J.; Shah, D.J.; Reardon, M.J. Primary cardiac tumors. Tex. Heart Inst. J. 2011, 38, 261–262. [Google Scholar]
- Llombart-Cussac, A.; Pivot, X.; Contesso, G.; Rhor-Alvarado, A.; Delord, J.P.; Spielmann, M.; Türsz, T.; Le Cesne, A. Adjuvant chemotherapy for primary cardiac sarcomas: The IGR experience. Br. J. Cancer. 1998, 78, 1624–1628. [Google Scholar] [CrossRef]
- Puppala, S.; Hoey, E.T.; Mankad, K.; Wood, A.M. Primary cardiac angiosarcoma arising from the interatrial septum: Magnetic resonance imaging appearances. Br. J. Radiol. 2010, 83, e230–e234. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Angeli, F.; Belmonte, M.; Foà, A.; Canton, L.; Fedele, D.; Armillotta, M.; Sansonetti, A.; Bodega, F.; et al. Cardiac Magnetic Resonance to Predict Cardiac Mass Malignancy: The CMR Mass Score. Circ. Cardiovasc. Imaging. 2024, 17, e016115. [Google Scholar] [CrossRef]
- Kassop, D.; Donovan, M.S.; Cheezum, M.K.; Nguyen, B.T.; Gambill, N.B.; Blankstein, R.; Villines, T.C. Cardiac Masses on Cardiac CT: A Review. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9281. [Google Scholar] [CrossRef]
- Rahbar, K.; Seifarth, H.; Schäfers, M.; Stegger, L.; Hoffmeier, A.; Spieker, T.; Tiemann, K.; Maintz, D.; Scheld, H.H.; Schober, O.; et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J. Nucl. Med. 2012, 53, 856–863. [Google Scholar] [CrossRef]
- Shmueli, H.; Thomas, F.; Flint, N.; Setia, G.; Janjic, A.; Siegel, R.J. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J. Am. Heart Assoc. 2020, 9, e017293. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Binda, G.; Rigamonti, E.; Vincenti, A.; Trevisan, R.; Nicolosi, G.L.; Zompatori, M.; Lombardo, M.; Anzà, C. A rare case of native pulmonary valve infective endocarditis complicated by septic pulmonary embolism. J. Cardiovasc. Med. (Hagerstown). 2019, 20, 152–155. [Google Scholar] [CrossRef]
- Vinod, G.V.; Kanjirakadavath, B.; Krishnan, M.N. Large mural vegetation from right ventricle, accompanying tricuspid valve endocarditis. Heart Asia 2013, 5, 82–83. [Google Scholar] [CrossRef]
- Koshy, A.G.; Kanjirakadavath, B.; Velayudhan, R.V.; Kunju, M.S.; Francis, P.K.; Haneefa, A.R.; Rajagopalan, R.V.; Krishnan, S. Images in cardiovascular medicine. Right ventricular mural bacterial endocarditis: Vegetations over moderator band. Circulation 2009, 119, 899–901. [Google Scholar] [CrossRef]
- Diaz-Navarro, R.A.; Kerkhof, P.L.M. Case report on right ventricular mural endocarditis, not diagnosed clinically, but histopathologically after cardiac surgery. Eur. Heart J. Case Rep. 2022, 6, ytac376. [Google Scholar] [CrossRef]
- Tomaszuk Kazberuk, A.; Sobkowicz, B.; Hirnle, T.; Lewczuk, A.; Sawicki, R.; Musiał, W. Giant right ventricular mural vegetation mimicking a cardiac tumour. Kardiol. Pol. 2011, 69, 587–589. [Google Scholar]
- Mandelkern, T.F.; Sultan, I.; Levenson, J.E. Righting on the Wall: A Case of Medically Managed Right Ventricular Mural Endocarditis. Circ. Cardiovasc. Imaging. 2023, 16, 591–593. [Google Scholar] [CrossRef]
- Sparrow, P.J.; Kurian, J.B.; Jones, T.R.; Sivananthan, M.U. MR imaging of cardiac tumors. Radiographics 2005, 25, 1255–1276. [Google Scholar] [CrossRef]
- Dursun, M.; Yılmaz, S.; Yılmaz, E.; Yılmaz, R.; Onur, İ.; Oflaz, H.; Dindar, A. The utility of cardiac MRI in diagnosis of infective endocarditis: Preliminary results. Diagn. Interv. Radiol. 2015, 21, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.; Yilmaz, S.; Ali Sayin, O.; Olgar, S.; Dursun, F.; Yekeler, E.; Tunaci, A. A rare cause of delayed contrast enhancement on cardiac magnetic resonance imaging: Infective endocarditis. J. Comput. Assist. Tomogr. 2005, 29, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Taylor, K.; Elkington, A.G.; Zeitz, C.J.; Beltrame, J.F. Images in cardiovascular medicine. Cardiac magnetic resonance imaging identifies the elusive perivalvular abscess. Circulation 2008, 118, e1–e3. [Google Scholar] [CrossRef]
- Thuny, F.; Grisoli, D.; Cautela, J.; Riberi, A.; Raoult, D.; Habib, G. Infective endocarditis: Prevention, diagnosis, and management. Can. J. Cardiol. 2014, 30, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Grob, A.; Thuny, F.; Villacampa, C.; Flavian, A.; Gaubert, J.Y.; Raoult, D.; Casalta, J.P.; Habib, G.; Moulin, G.; Jacquier, A. Cardiac multidetector computed tomography in infective endocarditis: A pictorial essay. Insights Imaging 2014, 5, 559–570. [Google Scholar] [CrossRef]
- Mitsis, A.; Alexi, A.; Constantinides, T.; Chatzantonis, G.; Avraamides, P. A Case of Right Ventricular Thrombus in a Patient With Recent COVID-19 Infection. Cureus 2022, 14, e25150. [Google Scholar] [CrossRef]
- Shenoy, C.; Grizzard, J.D.; Shah, D.J.; Kassi, M.; Reardon, M.J.; Zagurovskaya, M.; Kim, H.W.; Parker, M.A.; Kim, R.J. Cardiovascular magnetic resonance imaging in suspected cardiac tumour: A multicentre outcomes study. Eur. Heart J. 2021, 43, 71–80. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, J.; Xu, L.; Li, Y.; Liu, D.; Sun, Z.; Wen, Z. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant. Imaging Med. Surg. 2020, 10, 294–313. [Google Scholar] [CrossRef]
- Achuthanandan, S.; Harris, C.L.; Farooqui, A.A.; Hollander, G. Right Ventricular Thrombus Masquerading as a Tumor. Cureus 2022, 14, e26014. [Google Scholar] [CrossRef]
- Hiemetzberger, R.; Müller, S.; Bartel, T. Incremental use of tissue Doppler imaging and three-dimensional echocardiography for optimal assessment of intracardiac masses. Echocardiography 2008, 25, 446–447. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Prognostic Relevance of Left Ventricular Thrombus Motility: Assessment by Pulsed Wave Tissue Doppler Imaging. Angiology 2021, 72, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Muti-Schünemann, G.E.U.; Lombardo, M.; Muti, P. Could Pulsed Wave Tissue Doppler Imaging Solve the Diagnostic Dilemma of Right Atrial Masses and Pseudomasses? A Case Series and Literature Review. J. Clin. Med. 2024, 14, 86. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
12-lead electrocardiogram showing sinus rhythm with normal atrio-ventricular conduction, mild right ventricular conduction delay and deep T-wave inversion in right precordial leads and inferior leads, suggesting right ventricular overload.
Figure 1.
12-lead electrocardiogram showing sinus rhythm with normal atrio-ventricular conduction, mild right ventricular conduction delay and deep T-wave inversion in right precordial leads and inferior leads, suggesting right ventricular overload.
Figure 2.
Chest X-rays revealing diffuse fibrosing interstitial lung disease with multiple, bilateral parenchymal opacities, with no clear evidence of inflammatory foci.
Figure 2.
Chest X-rays revealing diffuse fibrosing interstitial lung disease with multiple, bilateral parenchymal opacities, with no clear evidence of inflammatory foci.
Figure 3.
Transthoracic echocardiography. Right ventricular focused apical four-chamber view. (A) Large sessile echogenic formation with hyperechoic edges (red arrow), attached to the mid-apical portion of the right ventricular free wall, protruding into the RV cavity. (B) Measurement of the right ventricular mass size. (C) Assessment of the right ventricular mass peak antegrade velocity by pulsed wave tissue Doppler imaging. The right ventricular mass showed a pattern of incoherent motion, totally discordant and independent from the surrounding myocardial tissue. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. The symbol * indicates the right ventricular mass peak antegrade velocity.
Figure 3.
Transthoracic echocardiography. Right ventricular focused apical four-chamber view. (A) Large sessile echogenic formation with hyperechoic edges (red arrow), attached to the mid-apical portion of the right ventricular free wall, protruding into the RV cavity. (B) Measurement of the right ventricular mass size. (C) Assessment of the right ventricular mass peak antegrade velocity by pulsed wave tissue Doppler imaging. The right ventricular mass showed a pattern of incoherent motion, totally discordant and independent from the surrounding myocardial tissue. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. The symbol * indicates the right ventricular mass peak antegrade velocity.
Figure 4.
Axial contrast-enhanced chest computed tomography showing a large filling defect (red arrow) involving the mid-apical region of the right ventricle. LV, left ventricle; RV, right ventricle.
Figure 4.
Axial contrast-enhanced chest computed tomography showing a large filling defect (red arrow) involving the mid-apical region of the right ventricle. LV, left ventricle; RV, right ventricle.
Figure 5.
(A) Transthoracic echocardiography. Right ventricular focused apical four-chamber view, demonstrating a slight reduction in right ventricular mass size (red arrow), characterized by anaechoic central space and hyperechoic border. (B) Right ventricular focused mid-esophageal section, showing the same echocardiographic features of the right ventricular mass (red arrow) observed from the transthoracic approach. Ao, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 5.
(A) Transthoracic echocardiography. Right ventricular focused apical four-chamber view, demonstrating a slight reduction in right ventricular mass size (red arrow), characterized by anaechoic central space and hyperechoic border. (B) Right ventricular focused mid-esophageal section, showing the same echocardiographic features of the right ventricular mass (red arrow) observed from the transthoracic approach. Ao, aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 6.
Coronal contrast-enhanced chest computed tomography revealing segmental and subsegmental filling defects (red arrow) in the right upper lobe.
Figure 6.
Coronal contrast-enhanced chest computed tomography revealing segmental and subsegmental filling defects (red arrow) in the right upper lobe.
Figure 7.
Axial late gadolinium enhancement cardiac magnetic resonance showing a right ventricular mass (red arrow) with a central area of colliquative necrosis and inhomogenous peripheral enhancement. LV, left ventricle; RV, right ventricle.
Figure 7.
Axial late gadolinium enhancement cardiac magnetic resonance showing a right ventricular mass (red arrow) with a central area of colliquative necrosis and inhomogenous peripheral enhancement. LV, left ventricle; RV, right ventricle.
Figure 8.
Transthoracic echocardiography. Apical four chamber view, demonstrating the total disappearance of the right ventricular mass. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 8.
Transthoracic echocardiography. Apical four chamber view, demonstrating the total disappearance of the right ventricular mass. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).