Submitted:
16 February 2025
Posted:
17 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
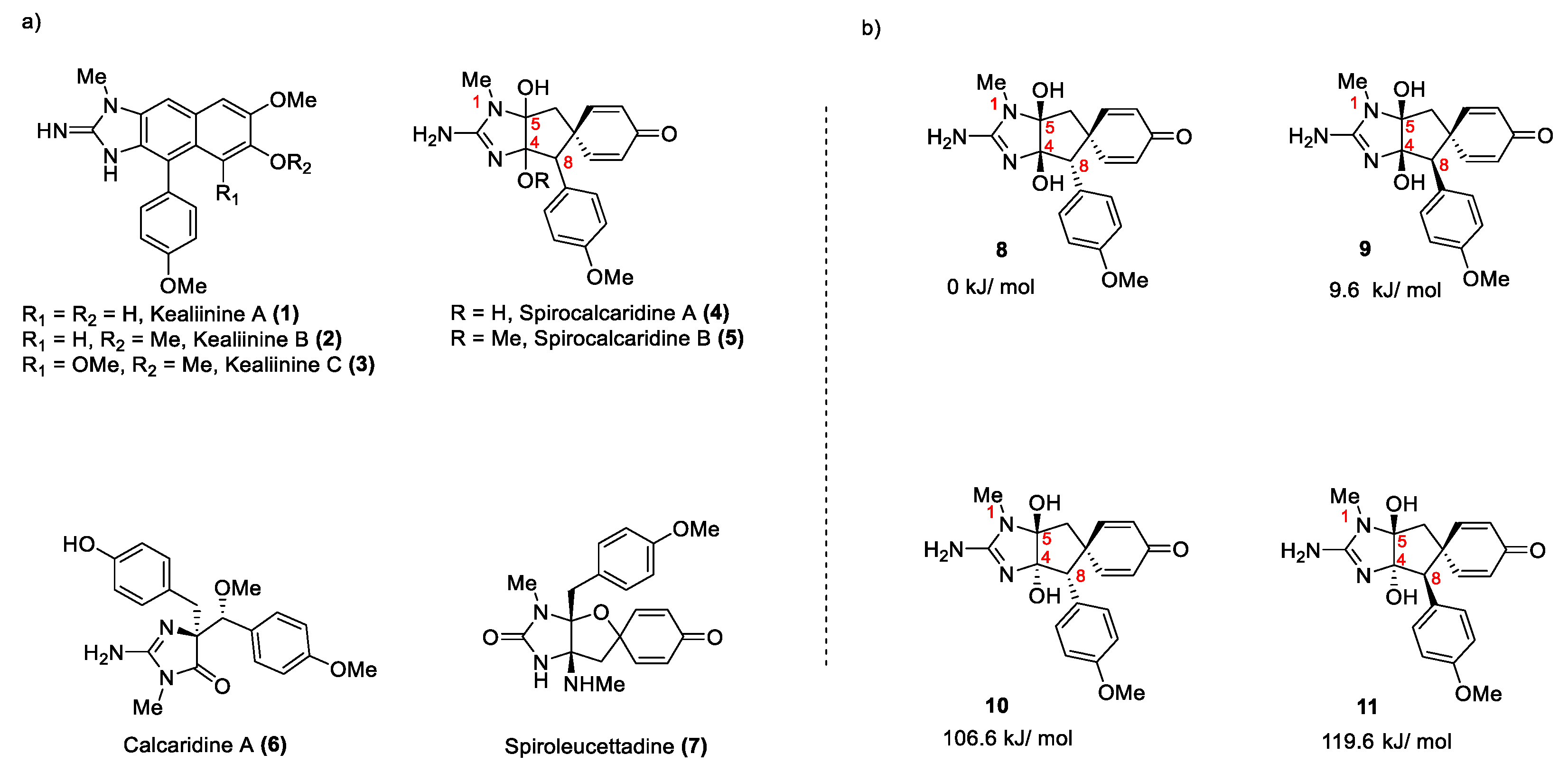
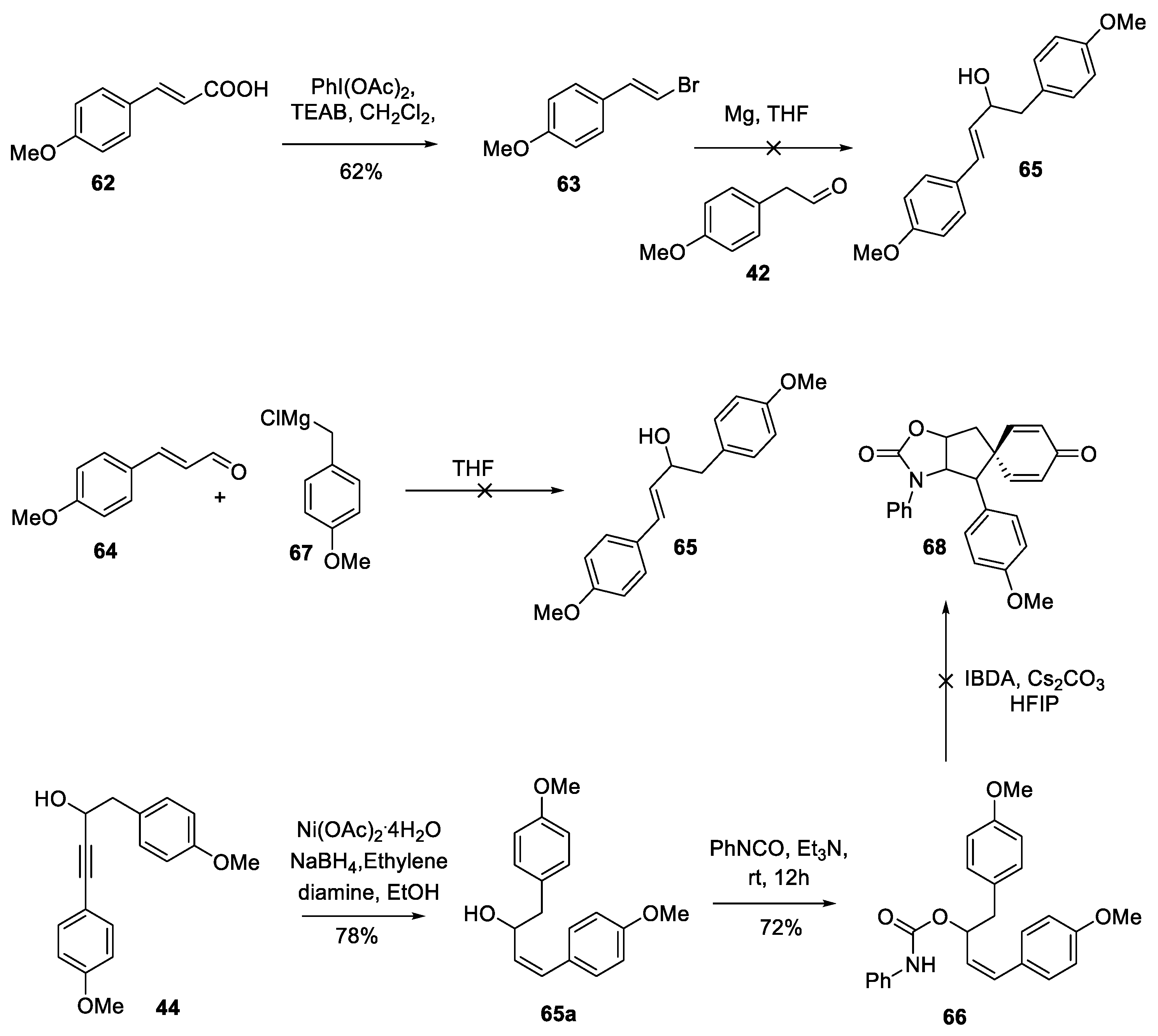
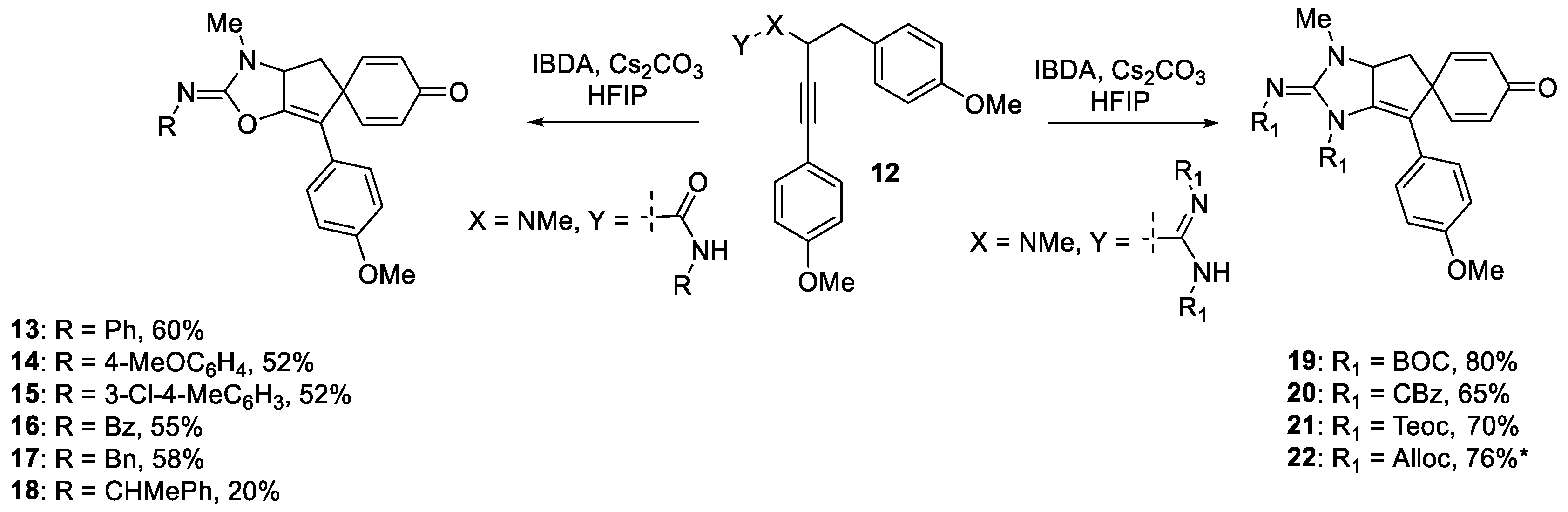
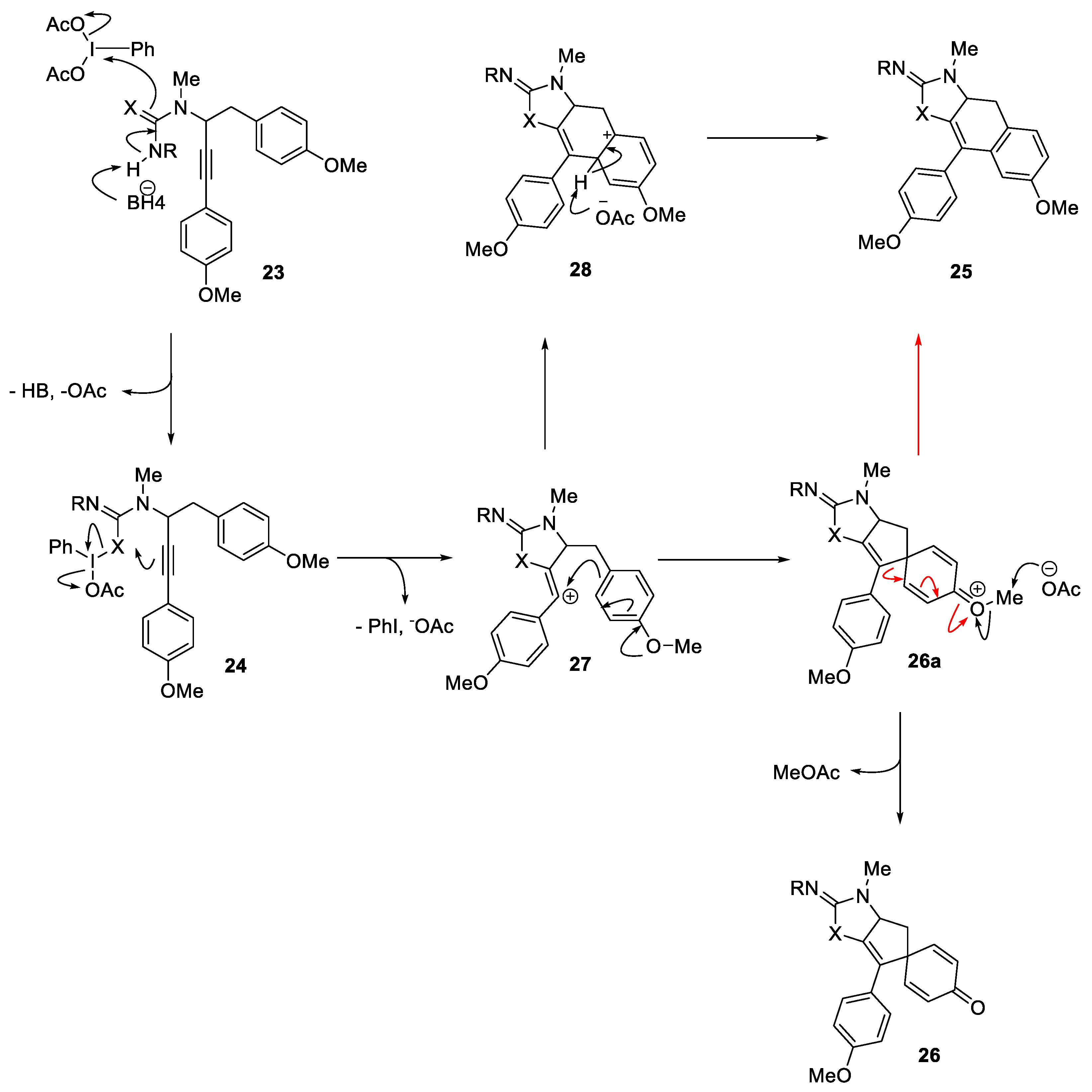
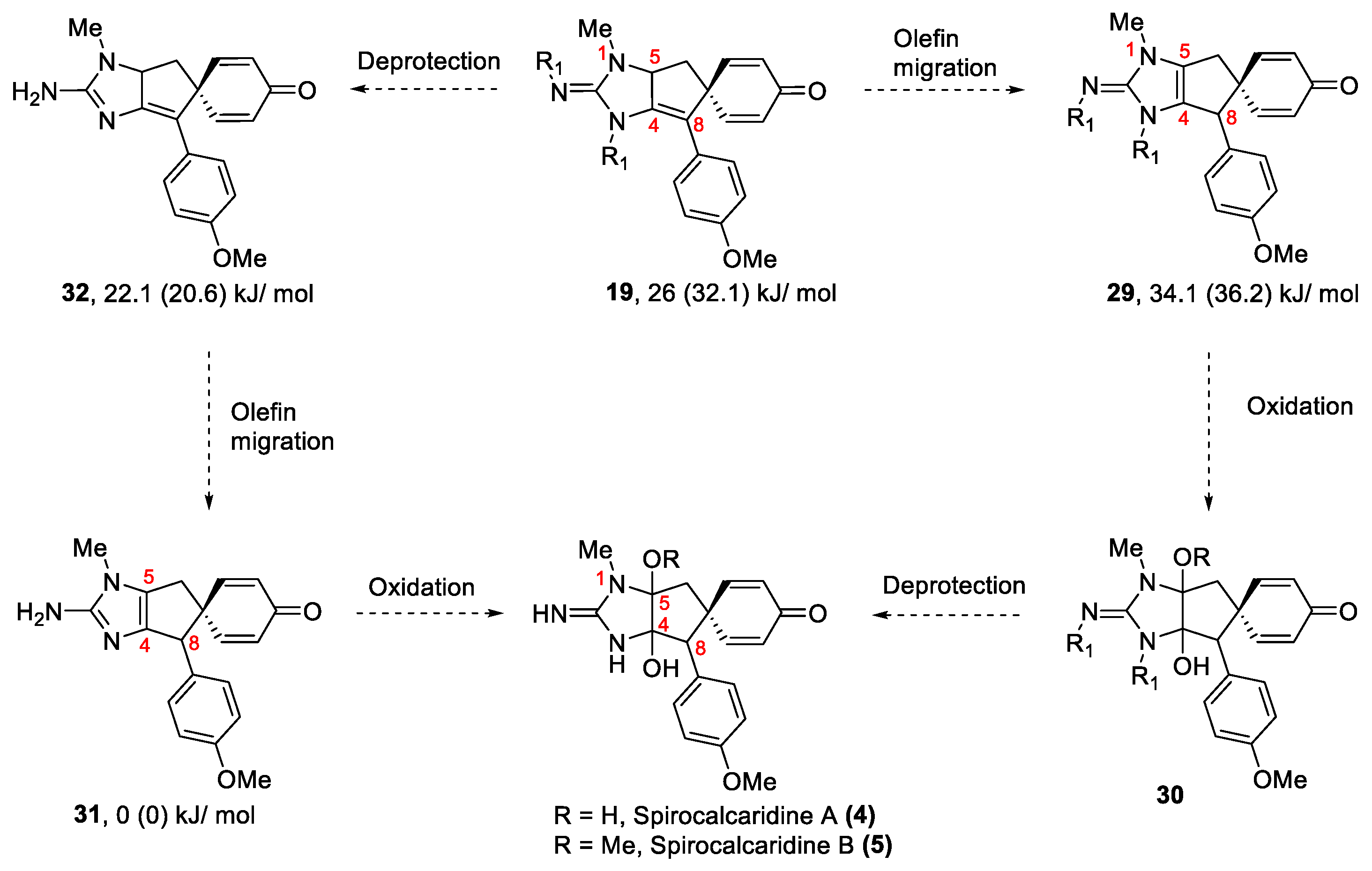
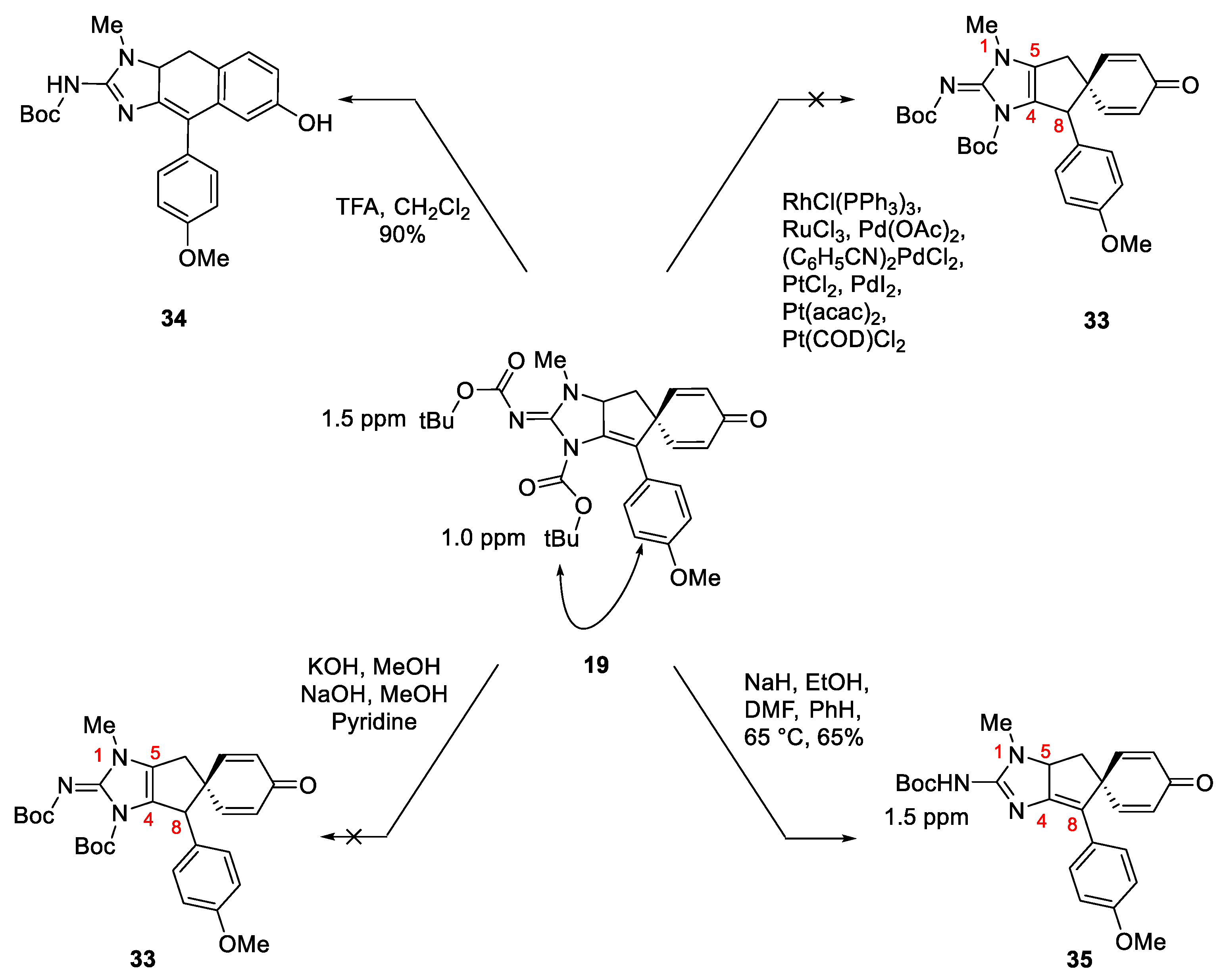
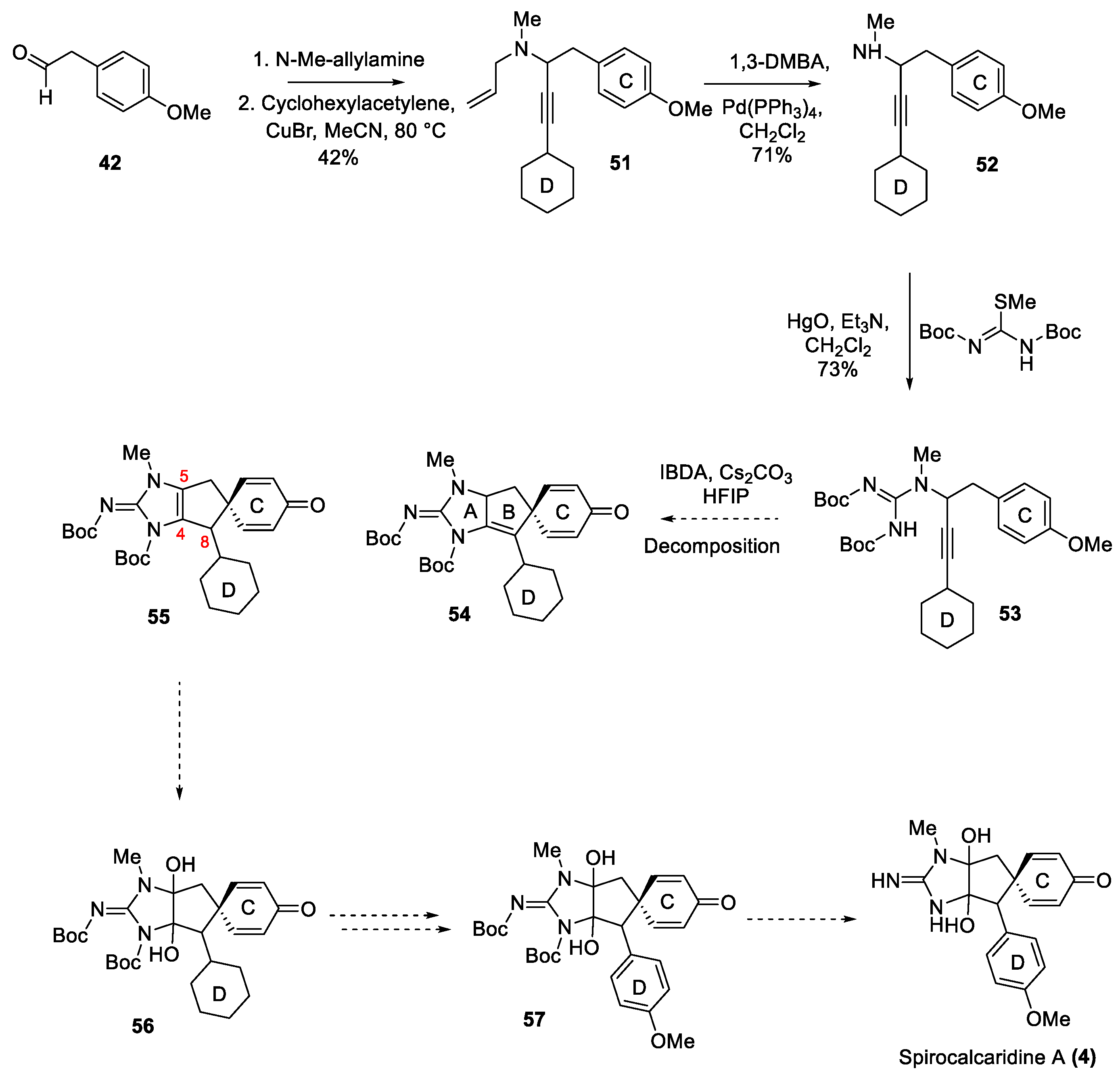
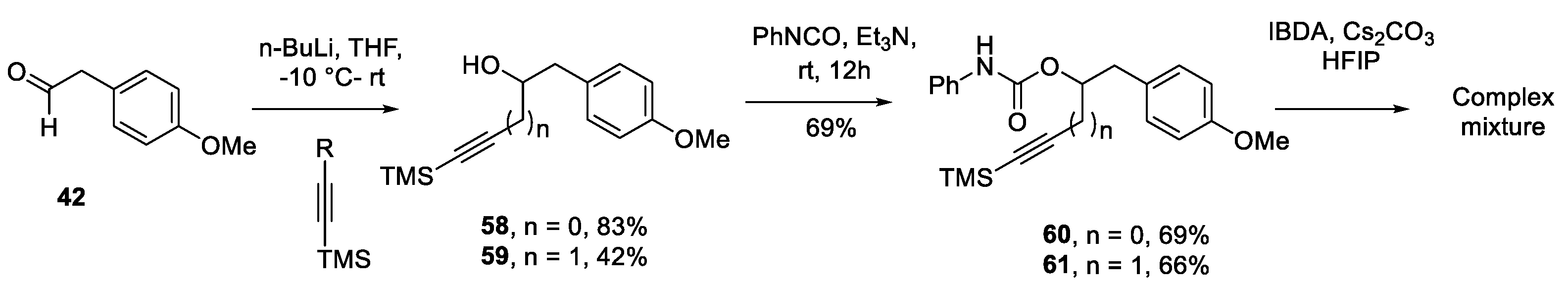
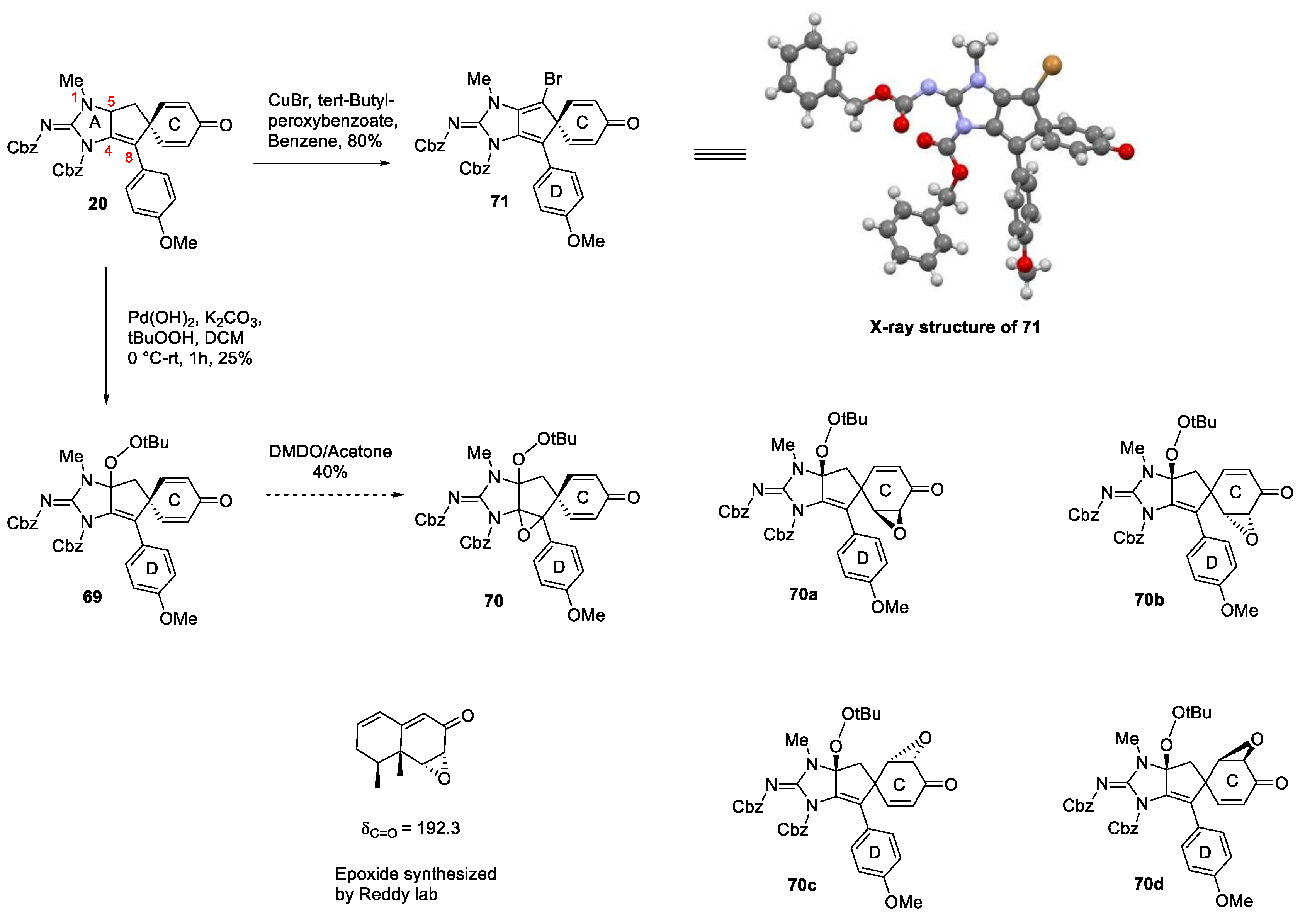
2. Results and Discussions
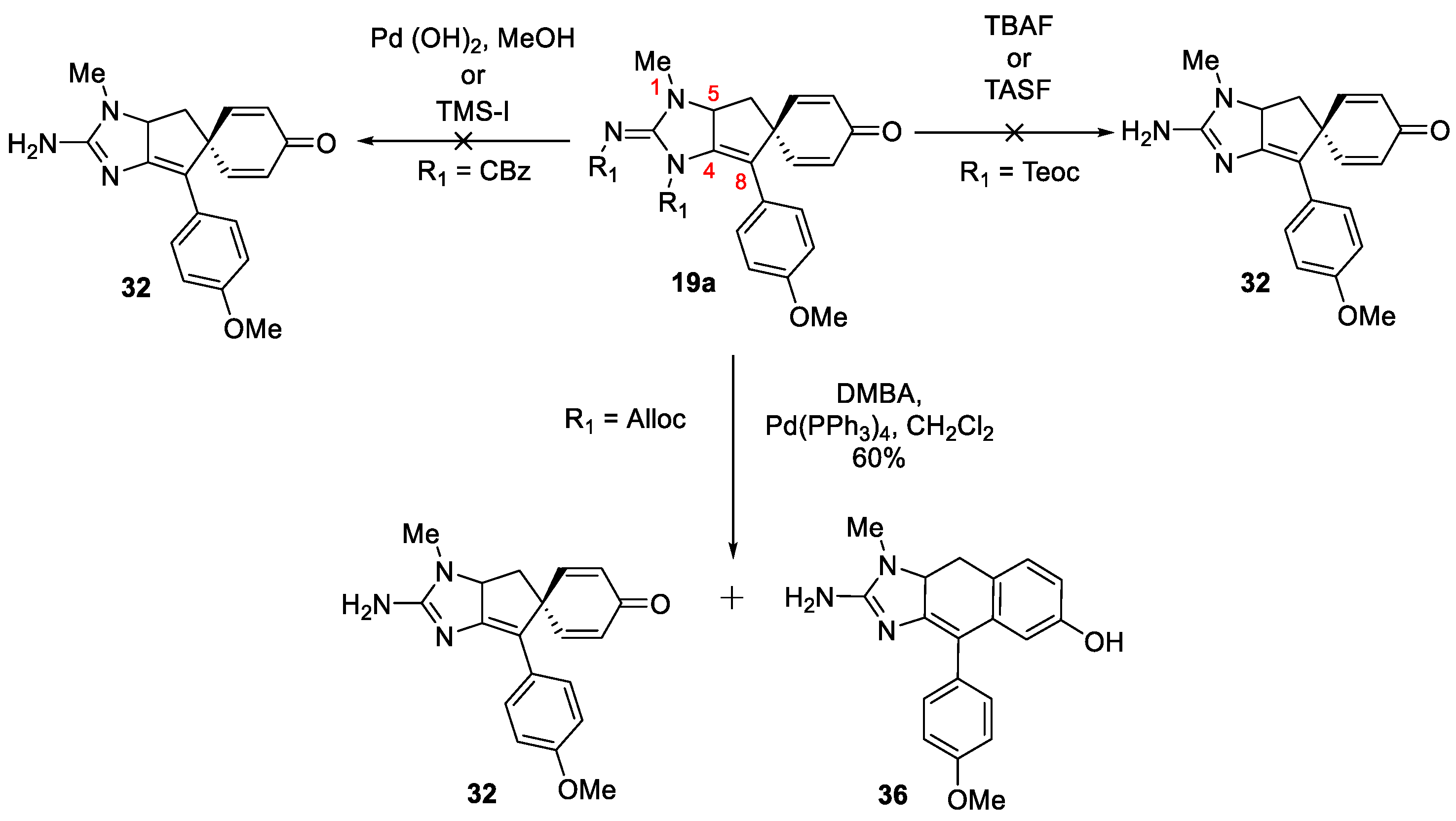
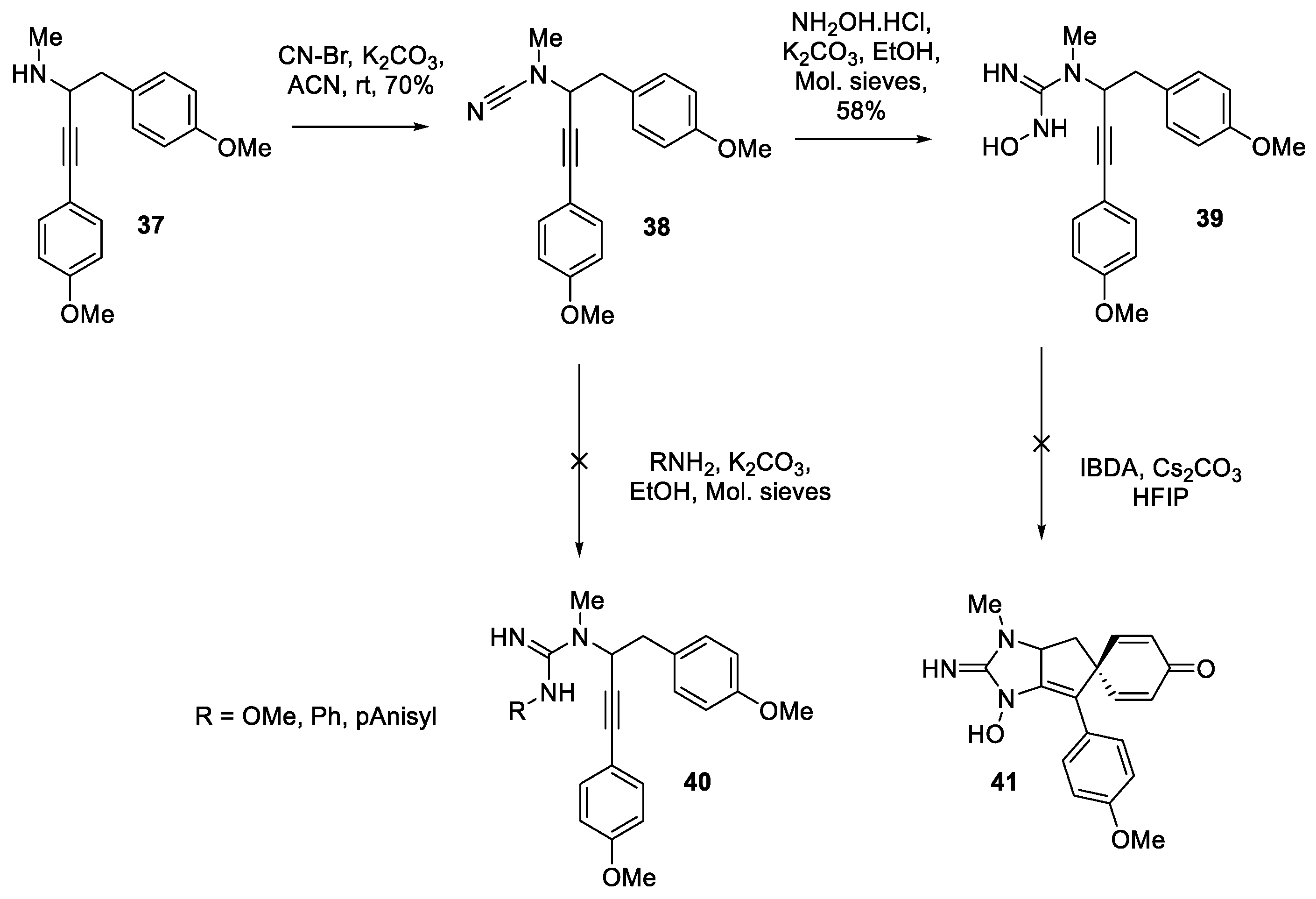
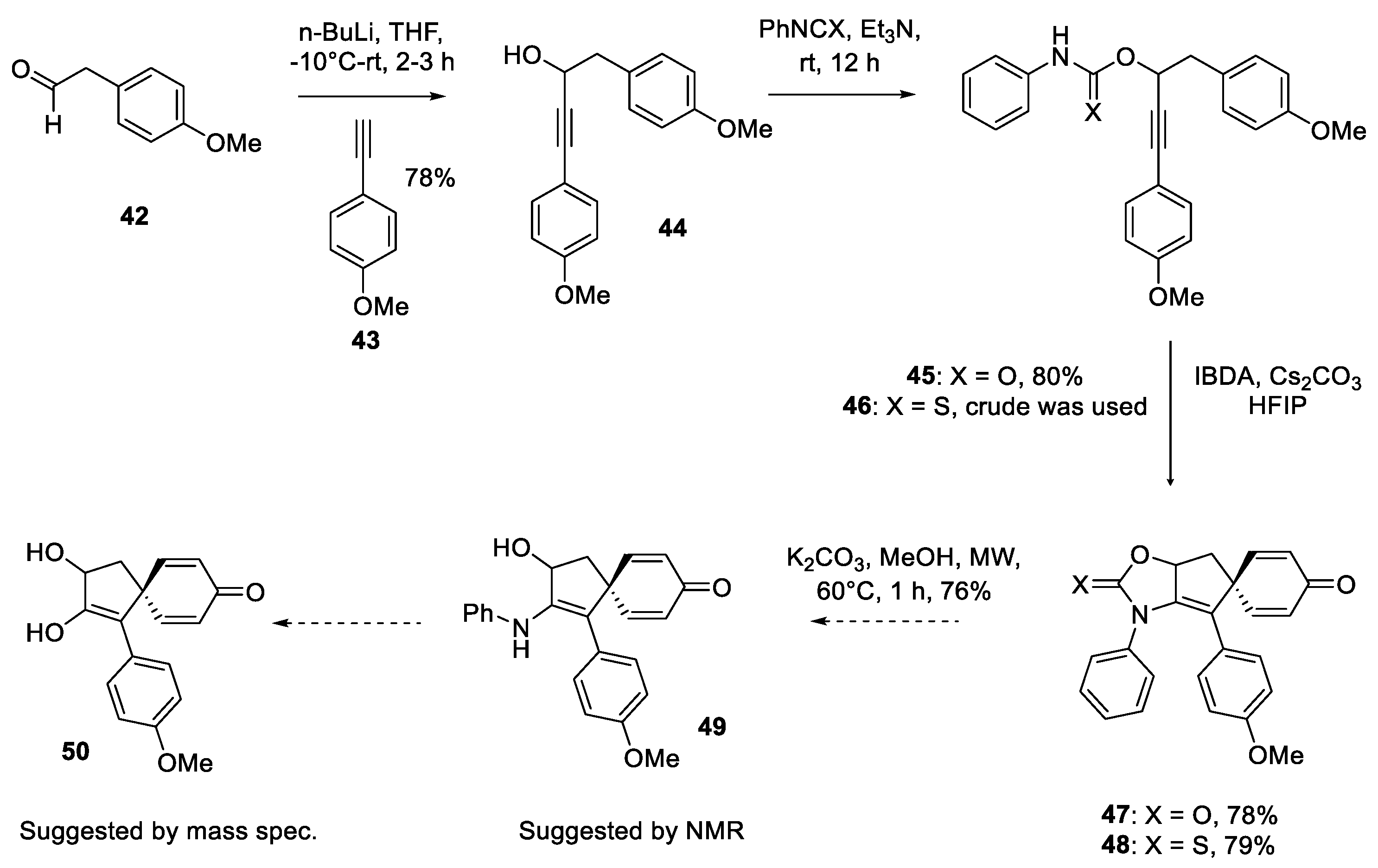
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Hou, Z.-W.; Li, P.; Wang, L. Electrochemical Dearomatizing Spirocyclization of Alkynes with Dimethyl 2-Benzylmalonates to Spiro[4.5]deca-trienones. J. Org. Chem. 2022, 87, 8697–8708. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Aberle, N.S.; Lucas, N.T.; Lessene, G.; Hawkins, B.C. Total Synthesis of (−)-Spiroleucettadine. Angew. Chem. Int. Ed. 2017, 56, 14663–14666. [Google Scholar] [CrossRef] [PubMed]
- Roose, T.R.; McSorley, F.; Groenhuijzen, B.; Saya, J.M.; Maes, B.U.W.; Orrù, R.V.A.; Ruijter, E. Dearomative Spirocyclization of Tryptamine-Derived Isocyanides via Iron-Catalyzed Carbene Transfer. J. Org. Chem. 2023, 88, 17345–17355. [Google Scholar] [CrossRef]
- Inprung, N.; Ho, H.E.; Rossi-Ashton, J.A.; Epton, R.G.; Whitwood, A.C.; Lynam, J.M.; Taylor, R.J.K.; James, M.J.; Unsworth, W.P. Indole-ynones as Privileged Substrates for Radical Dearomatizing Spirocyclization Cascades. Org. Lett. 2022, 24, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Gao, Y.; Yang, L.; Zhou, C.; Zhang, M.; Cheng, P.; Li, G. Dearomative spirocyclization via visible-light-induced reductive hydroarylation of non-activated arenes. Chin. Chem. Lett. 2022, 33, 225–228. [Google Scholar] [CrossRef]
- Jin, Z. Muscarine, imidazole, oxazole, and thiazolealkaloids. Nat. Prod. Rep. 2011, 28, 1143–1191. [Google Scholar] [CrossRef]
- Koswatta, P.B.; Lovely, C.J. Structure and synthesis of 2-aminoimidazole alkaloids from Leucetta and Clathrina sponges. Nat. Prod. Rep. 2011, 28, 511–528. [Google Scholar] [CrossRef]
- Sullivan, J.D.; Giles, R.L.; Looper, R.E. 2-Aminoimidazoles from Leucetta Sponges: Synthesis and Biology of an Important Pharmacophore. Curr. Bioact. Compd. 2009, 5, 39–78. [Google Scholar] [CrossRef]
- Roué, M.; Quévrain, E.; Domart-Coulon, I.; Bourguet-Kondracki, M.-L. Assessing calcareous sponges and their associated bacteria for the discovery of new bioactive natural products. Nat. Prod. Rep. 2012, 29, 739–751. [Google Scholar] [CrossRef]
- Tang, W.-Z.; Yang, Z.-Z.; Sun, F.; Wang, S.-P.; Yang, F.; Jiao, W.-H.; Lin, H.-W. (-)-Calcaridine B, a new chiral aminoimidazole-containing alkaloid from the marine sponge Leucetta chagosensis. J. Asian Nat. Prod. Res. 2019, 21, 1123–1128. [Google Scholar] [CrossRef]
- Campos, P.-E.; Herbette, G.; Fougère, L.; Clerc, P.; Tintillier, F.; de Voogd, N.J.; Le Goff, G.; Ouazzani, J.; Gauvin-Bialecki, A. An Aminopyrimidone and Aminoimidazoles Alkaloids from the Rodrigues Calcareous Marine Sponge Ernsta naturalis Mar. Drugs [Online], 2022, p. 637.
- Edrada, R.A.; Stessman, C.C.; Crews, P. Uniquely Modified Imidazole Alkaloids from a Calcareous Leucetta Sponge. J. Nat. Prod. 2003, 66, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Aberle, N.; Ovenden, S.P.B.; Lessene, G.; Watson, K.G.; Smith, B.J. Spiroleucettadine: synthetic studies and investigations towards structural revision. Tetrahedron Lett. 2007, 48, 2199–2203. [Google Scholar] [CrossRef]
- Chang, J.J.; Chan, B.; Ciufolini, M.A. Synthetic studies toward spiroleucettadine. Tetrahedron Lett. 2006, 47, 3599–3601. [Google Scholar] [CrossRef]
- White, K.N.; Amagata, T.; Oliver, A.G.; Tenney, K.; Wenzel, P.J.; Crews, P. Structure Revision of Spiroleucettadine, a Sponge Alkaloid with a Bicyclic Core Meager in H-Atoms. J. Org. Chem. 2008, 73, 8719–8722. [Google Scholar] [CrossRef]
- Singh, R.P.; Das, J.; Yousufuddin, M.; Gout, D.; Lovely, C.J. Tandem Oxidative Dearomatizing Spirocyclizations of Propargyl Guanidines and Ureas. Org. Lett. 2017, 19, 4110–4113. [Google Scholar] [CrossRef]
- Das, J.; Koswatta, P.B.; Jones, J.D.; Yousufuddin, M.; Lovely, C.J. Total Syntheses of Kealiinines A–C. Org. Lett. 2012, 14, 6210–6213. [Google Scholar] [CrossRef] [PubMed]
- Koswatta, P.B.; Sivappa, R.; Dias, H.V.R.; Lovely, C.J. Total Synthesis of (±)-Calcaridine A and (±)-epi-Calcaridine A. Org. Lett. 2008, 10, 5055–5058. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhandari, M.R.; Torres, F.M.; Doundoulakis, T.; Gout, D.; Lovely, C.J. Total Synthesis of (±)-2-Debromohymenin via Gold-Catalyzed Intramolecular Alkyne Hydroarylation. Org. Lett. 2020, 22, 3412–3417. [Google Scholar] [CrossRef]
- Zhang, X.; Larock, R.C. Synthesis of Spiro[4.5]trienones by Intramolecular ipso-Halocyclization of 4-(p-Methoxyaryl)-1-alkynes. J. Am. Chem. Soc. 2005, 127, 12230–12231. [Google Scholar] [CrossRef]
- Tang, B.-X.; Tang, D.-J.; Tang, S.; Yu, Q.-F.; Zhang, Y.-H.; Liang, Y.; Zhong, P.; Li, J.-H. Selective Synthesis of Spiro[4,5]trienyl Acetates via an Intramolecular Electrophilic ipso-Iodocyclization Process. Org. Lett. 2008, 10, 1063–1066. [Google Scholar] [CrossRef]
- Koswatta, P.B.; Das, J.; Yousufuddin, M.; Lovely, C.J. Studies towards the Leucetta-Derived Alkaloids Spirocalcaridine A and B – Possible Biosynthetic Implications. Eur. J. Org. Chem. 2015, 2015, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Gout, D.; Lovely, C.J. Tandem Thioacylation-Intramolecular Hydrosulfenylation of Propargyl Amines – Rapid Access to 2-Aminothiazolidines. European Journal of Organic Chemistry 2019, 2019, 1726–1740. [Google Scholar] [CrossRef]
- Singh, R.P.; Aziz, M.N.; Gout, D.; Fayad, W.; El-Manawaty, M.A.; Lovely, C.J. Novel thiazolidines: Synthesis, antiproliferative properties and 2D-QSAR studies. Bioorg. Med. Chem. 2019, 27, 115047. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.N.; Singh, R.P.; Gout, D.; Lovely, C.J. Dearomatizing spirocyclization of thioureas, ureas and guanidines. Tetrahedron Lett. 2021, 72, 153054. [Google Scholar] [CrossRef]
- Singh, R.P.; Lovely, C.J. Chapter 3 - The Leucetta alkaloids: Synthetic aspects. In Stud. Nat. Prod. Chem., Atta ur, R. Ed. Elsevier: 2019; Vol. 63, pp 43-79.
- Yousufuddin, M.; Morales, R.; Cassis, W.; Brown, G.W.; Singh, R.P.; Lovely, C.J. [1,4-Bis(4-meth oxy phen yl)but-3-yn-2-yl](cyano) methyl amine. IUCrData 2018, 3, x180389. [Google Scholar] [CrossRef]
- Morris, L.L.; Alvarado, C.A.; Goncalves, J.M.; Singh, R.P.; Lovely, C.J.; Yousufuddin, M. [4-(4-Meth oxy phen yl)-8-oxo-3-(phenyl selan yl)spiro [4.5]deca-3,6,9-trien-2-yl]methyl cyanamide. IUCrData 2020, 5, x200078. [Google Scholar] [CrossRef]
- Singh, R.P.; Spears, J.A.; Dalipe, A.; Yousufuddin, M.; Lovely, C.J. Dearomatizing spirocyclization reactions of alkynyl cyanamides. Tetrahedron Lett. 2016, 57, 3096–3099. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef]
- Catalán, J.; López, V.; Pérez, P. Solvent dipolarity/polarizability (SPP) of alcoholic solvents. Liebigs Annalen 1995, 1995, 793–795. [Google Scholar] [CrossRef]
- Lovely, C.J.; Du, H.; Sivappa, R.; Bhandari, M.R.; He, Y.; Dias, H.V.R. Preparation and Diels−Alder Chemistry of 4-Vinylimidazoles. J. Org. Chem. 2007, 72, 3741–3749. [Google Scholar] [CrossRef]
- Sivappa, R.; Koswatta, P.; Lovely, C.J. Oxidative reactions of tetrahydrobenzimidazole derivatives with N-sulfonyloxaziridines. Tetrahedron Lett. 2007, 48, 5771–5775. [Google Scholar] [CrossRef]
- Grieco, P.A.; Nishizawa, M.; Marinovic, N.; Ehmann, W.J. Remote double bond migration via rhodium catalysis: a novel enone transposition. J. Am. Chem. Soc. 1976, 98, 7102–7104. [Google Scholar] [CrossRef]
- Murai, M.; Nishimura, K.; Takai, K. Palladium-catalyzed double-bond migration of unsaturated hydrocarbons accelerated by tantalum chloride. Chem Commun (Camb) 2019, 55, 2769–2772. [Google Scholar] [CrossRef]
- Seto, H.; Fujioka, S.; Koshino, H.; Takatsuto, S.; Yoshida, S. Stereo and chemical course of acid-catalyzed double bond migration of cholesta-5,7-dien-3β-ol to 5α-cholesta-8,14-dien-3β-ol. J. Chem. Soc. Perkin Trans. 1 2000, 1697–1703. [Google Scholar] [CrossRef]
- Noyce, D.S.; Evett, M. Mechanism of the acid-catalyzed double bond migration in 3-cyclohexen-1-one and 3-methyl-3-cyclohexen-1-one. J. Org. Chem. 1972, 37, 394–397. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Zhao, Y.; Li, D.; Zhao, J.; Wang, Z.; Qu, J. Base-Promoted Double-Bond-Migration/Hydrolysis/Isomerization of 4-Aryl-1,1,1-trifluorobut-2-en-2-yl Trifluoromethanesulfonates: A Metal-Free Approach to β-Trifluoromethyl Ketones. Eur. J. Org. Chem. 2018, 2018, 6217–6222. [Google Scholar] [CrossRef]
- Kloosterziel, H.; van Drunen, J.A.A. Stereochemistry of base-catalysed double-bond migration in allyl ethers. Recl. Trav. Chim. Pays-Bas 1970, 89, 32–36. [Google Scholar] [CrossRef]
- Walker, D.P.; Eklov, B.M.; Bedore, M.W. Practical Synthesis of 3-Oxa-6-azabicyclo[3.1.1]heptane Hydrotosylate; A Novel Morpholine-Based Building Block. Synthesis 2012, 44, 2859–2862. [Google Scholar] [CrossRef]
- Krompiec, S.; Krompiec, M.; Penczek, R.; Ignasiak, H. Double bond migration in N-allylic systems catalyzed by transition metal complexes. Coord. Chem. Rev. 2008, 252, 1819–1841. [Google Scholar] [CrossRef]
- Fiorito, D.; Scaringi, S.; Mazet, C. Transition metal-catalyzed alkene isomerization as an enabling technology in tandem, sequential and domino processes. Chem. Soc. Rev. 2021, 50, 1391–1406. [Google Scholar] [CrossRef]
- Grigg, R.; Stevenson, P.J. Rhodium(I)-Catalysed Isomerisation of N-Allylimines to 2-Aza-1,3-dienes. Synthesis 1983, 1983, 1009–1010. [Google Scholar] [CrossRef]
- Smadja, W.; Valery, J.-M.; Ville, G.; Bernassau, J.-M. RuCl3, NaOH-catalyzed isomerization of allylic alcohols to saturated ketones: Part II Regiochemistry. J. Mol. Catal. 1985, 30, 389–394. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Kamimura, R.; Shono, K.; Kawakami, T.; Nakayama, K.; Nishino, K.; Nakayama, T.; Takahashi, K.; Nakamura, A.; Hosokawa, T. Efficient double bond migration of allylbenzenes catalyzed by Pd(OAc)2–HFIP system with unique substituent effect. Tetrahedron Lett. 2010, 51, 3590–3592. [Google Scholar] [CrossRef]
- Golborn, P.; Scheinmann, F. Isomerisation of allyl phenyl ethers and allylphenols with transition metal catalysts. J. Chem. Soc. Perkin Trans. 1 1973, 2870–2875. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, X.; Huang, J.; Yuan, J. Platinum-Catalyzed Tandem Cycloisomerization Reaction of Benzoendiynyl Esters: Regioselective Long-Range 1,5-Acyl Migration. J. Org. Chem. 2014, 79, 10674–10681. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.V.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr. J. A.; Peralta, J.E.; Ogliaro, F.; Bearpark, M.J.; Heyd, J.J.; Brothers, E.N.; Kudin, K.N.; Staroverov, V.N.; Keith, T.A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.P.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B.; Fox, D.J. Gaussian 16 Rev. C.01, Wallingford, CT, 2016.
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Renodon-Cornière, A.; Dijols, S.; Perollier, C.; Lefevre-Groboillot, D.; Boucher, J.-L.; Attias, R.; Sari, M.-A.; Stuehr, D.; Mansuy, D. N-Aryl N‘-Hydroxyguanidines, A New Class of NO-Donors after Selective Oxidation by Nitric Oxide Synthases: Structure−Activity Relationship. J. Med. Chem. 2002, 45, 944–954. [Google Scholar] [CrossRef]
- Noshita, M.; Shimizu, Y.; Morimoto, H.; Ohshima, T. Diethylenetriamine-Mediated Direct Cleavage of Unactivated Carbamates and Ureas. Org. Lett. 2016, 18, 6062–6065. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, Z.; Lesieur, D.; Lespagnol, C.; Sauzieres, J.; Olivier, P. Acyl-7 dihydro-2,3 benzoxazin-1,4 ones-3 et propriétés normolipémiantes7-Acyl-2,3-dihydro-1,4-benzoxazin-3-ones and normolipemic properties. Eur. J. Org. Chem. 1989, 24, 55–60. [Google Scholar]
- Buckle, D.R.; Cantello, B.C.C.; Smith, H.; Smith, R.J.; Spicer, B.A. Synthesis and antiallergic activity of 2-hydroxy-3-nitro-1,4-naphthoquinones. J. Med. Chem. 1977, 20, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Lovely, C.J.; Du, H.; He, Y.; Rasika Dias, H.V. Oxidative Rearrangement of Imidazoles with Dimethyldioxirane. Org. Lett. 2004, 6, 735–738. [Google Scholar] [CrossRef]
- Watson, L.J.; Harrington, R.W.; Clegg, W.; Hall, M.J. Diastereoselective intermolecular ene reactions: synthesis of 4,5,6,7-tetrahydro-1H-benzo[d]imidazoles. Org. Biomol. Chem. 2012, 10, 6649–6655. [Google Scholar] [CrossRef]
- Larionov, E.; Li, H.; Mazet, C. Well-defined transition metal hydrides in catalytic isomerizations. Chem Commun (Camb) 2014, 50, 9816–9826. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Koyama, Y.; Moro-Oka, Y.; Ikawa, T. Novel peparation of silyl enol ethers from allyl alcohols. Tetrahedron Lett. 1979, 20, 1415–1418. [Google Scholar] [CrossRef]
- Jin, X.; Taniguchi, K.; Yamaguchi, K.; Nozaki, K.; Mizuno, N. A Ni–Mg–Al layered triple hydroxide-supported Pd catalyst for heterogeneous acceptorless dehydrogenative aromatization. Chem Commun (Camb) 2017, 53, 5267–5270. [Google Scholar] [CrossRef]
- Giustra, Z.X.; Chou, L.-Y.; Tsung, C.-K.; Liu, S.-Y. Kinetics of −CH2CH2– Hydrogen Release from a BN-cyclohexene Derivative. Organometallics 2016, 35, 2425–2428. [Google Scholar] [CrossRef]
- Tian, G.; Fedoseev, P.; Van der Eycken, E.V. Hypervalent Iodine(III)-Mediated Cascade Cyclization of Propargylguanidines and Total Syntheses of Kealiinine B and C. Chem. Eur. J 2017, 23, 5224–5227. [Google Scholar] [CrossRef]
- Chenna Reddy, M.L.; Patil, V.B.; Nawaz Khan, F.R.; Saravanan, V. Synthesis of Imidazo[1,2-a]pyridines and Imidazo[2,1-b]thiazoles Attached to a Cycloalkyl or Saturated Heterocycle Containing a Tertiary Hydroxy Substitution. J. Heterocycl. Chem. 2019, 56, 1486–1497. [Google Scholar] [CrossRef]
- Fursule, R.A.; Patil, P.O.; Shewale, B.D.; Kosalge, S.B.; Deshmukh, P.K.; Patil, D.A. Novel system for decarboxylative bromination of alpha,beta-unsaturated carboxylic acids with diacetoxyiodobenzene. Chem. Pharm. Bull. 2009, 57, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ojima, I. Asymmetric synthesis of 1-vinyltetrahydroisoquinoline through Pd-catalyzed intramolecular allylic amination. Tetrahedron 2007, 63, 8563–8570. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Sun, X.; Du, Y. An efficient cis-reduction of alkyne to alkene in the presence of a vinyl iodide: stereoselective synthesis of the C22–C31 fragment of leiodolide A. Tetrahedron 2013, 69, 1553–1558. [Google Scholar] [CrossRef]
- Yu, J.-Q.; Corey, E.J. A Mild, Catalytic, and Highly Selective Method for the Oxidation of α,β-Enones to 1,4-Enediones. J. Am. Chem. Soc. 2003, 125, 3232–3233. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, L.M.; Aguirre, J.M.; Lantaño, B.; Alesso, E.N.; Iglesias, G.Y.M. ZnI2/NaCNBH3 as an Efficient Reagent for Regioselective Ring Opening of the Benzylic Epoxide Moiety. Synth. Commun. 2004, 34, 895–901. [Google Scholar] [CrossRef]
- Handore, K.L.; Jadhav, P.D.; Hazra, B.; Basu, A.; Reddy, D.S. Total Syntheses and Biological Evaluation of (±)-Botryosphaeridione, (±)-Pleodendione, 4-epi-Periconianone B, and Analogues. ACS Med. Chem. Lett. 2015, 6, 1117–1121. [Google Scholar] [CrossRef]
- Zhu, N.; Qian, B.; Xiong, H.; Bao, H. Copper-catalyzed regioselective allylic oxidation of olefins via C–H activation. Tetrahedron Lett. 2017, 58, 4125–4128. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Sosnovsky, G. THE REACTIONS OF t-BUTYL PERBENZOATE AND OLEFINS—A STEREOSPECIFIC REACTION. J. Am. Chem. Soc. 1958, 80, 756–756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




