Introduction
Skin cancer presents a life-threatening health issue if left untreated (Hu et al., 2022; Sol et al., 2024). Although the skin is the most visible organ, many lesions can easily be misidentified as skin cancer. It is particularly concerning that skin cancer is often underdiagnosed until it progresses to advanced stages, as clinical characteristics are not always sufficient to identify a definitive signature of the disease. It can be categorized into two groups: non-melanoma skin cancer (NMSC) and melanoma. Among the former, 95% of cases are basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), but other rare malignant skin tumors also fall under the NMSC classification (Ciążyńska et al., 2021; Sol et al., 2024).
Research from various electronic databases indicates that the types and subtypes of skin cancer are becoming increasingly common among clinically diagnosed cancers worldwide (Roky et al., 2024). Despite darker-skinned patients being affected at lower rates compared to their lighter-skinned counterparts, this group reports higher mortality rates. Biological differences, such as genomic and melanin-related variations (Manganelli et al., 2021; Zambrano-Román et al., 2022), along with disparities in healthcare utilization, significantly contribute to these outcomes. Melanin pigmentation protects the skin from the harmful effects of UV radiation, serves various functions, and has diverse structures and forms. Pigmentary traits are genetically determined and exhibit a polygenic inheritance pattern (Bhattacharya et al., 2021). In dermatology practice, the structure and functional behavior of the skin are influenced by phototype, and environmental factors also play an essential role (Bolick et al., 2022). Ancestral background is critical because natural selection shapes the overall genetic architecture of skin pigmentation (Rangel-Villalobos et al., 2008).
Dermatoscopy is a non-invasive, in vivo technique that adds a new dimension to evaluating subsurface skin structures in the epidermis, dermo-epidermal junction, and upper dermis (Sławińska et al., 2023). The correlation between dermatoscopic and histopathological findings enhances the quality of skin cancer diagnoses and boosts dermatologists' confidence. Dermatoscopy operates parallel to the skin surface, analyzing structures on a horizontal plane, while pathology examines sections on a vertical plane. The color of dermatoscopic features varies based on the histopathological levels within the epidermis and superficial dermis. Pigmented structures appear black in the cornified layer, brown at the dermo-epidermal junction, and gray-blue in the papillary dermis (Sławińska et al., 2023). Dermatoscopic-pathologic correlation has revealed additional features such as brown globules, hypopigmented areas, white regions, and whitish veils that correlate well with melanocytic lesions (Behera et al., 2021). The situation is less clear for non-melanocytic lesions.
Visualizing and identifying vessels with a characteristic morphology can be the key to diagnosis, especially in NMSC, where vascular features are often the only clues. A significant body of evidence highlights the importance of aberrant angiogenesis in cancer pathogenesis. Growing tumors feed on newly formed capillaries. Identifying and evaluating vascular structures on dermatoscopy depends mainly on the optical system and examination technique. The method (contact dermatoscopy vs. polarized light dermatoscopy), the dermatoscope resolution, and the choice of immersion fluid are considered (Wojtowicz & Żychowska, 2024). Thanks to its high viscosity, a range of immersion fluids can be used, making the ultrasound gel the favorite for some authors when examining vessels without polarized light. A magnification of at least 30x is recommended for visualizing tiny capillaries (Martín et al., 2012). The most crucial chromophore in non-pigmented cutaneous tumors is hemoglobin.
Some vascular features are small and usually occluded by other structures, making their detection challenging. The predominant vascular pattern will also depend on the depth of lesions, the volume of the tumor, and its proliferation pattern. Tumor topography and morphology, phototype, and age are essential clinical aspects. The vessel morphology, architectural arrangement, and additional defects should be analyzed. Several studies have sought, by various means, to identify vascular features associated with the more aggressive NMSC phenotypes or initial, subtle lesions.
We undertook this work to determine whether the melanin and vascular patterns observed in dermatoscopy might reflect the biological behavior of NMSC in a Mexican population. Given the large Indigenous populations, ethnicity in Mexico is regarded as a “risk factor” for several diseases, warranting special attention and concern. The paternal ancestry estimated in western Mexico was predominantly European, followed by Amerindian and African (approximately 60%, 25%, and 15%) (Rangel-Villalobos et al., 2008). Significant genetic heterogeneity was established. Analyzing the pigment and vascular morphological and distribution patterns in Mexican patients with NMSC can further support diagnosis, assessment, or monitoring.
Methodology
Selection of Patients
We conducted an institutionally approved transversal study that included patients diagnosed with NMSC in an academic dermatology setting (Dermatology Department in the Sonora State General Hospital) from April 2024 to July 2024. Two independent reviewers obtained data from Electronic Medical Records (EMR). The prevalence of NMSC in the general adult population was taken as a reference, where it is reported to be around 3%. The equation used was n=Z2×P×1-P∕d2. Patients with a clinical suspicion were evaluated under dermatoscopy using a Dermlite DL5 dermatoscope. At least three images were captured under PD and UV light (365 nm). The images were obtained using an iPhone 15 Pro Max and were stored in the database until analysis. A total of fifty-three patients with NMSC were identified.
Two expert dermatologists independently analyzed the images. Then, a 5 mm punch biopsy was taken to confirm the preliminary diagnosis in each patient. A positive clinicopathological correlation for any NMSC underwent excisional surgery and defect repair by direct closure, flap, or graft. Two certified histopathologists performed a histopathological analysis of the entire sample. This study was conducted according to the Helsinki Declaration. Informed written consent was obtained from all subjects to participate in the research at every step and to publish images.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism v8.0. A probability (p) value of less than 0.05 was considered significant. Categorical variables were expressed as percentages and counts. The Shapiro-Wilk normality test was applied to verify the distribution of the data. Continuous variables are expressed as mean and standard deviation (parametric distribution) or median with an interquartile range (nonparametric distribution) depending on the distribution. Differences in frequencies were compared using Chi-square (p) or Fisher's exact test (p´). Mann–Whitney U test was used to evaluate differences between two groups, whereas Kruskal–Wallis test was used to analyze three or more groups. Spearman test was used to assess correlations between quantitative data.
Results
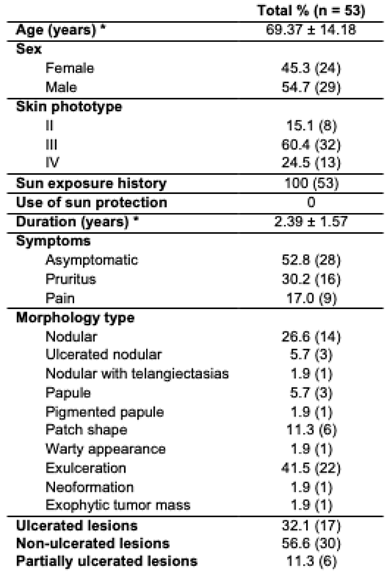
The average age of the patients was 69 years, with the majority classified as phototype III (60.4%). Tumors had an average duration of 2.39 years. Most were asymptomatic (52.8%) or presented with pruritus (30.2%) or pain (17%), primarily in the nodular or ulcerated forms (
Table 1). No correlations were found between age and clinical variables such as duration of lesions (years), lesion size (cm), or depth of invasion (mm). A positive correlation was identified between lesion duration (years) and lesion size (cm) (
p <0.0001, r = 0.6934, 95% confidence interval 0.514 - 0.814).
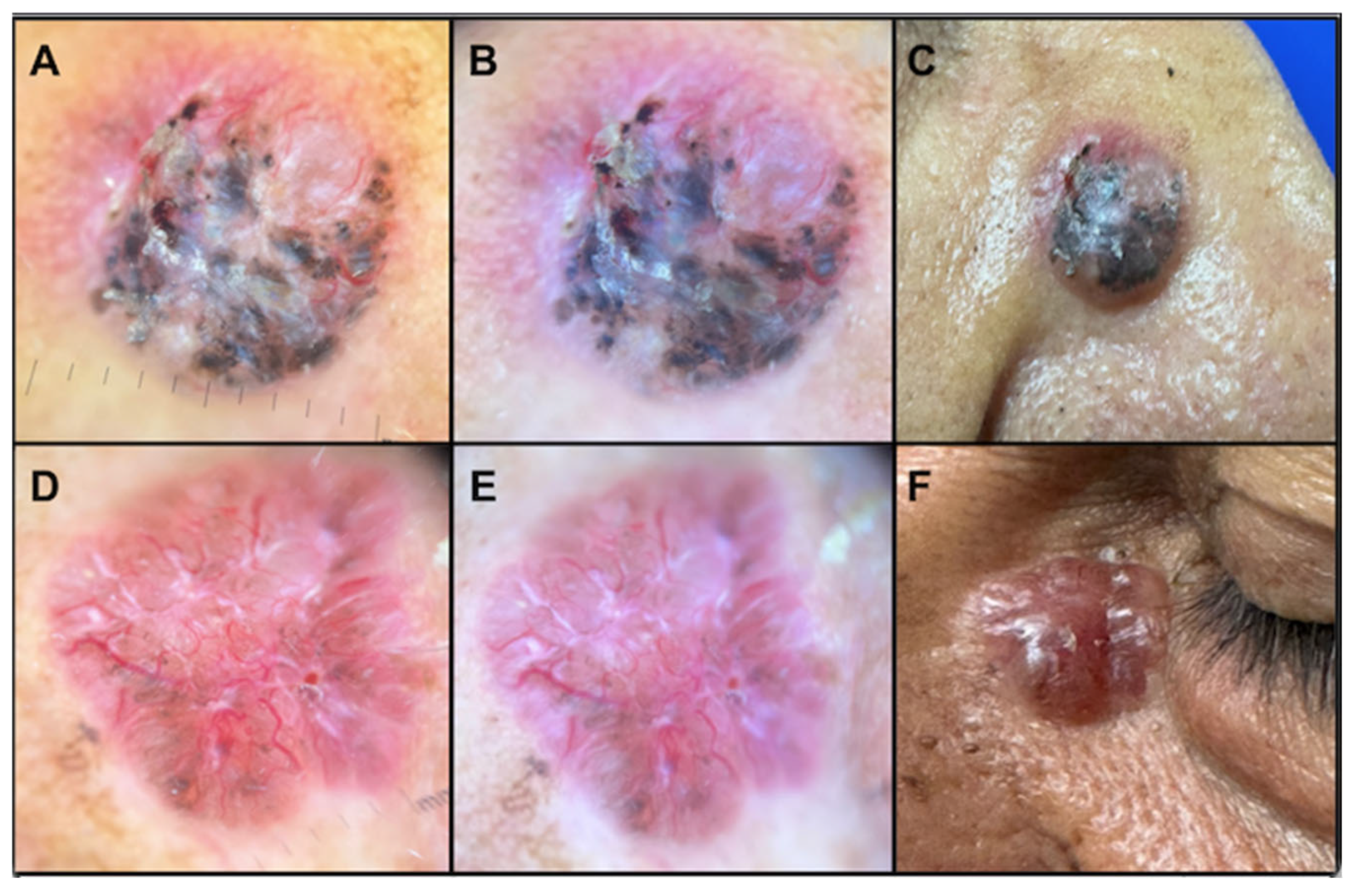
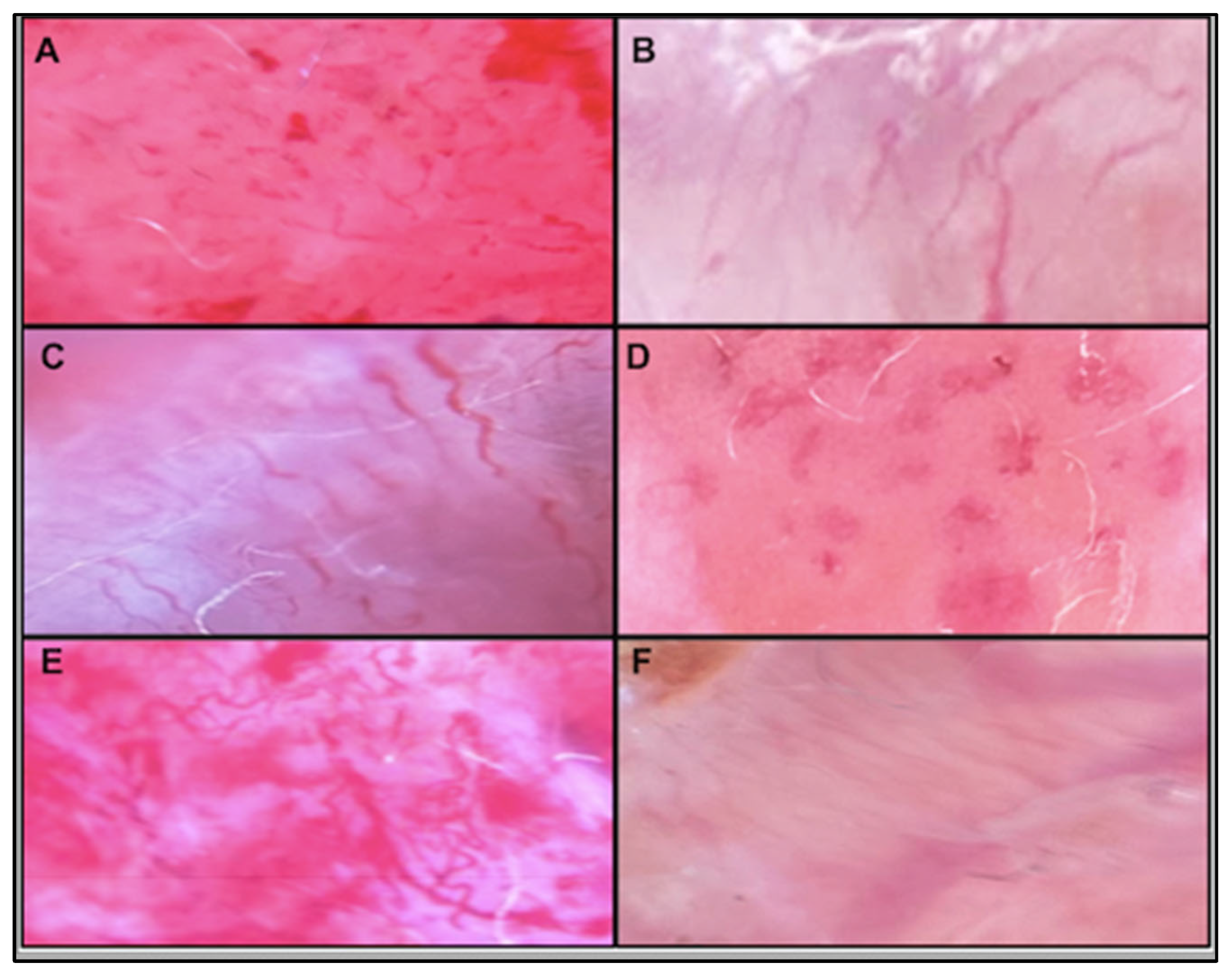
Figure 1 presents two clinical examples.
Women, on average, were older (72 ± 14.8) than men (67 ± 13.5), with no statistical differences (p = 0.1). However, a trend in the data indicated a longer tumor duration in men (2.05 ± 1.12 years versus 2.69 ± 1.83 years, p = 0.1), as well as larger lesion sizes: 1.99 ± 1.66 cm in women compared to 3.59 ± 3.55 cm in men (p = 0.09). In general, patients with ≥ 3 years of evolution had larger lesions (4.82 ± 3.53) than those with 1-2 years (1.49 ± 1.23) (p <0.0001).
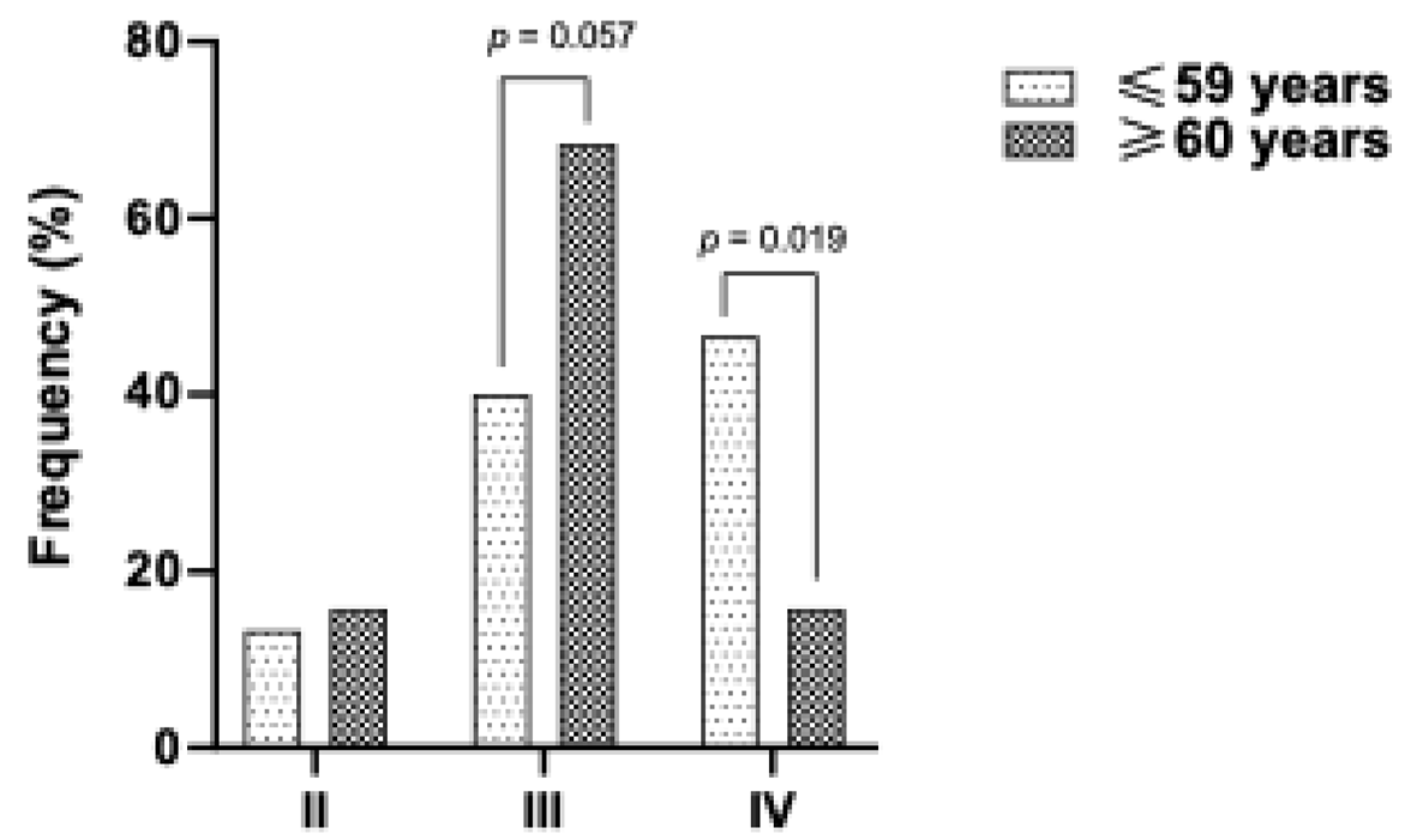
Interestingly, patients with darker phototypes were younger at diagnosis (
Figure 2), suggesting potential underlying factors in the Mexican population that may promote skin cancer development, independent of UV radiation exposure.
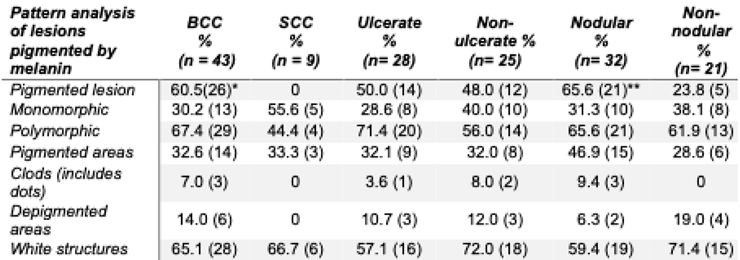
BCC represented the most prevalent type of NMSC, comprising 81.1%. SCC accounted for 16.98%, while sarcoma comprised 1.89% of the diagnosed cases. Pigmentation was noted in 60.5% of BCC patients (
p = 0.001), while keratinization was observed in 77.8% of SCC patients (
p < 0.0001) (
Table 2). Gray or blue coloration, milium-type cysts, and pseudo follicular openings were exclusively reported in patients with BCC, without statistical differences (
Figure 3).
Analyzing the topography, BCC was most commonly found on the nose (27.9%), cheek (25.6%), and forehead (11.6%), with no statistical difference. In contrast, SCC was most frequently located on the lips (33.3%, p < 0.0001), chin (11.1%, p = 0.027), and thumb (11.1%, p = 0.027). Patients with ulcerated lesions experienced a longer duration of lesions and larger lesion sizes (2.89 ± 1.87 years and 1.84 ± 0.89 cm) compared to those without ulcers (1.84 ± 0.89 years and 2.07 ± 1.91 cm; p = 0.014 and p = 0.058, respectively). Patients with nodular lesions were younger (66 ± 14.6 years) and had smaller lesion sizes (2.25 ± 1.95 cm) than those with non-nodular lesions (74.5 ± 12.1 years and 3.81 ± 3.88 cm), though no statistically significant differences were noted.
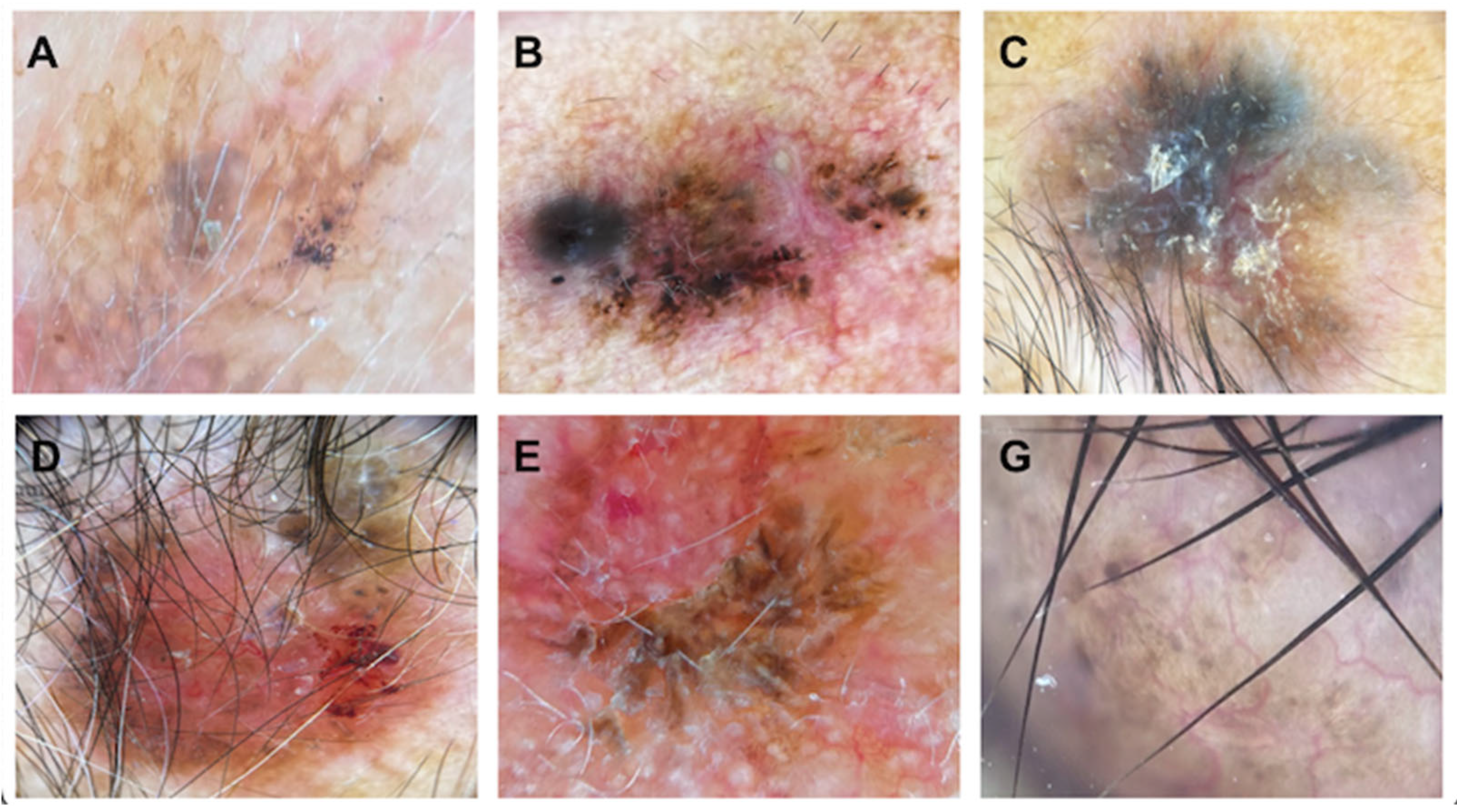
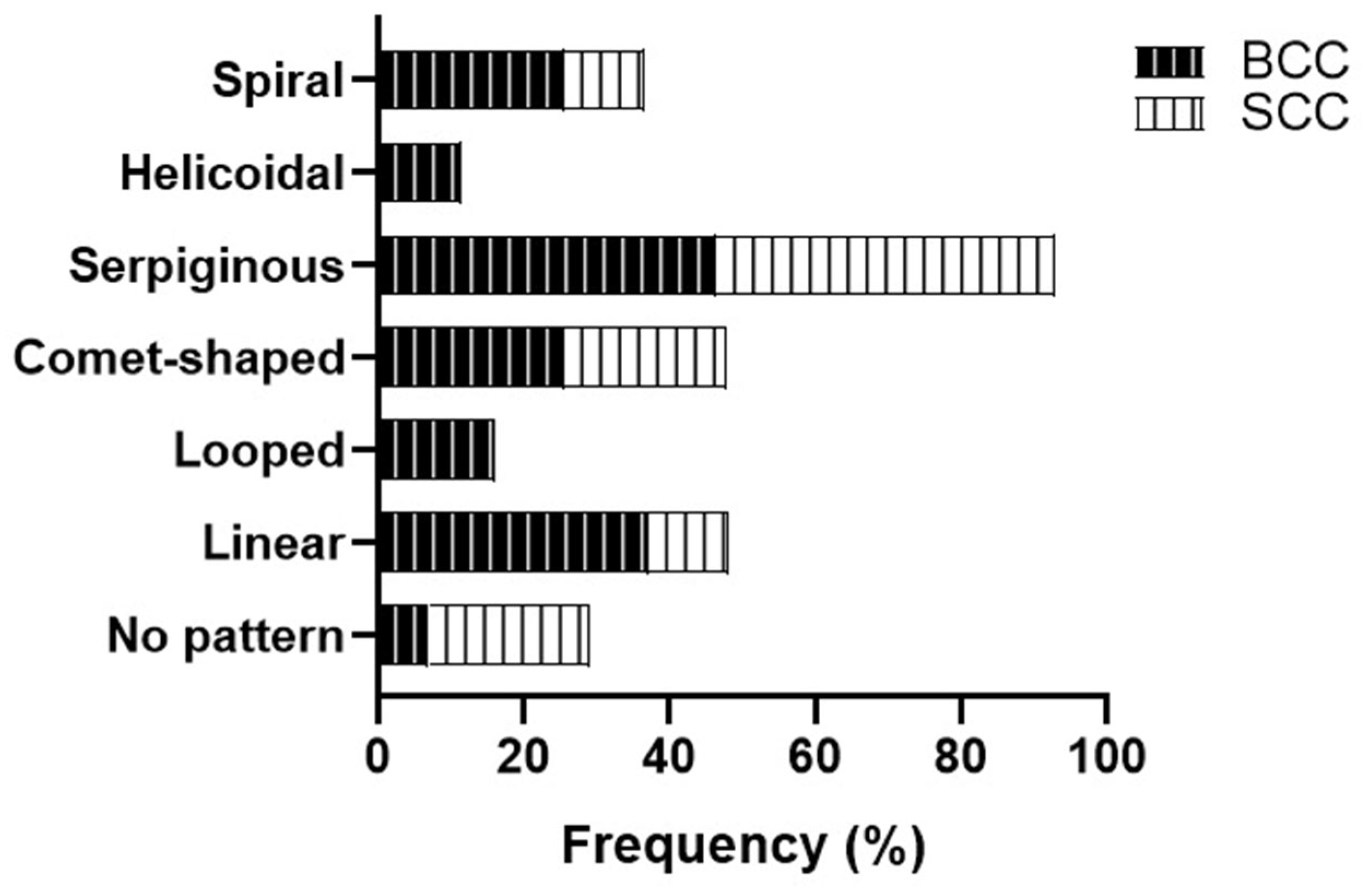
Based on the vascular pattern, serpiginous vessels were the most common finding in both BCC and SCC (46.5%), while the second most prevalent pattern in BCC was the linear pattern (37.2%). In SCC, the comet morphology of vessels emerged as the second typical pattern (22.2%). Patients lacking a specific vascular pattern were those with SCC (22.2%), showing no significant statistical differences (
Figure 4).
BCC neoplasms exhibit a higher frequency of polymorphic vessel patterns (67.5%), while those with SCC tend to present a monomorphic pattern (55.6%). Most patients with BCC showed serpiginous (44.2%) and linear (37.2%) structures within the linear blood vessel subtype. Meanwhile, many SCC patients did not exhibit a specific subtype (33.3%,
p = 0.055). Both groups' blood vessel arrangements and specific patterns were primarily radial (65.1% in BCC and 44.4% in SCC) (
Figure 5).
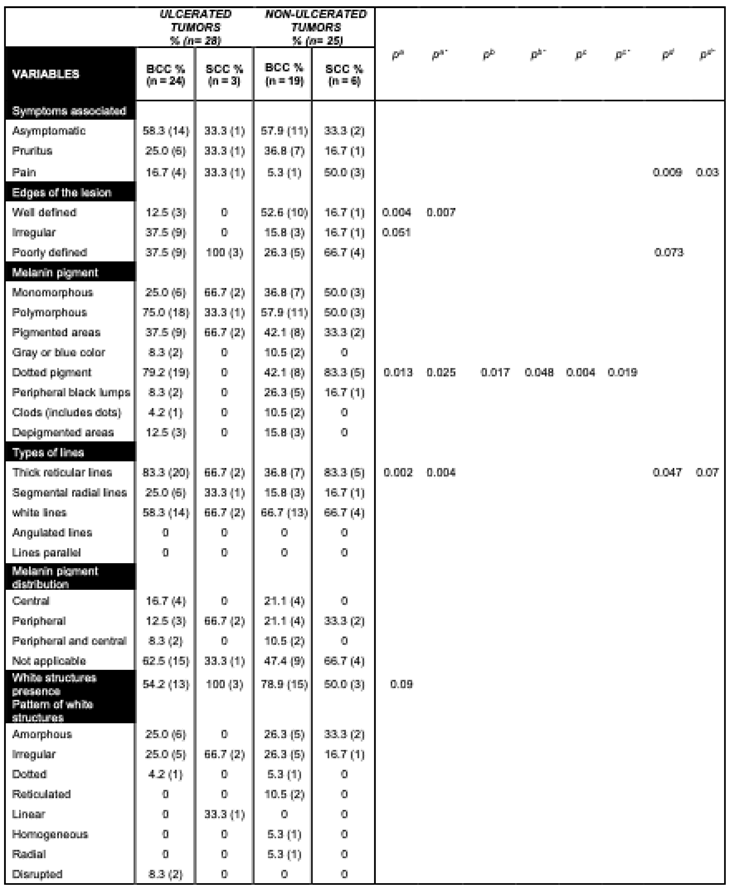
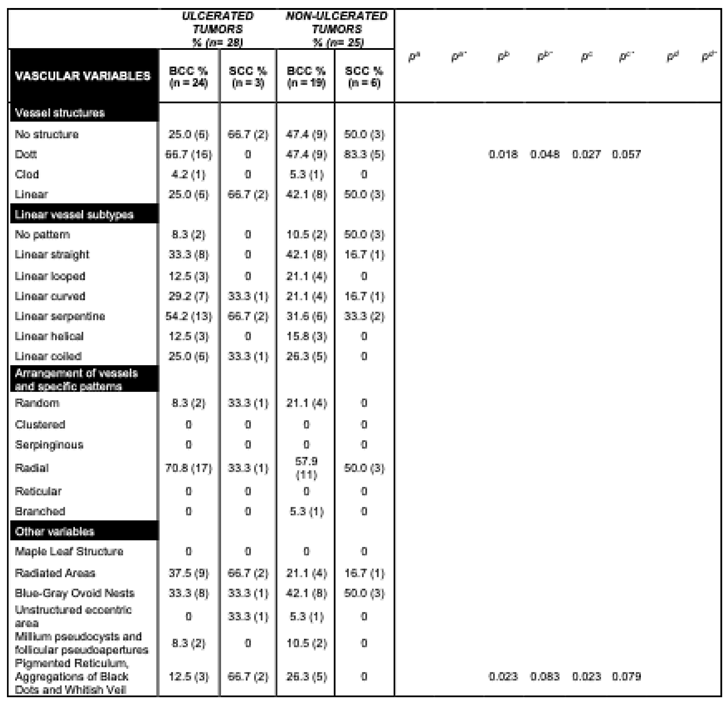
Ulcerated tumors were common in our sample (52.8% of patients). In 82.1% of cases, they displayed prominent reticular lines associated with perineural invasion in the histopathology images from 10.7% of patients. These characteristics were found more frequently in ulcerated than non-ulcerated lesions (
p = 0.009 and
p = 0.09, respectively). In contrast, non-ulcerated lesions had a higher frequency of peripheral black lumps, 24.0% (
p = 0.009), in addition to milium-like cysts, pseudo follicular openings (
p = 0.09), and pigmentation (
p = 0.003) (
Table 3).
Furthermore, 60.4% of the tumors had nodular morphology, which showed a higher frequency of the linear helical blood vessel subtype (
p = 0.035). Patients with nodular lesions more frequently had ulcerated surfaces than those with non-nodular lesions, which had rough surfaces (
p = 0.0038,
p = 0.009). Also, in non-nodular lesions, it was more common to see a lack of specific blood vessel arrangement patterns (
p = 0.06) and keratinization (
p = 0.0008) (
Table 4).
Discussion
Despite the lack of recognition of high skin cancer rates among non-Whites, our increasing incidence underscores the need for enhanced preventive and diagnostic programs. The rising life expectancy of the general population is likely contributing to the prevalence of dermatological NMSC in elderly patients. However, a significant portion of our study population was under 60. Notably, the younger demographic exhibited higher phototypes. These findings emphasize the need to consider additional factors beyond UV radiation as risks for skin cancer.
A comprehensive genome-wide association study (GWAS) and Polygenic Risk Score (PRS) analysis of 13 pigmentary-related traits was conducted to assess phototype as a genetic proxy for skin functionality and disease in open mixed populations (Farré et al., 2023). This study revealed a strong link between fair phototypes and NMSC, OR = 0.93; BCC, OR = 0.97). Abnormal melanin physiology has been associated with the immunomodulation of the tumor microenvironment (Cabaço et al., 2022). Moreover, variations in sun-protective behaviors can be observed among sun-sensitive individuals based on race and ethnicity (Bolick et al., 2022). A history of severe sunburns has been connected to both BCC and SCC (Savoye et al., 2018).
BCC was the most common form of NMSC, with the nodular subtype being the most prevalent. Pigmented lesions of BCC were predominant. However, the pattern of lesions displaying melanin and BCC-related pigmented structures, such as large gray-blue ovoid nests, multiple gray-blue globules, maple leaf-like areas, and spoke-wheel areas (Behera et al., 2021; Menzies et al., 2000), did not dominate the dermatoscopic features. A less recognized dermatoscopic feature, the blue-white veil, was observed in some of our patients and was significantly associated with non-ulcerated BCC. In one study, short fine telangiectasias, leaf-like areas, spoke-wheel areas, small erosions, and concentric structures were significantly linked only to BCC's superficial variant (Suppa et al., 2015).
Other rare findings, such as milium-type cysts and pseudo-follicular openings, were exclusively noted in patients with BCC, showing no statistical differences. Among the six positive characteristics of Menzies’ algorithm (arborizing telangiectasias, ulceration, blue-gray ovoid nests, blue-gray globules, leaf-like areas, and spoke-wheel areas) (Behera et al., 2021), the presence of ulcers was significantly associated, particularly in patients with a longer duration of lesions at the time of diagnosis.
Despite heavily pigmented structures, we observed a high occurrence of vascular patterns. An inverse relationship has been reported between the degree of pigmentation in BCC and the presence of any vascular structure (Suppa et al., 2015). Consistent with previous findings, at least one vascular pattern was noted in all lesions. In contrast to Arpaia et al. ‘s work, in which the arborizing pattern in the ulcerated portion was associated with a correct diagnosis (Arpaia et al., 2017), our sample had no prevalent vascular pattern. The polymorphic vessel patterns were described only in the BCC. The distribution of specific NMSC-associated patterns did not differ between BCCs and SCCs. Except for the melanin-dotted pigment presence and the thick reticular lines, both tumors displayed roughly the same associations with single dermatoscopic criteria.
Conclusions
Several research groups have studied the use of dermatoscopy to enhance the detection and differentiation of skin cancer. However, aside from melanoma, there are no well-defined algorithms for distinguishing between the specific types of NMSC, particularly in patients with skin phototypes III and IV. Our study did not confirm the effectiveness of the NMSC-associated dermatoscopy criteria in differentiating the two most common clinicopathologic tumor types. However, certain factors like pigmentation and linear vessels seem more prevalent in nodular BCCs. Generally, BCCs exhibit polymorphic patterns of blood vessels, while SCCs tend to be monomorphic. Nonetheless, it’s important to note that our sample included a limited number of patients with SCC.
Limitations
This study's limitations include its cross-sectional nature and small sample size; further controlled studies are needed to confirm our preliminary results.
References
- Arpaia, N., Filoni, A., Bonamonte, D., Giudice, G., Fanelli, M., & Vestita, M. (2017). Vascular Patterns in Cutaneous Ulcerated Basal Cell Carcinoma: A Retrospective Blinded Study Including Dermoscopy. Acta Dermato Venereologica, 97(5), 612–616. [CrossRef]
- Behera, B., Kumari, R., Thappa, D. M., Gochhait, D., Srinivas, B. H., & Ayyanar, P. (2021). Dermoscopic features of basal cell carcinoma in skin of color: A retrospective cross-sectional study from Puducherry, South India. Indian Journal of Dermatology, Venereology and Leprology, 89, 254. [CrossRef]
- Bhattacharya, B., Chauhan, D., Singh, A. K., & Chatterjee, M. (2021). Melanin Based Classification of Skin Types and Their Susceptibility to UV-Induced Cancer. In Skin Cancer: Pathogenesis and Diagnosis (pp. 41–67). Springer Singapore. [CrossRef]
- Bolick, N. L., Huang, L., Trepanowski, N., & Hartman, R. I. (2022). Sun protective behaviors in sun-sensitive individuals: a cross-sectional study examining for ethnic and racial differences. Archives of Dermatological Research, 315(4), 1023–1027. [CrossRef]
- Cabaço, L. C., Tomás, A., Pojo, M., & Barral, D. C. (2022). The Dark Side of Melanin Secretion in Cutaneous Melanoma Aggressiveness. Frontiers in Oncology, 12. [CrossRef]
- Ciążyńska, M., Kamińska-Winciorek, G., Lange, D., Lewandowski, B., Reich, A., Sławińska, M., Pabianek, M., Szczepaniak, K., Hankiewicz, A., Ułańska, M., Morawiec, J., Błasińska-Morawiec, M., Morawiec, Z., Piekarski, J., Nejc, D., Brodowski, R., Zaryczańska, A., Sobjanek, M., Nowicki, R. J., … Lesiak, A. (2021). The incidence and clinical analysis of non-melanoma skin cancer. Scientific Reports, 11(1), 4337. [CrossRef]
- Farré, X., Blay, N., Cortés, B., Carreras, A., Iraola-Guzmán, S., & de Cid, R. (2023). Skin Phototype and Disease: A Comprehensive Genetic Approach to Pigmentary Traits Pleiotropy Using PRS in the GCAT Cohort. Genes, 14(1), 149. [CrossRef]
- Hu, W., Fang, L., Ni, R., Zhang, H., & Pan, G. (2022). Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer, 22(1), 836. [CrossRef]
- Manganelli, M., Guida, S., Ferretta, A., Pellacani, G., Porcelli, L., Azzariti, A., & Guida, G. (2021). Behind the Scene: Exploiting MC1R in Skin Cancer Risk and Prevention. Genes, 12(7), 1093. [CrossRef]
- Martín, J. M., Bella-Navarro, R., & Jordá, E. (2012). Vascular Patterns in Dermoscopy. Actas Dermo-Sifiliográficas (English Edition), 103(5), 357–375. [CrossRef]
- Menzies, S. W., Westerhoff, K., Rabinovitz, H., Kopf, A. W., McCarthy, W. H., & Katz, B. (2000). Surface Microscopy of Pigmented Basal Cell Carcinoma. Archives of Dermatology, 136(8). [CrossRef]
- Rangel-Villalobos, H., Muñoz-Valle, J. F., González-Martín, A., Gorostiza, A., Magaña, M. T., & Páez-Riberos, L. A. (2008). Genetic admixture, relatedness, and structure patterns among Mexican populations revealed by the Y-chromosome. American Journal of Physical Anthropology, 135(4), 448–461. [CrossRef]
- Roky, A. H., Islam, M. M., Ahasan, A. M. F., Mostaq, M. S., Mahmud, M. Z., Amin, M. N., & Mahmud, M. A. (2024). Overview of skin cancer types and prevalence rates across continents. Cancer Pathogenesis and Therapy. [CrossRef]
- Savoye, I., Olsen, C. M., Whiteman, D. C., Bijon, A., Wald, L., Dartois, L., Clavel-Chapelon, F., Boutron-Ruault, M.-C., & Kvaskoff, M. (2018). Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: the E3N-SunExp Study. Journal of Epidemiology, 28(1), 27–33. [CrossRef]
- Sławińska, M., Żółkiewicz, J., Behera, B., Ding, D. D., Lallas, A., Chauhan, P., Khare, S., Enechukwu, N. A., Akay, B. N., Ankad, B. S., Bhat, Y. J., Jha, A. K., Kaliyadan, F., Kelati, A., Neema, S., Parmar, N. V, Stein, J., Usatine, R. P., Vinay, K., … Errichetti, E. (2023). Dermoscopy of Inflammatory Dermatoses (Inflammoscopy) in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatology Practical & Conceptual, e2023297S. [CrossRef]
- Sol, S., Boncimino, F., Todorova, K., Waszyn, S. E., & Mandinova, A. (2024). Therapeutic Approaches for Non-Melanoma Skin Cancer: Standard of Care and Emerging Modalities. International Journal of Molecular Sciences, 25(13), 7056. [CrossRef]
- Suppa, M., Micantonio, T., Di Stefani, A., Soyer, H. P., Chimenti, S., Fargnoli, M. C., & Peris, K. (2015). Dermoscopic variability of basal cell carcinoma according to clinical type and anatomic location. Journal of the European Academy of Dermatology and Venereology, 29(9), 1732–1741. [CrossRef]
- Wojtowicz, I., & Żychowska, M. (2024). Application of Ultraviolet-Enhanced Fluorescence Dermoscopy in Basal Cell Carcinoma. Cancers, 16(15), 2685. [CrossRef]
- Zambrano-Román, M., Padilla-Gutiérrez, J. R., Valle, Y., Muñoz-Valle, J. F., & Valdés-Alvarado, E. (2022). Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers, 14(10), 2371. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).