1. Introduction
Intracranial dermoid cysts (IDC) are rare, benign, slow-growing congenital lesions originating from ectodermal elements during neural tube closure, typically between the 5th and 6th weeks of fetal development [
1]. By definition, intracranial dermoid cysts are derived from ectodermal tissue. Histologically, dermoids, unlike epidermoids, not only contain squamous epithelium but may also contain hair follicles, stratified squamous epithelium, apocrine, eccrine, and sebaceous glands [
2]. On the contrary, teratomas are neoplastic structures originating from aberrant embryonic germ cells, typically composed of tissues derived from multiple germ layers [
2]. IDC differential diagnosis includes epidermoid cyst, lipoma, teratoma, craniopharyngioma, etc
. Epidermoids are typically located off the midline, while dermoids are generally found in or near the midline.
According to the available literature, IDC accounts for approximately 0.5% of all primary intracranial tumours [
3]. Intracranial dermoid cyst ruptures account for approximately 0.18% of all surgically treated CNS tumours, based on data from a 12-year retrospective review at the University of Utah School of Medicine [
4]. Ruptures are often described in adults, mostly at around 30–60 years of age, and rarely in children, mostly under 10 years old. Men are slightly more affected than women [
5].
On a non-enhanced computed tomography (NECT), IDC typically are noted as hypodense lesions with fat-density material [
1]. In brain magnetic resonance imaging (MRI) studies, dermoids are typically hyperintense lesions seen on T1-weighted images and hyper- to hypointense structures on T2-weighted images [
1]. Published studies so far show that IDC can develop in various locations, and the most common sites include midline sellar and suprasellar, parasellar, posterior fossa, and frontonasal regions. If the size of the cyst affects the surrounding tissues, it may cause various neurological symptoms, such as headaches, seizures, numbness, weakness, visual impairment, neck stiffness, and facial pain. On the contrary, the rupture of IDC is a rare complication that may lead to severe outcomes, such as aseptic meningitis caused by the leakage of cyst contents into the subarachnoid space. Intracranial dermoid cysts are usually well-defined, lobulated midline masses.
2. Case Presentation
A thirty-two-year-old Caucasian male presented to a tertiary university hospital due to recurrent headache and paroxysmal visual impairment during the last twelve months. The MRI brain scan revealed a likely benign intracranial dermoid or epidermoid cyst affecting the optic nerve. Initially, the MRI results were unclear and did not distinguish between the two. The patient was consulted by a neurosurgeon, and a planned neurosurgical treatment was scheduled. The patient had no prior documented medical conditions.
A month later, the patient was hospitalized for planned surgery. During this time, the patient claimed his headache and vision disturbances had gotten worse. The patient’s overall health condition was stable. Neurologically, Glasgow Coma Scale (GCS) was 15, the patient was alert. Pupils were symmetrical, reactive to light. No paresis was noted. Deep tendon reflexes were symmetrical, no pathological reflexes were seen. The patient did not demonstrate any coordination difficulties. No sensory impairment. The gait was stable. Meningeal signs were negative.
An MRI brain scan was performed on an outpatient basis. MRI revealed most likely a ruptured dermoid cyst located in the anterior fossa, with mass effect on the optic chiasm, prechiasmatic part (
Figure 1 and
Figure 2). Dermoid cyst dimensions: 4.6 x 4.0 x 2.5 cm (AP x LL x CC) (
Figure 1). Susceptibility weighted imaging (SWI) sequence showed hypointensity with blooming artifact (
Figure 3). The radiologist initially considered a diagnosis of dermoid or epidermoid cyst based on what was seen in the diffusion-weighted imaging (DWI) sequence and apparent diffusion coefficient (ADC) maps, which was later ruled out following the histology results (
Figure 4). The patient underwent a subtotal cyst surgical resection via right-side osteoplastic craniotomy, and histopathological analysis confirmed the diagnosis. The postoperative period was uneventful. Histological examination showed a cyst containing fibrous tissue, multiple squamous epithelium with hyperkeratosis, subepithelial sebaceous glands, hair follicles, focal hemorrhage, and focal lymphocytic infiltration. The morphological appearance corresponded to a dermoid cyst (
Figure 6).
The patient was discharged from the hospital without any complaints. The patient’s overall health status was satisfactory, neurologically GCS was 15 points, no focal neurological deficit was seen. The surgical wound healed primarily. No recurrence and dermoid progression was noted on follow-up MRI imaging at 4 months, one year, and 2 years. MRI follow-up, performed 1 year after surgery, showed residual tissue of the tumour without volume increase, residual calcifications were also seen along the suprasellar region on the left side. Many punctate fat-containing lesions both along the ventricle wall and subarachnoid, similar to the previous MR examination (
Figure 5). Scarring changes, with encephalomalacia more in the right frontal lobe in the basal medial parts, extending more at the level of the gyrus recti, with minimal blood products. The optic nerve was not scarred, and no abnormal tissue was found around the optic nerve following neurosurgical treatment. It is important to monitor for any signs of optic nerve atrophy during follow-up MRI (
Figure 5).
During follow-up four months after surgery, the patient reported a loss of smell and altered taste. Additionally, two seizure episodes occurred, most likely provoked due to ethanol intoxication. After the seizures, an EEG was performed to rule out epileptic activity. The EEG revealed focal changes in the right frontal area - a possible epileptiform focus. Follow-up MRI DWI showed no indications of possible ictal injury.
Figure 1.
A: MRI of the brain (T2-FLAIR axial sequence) showed a slightly hyperintense lesion, most likely a dermoid cyst, located in the basal dorsal anterior fossa. The cyst extended into the suprasellar cisterns, positioned above the chiasma opticum and the prechiasmal part of the optic nerve. It was situated under the anterior cerebral arteries and caused compression and displacement of the anterior parts of the third ventricle. Despite this, the foramen of Monro remained open. Additionally, there was an expansion of the lateral and third ventricles. B: MRI of the brain (T2W-TSE axial sequence) showed an intracranial cyst located in the anterior fossa. The lateral ventricles were dilated with the temporal horns measuring up to 8.5 mm, indicative of hydrocephalus. The likely cause of hydrocephalus development was a combination of the ruptured dermoid cyst and its intraventricular spread, exerting mass effect on the third ventricle and aqueductus cerebri. TV – third ventricle, * – intracranial dermoid cyst.
Figure 1.
A: MRI of the brain (T2-FLAIR axial sequence) showed a slightly hyperintense lesion, most likely a dermoid cyst, located in the basal dorsal anterior fossa. The cyst extended into the suprasellar cisterns, positioned above the chiasma opticum and the prechiasmal part of the optic nerve. It was situated under the anterior cerebral arteries and caused compression and displacement of the anterior parts of the third ventricle. Despite this, the foramen of Monro remained open. Additionally, there was an expansion of the lateral and third ventricles. B: MRI of the brain (T2W-TSE axial sequence) showed an intracranial cyst located in the anterior fossa. The lateral ventricles were dilated with the temporal horns measuring up to 8.5 mm, indicative of hydrocephalus. The likely cause of hydrocephalus development was a combination of the ruptured dermoid cyst and its intraventricular spread, exerting mass effect on the third ventricle and aqueductus cerebri. TV – third ventricle, * – intracranial dermoid cyst.
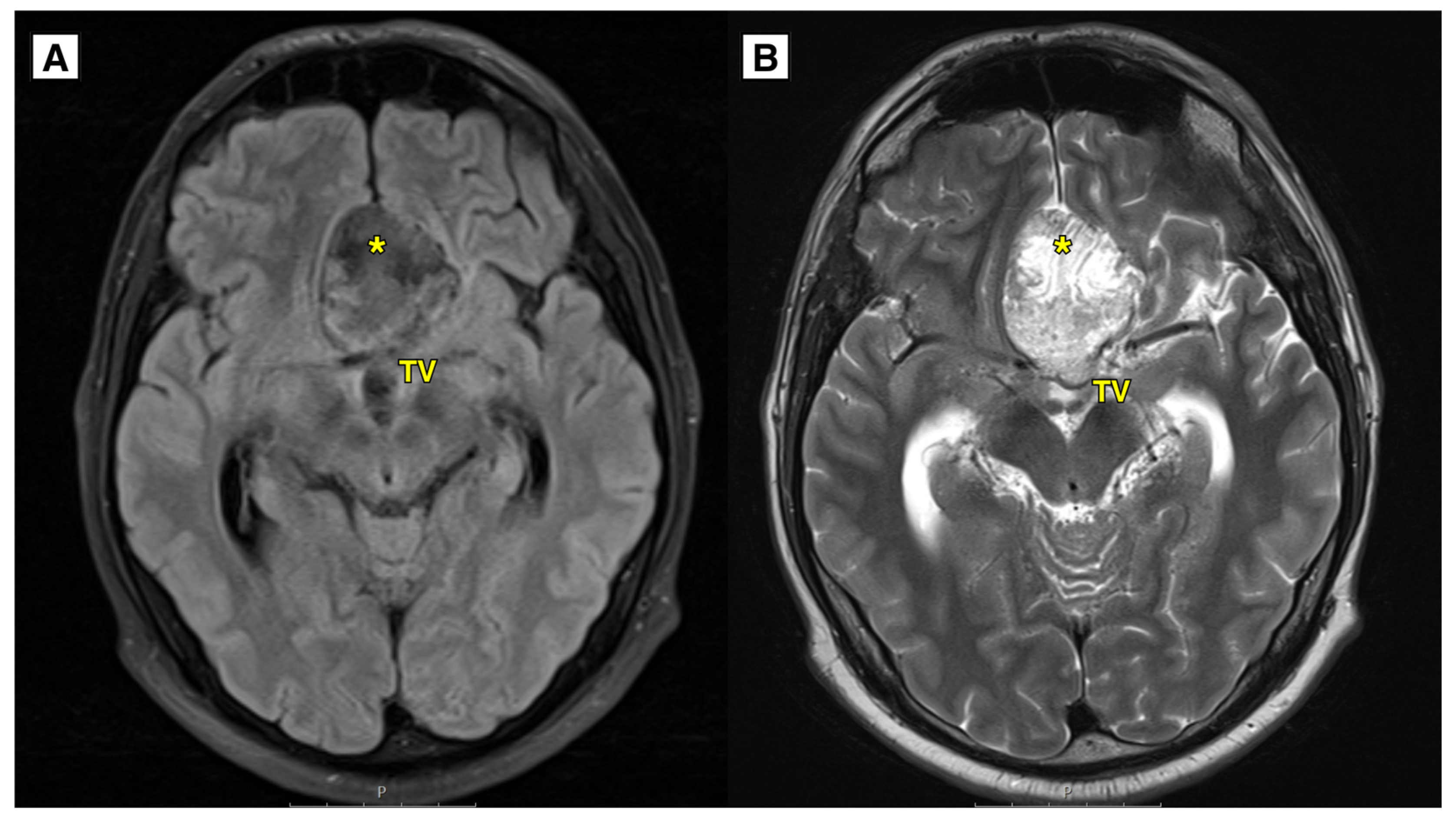
Figure 2.
A: MRI of the brain T1W sag. The intracranial dermoid cyst located in the anterior fossa with hyperintense fat-containing lesions (small arrows), and extracellular methemoglobin containing hemorrhage (arrowhead), representing a small subacute hemorrhage due to cyst rupture.. B: MRI of the brain T1W ax. sequence showing the intracranial dermoid cyst located in the anterior fossa with mass effect on the chiasma opticum. Dermoid cyst rupture with massive subarachnoid fat inclusions of various sizes in the frontal lobes, cisterns, occipital, temporal lobes, and upper parts of the cerebellar hemispheres. CC – corpus callosum, CO – chiasma opticum, * – intracranial dermoid cyst, Arrows – fat-dissemination lesions, Arrowhead – extracellular methemoglobin containing subacute hemorrhage.
Figure 2.
A: MRI of the brain T1W sag. The intracranial dermoid cyst located in the anterior fossa with hyperintense fat-containing lesions (small arrows), and extracellular methemoglobin containing hemorrhage (arrowhead), representing a small subacute hemorrhage due to cyst rupture.. B: MRI of the brain T1W ax. sequence showing the intracranial dermoid cyst located in the anterior fossa with mass effect on the chiasma opticum. Dermoid cyst rupture with massive subarachnoid fat inclusions of various sizes in the frontal lobes, cisterns, occipital, temporal lobes, and upper parts of the cerebellar hemispheres. CC – corpus callosum, CO – chiasma opticum, * – intracranial dermoid cyst, Arrows – fat-dissemination lesions, Arrowhead – extracellular methemoglobin containing subacute hemorrhage.
Figure 3.
A: MRI of the brain SWI Susceptibility weighted imaging sequence showing the intracranial dermoid cyst located in the anterior fossa with fat-containing lesions - reveals blooming artifact (yellow arrows). B: MRI T1W post- contrast axial sequence showing leptomeningeal enhancement around the uncus most likely due to chemical meningitis (arrowhead). Arrows – punctate fat-containing lesions, * – intracranial dermoid cyst.
Figure 3.
A: MRI of the brain SWI Susceptibility weighted imaging sequence showing the intracranial dermoid cyst located in the anterior fossa with fat-containing lesions - reveals blooming artifact (yellow arrows). B: MRI T1W post- contrast axial sequence showing leptomeningeal enhancement around the uncus most likely due to chemical meningitis (arrowhead). Arrows – punctate fat-containing lesions, * – intracranial dermoid cyst.
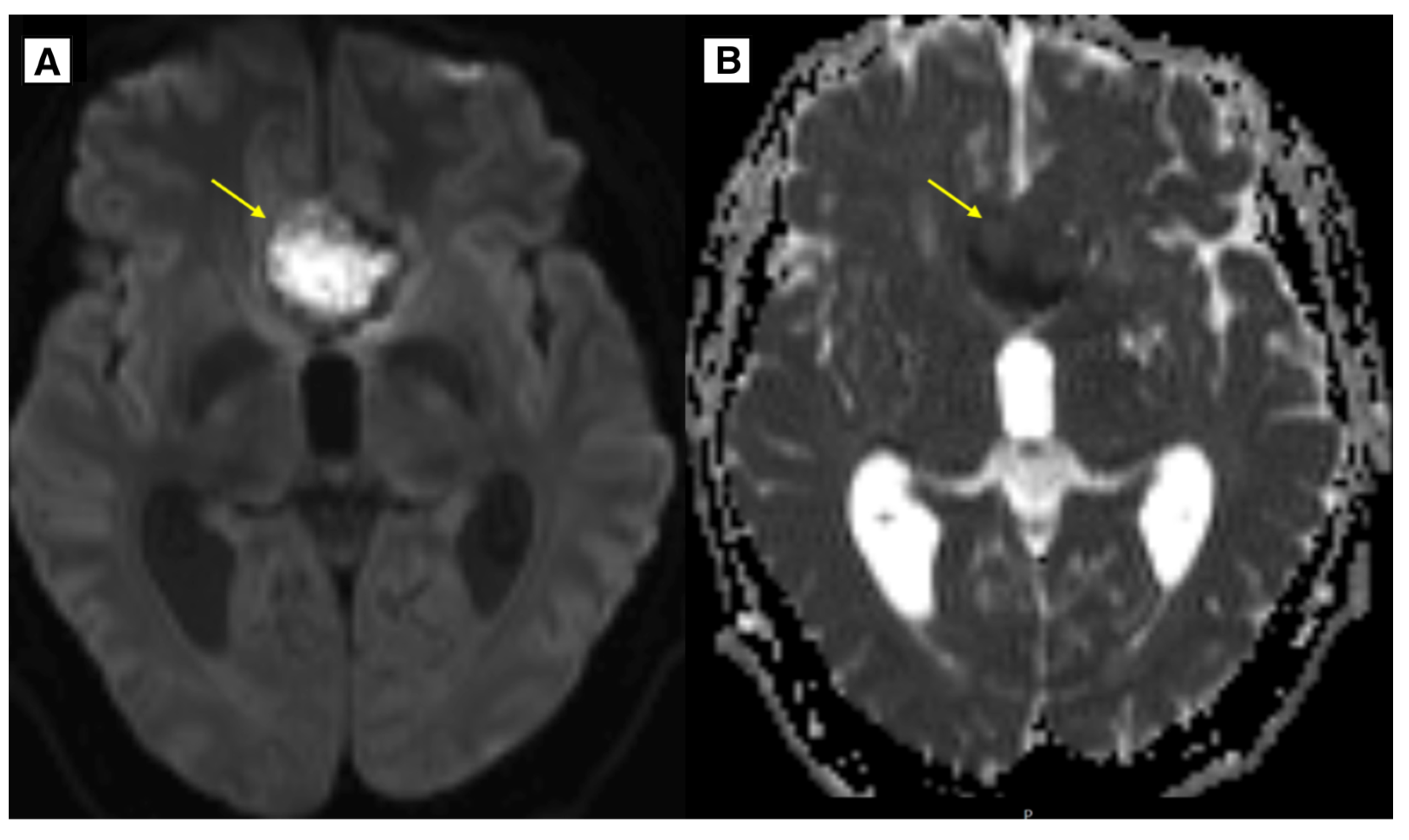
Figure 4.
In the image, A (DWI sequence) and B (ADC map), there is a lesion located in the anterior cranial fossa along the midline, demonstrating slight diffusion restriction and minimally decreased ADC values. Preoperatively, this lesion mimicked an epidermoid cyst; however, histology confirmed it to be a dermoid cyst.
Figure 4.
In the image, A (DWI sequence) and B (ADC map), there is a lesion located in the anterior cranial fossa along the midline, demonstrating slight diffusion restriction and minimally decreased ADC values. Preoperatively, this lesion mimicked an epidermoid cyst; however, histology confirmed it to be a dermoid cyst.
The observed signal characteristics could be explained by the lesion being keratin-rich, which can cause diffusion restriction. Additionally, it is hypothesized that the fatty content of the dermoid cyst was less dense, contributing to the atypical imaging appearance.
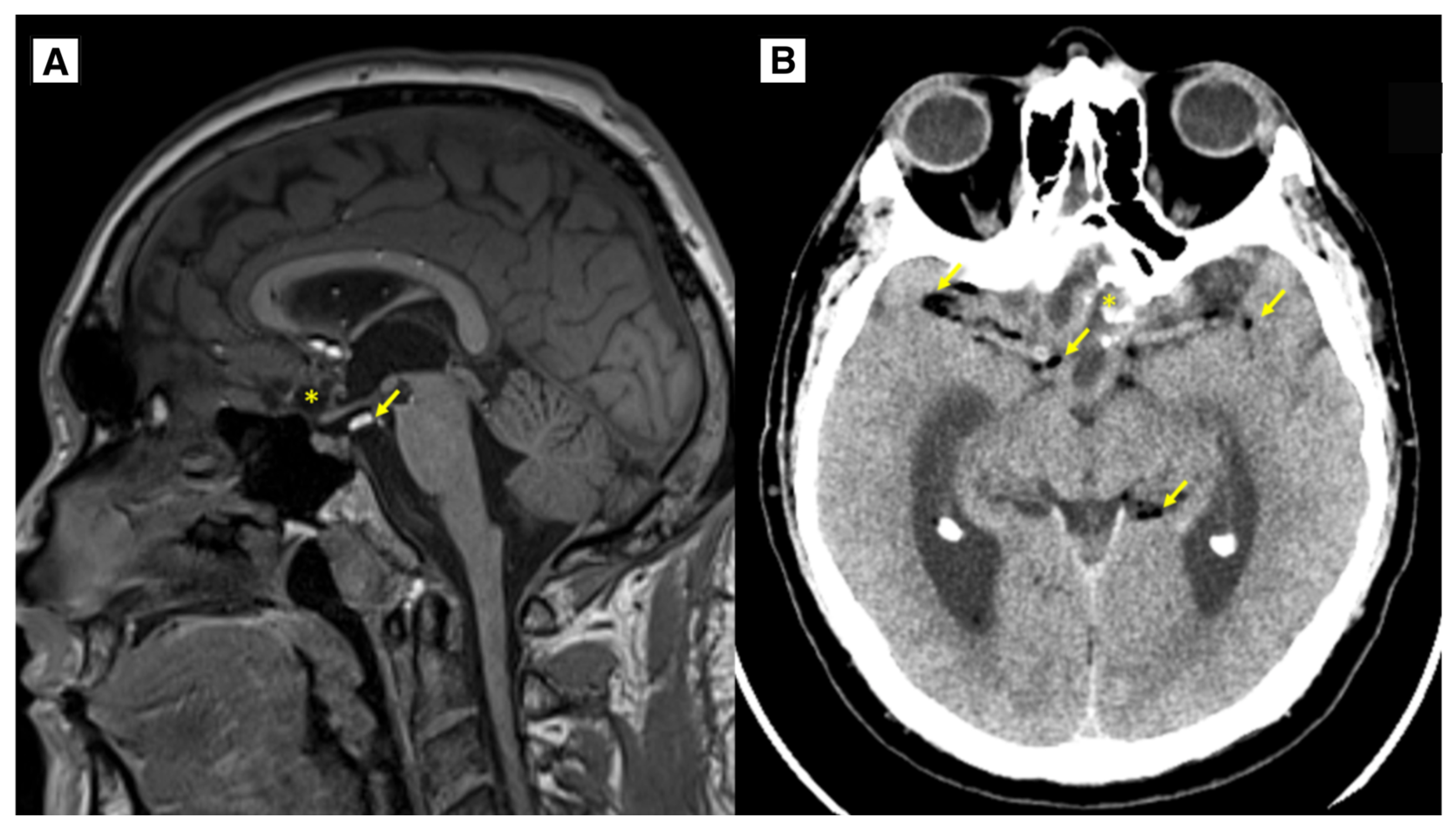
Figure 5.
Follow up. A: MRI of the brain T1W sagittal 1-year follow-up showing the dermoid cyst has been subtotally resected (*), there are very small fat lesions (yellow arrows), as well as residual calcifications of the dermoid along the left side of the post-operative lobe, as in the previous MR examination, small scarring changes with encephalomalacia in the dorsal parts of the gyrus recti of both frontal lobes. No data on progressions of dermoid cyst. B: CT of the brain 2-year follow-up showing intracranial dermoid cyst has been largely resected (*), there are very small fat lesions (yellow arrows). No data on progression of dermoid cyst. * – intracranial dermoid cyst, Arrows – punctate fat-containing lesions.
Figure 5.
Follow up. A: MRI of the brain T1W sagittal 1-year follow-up showing the dermoid cyst has been subtotally resected (*), there are very small fat lesions (yellow arrows), as well as residual calcifications of the dermoid along the left side of the post-operative lobe, as in the previous MR examination, small scarring changes with encephalomalacia in the dorsal parts of the gyrus recti of both frontal lobes. No data on progressions of dermoid cyst. B: CT of the brain 2-year follow-up showing intracranial dermoid cyst has been largely resected (*), there are very small fat lesions (yellow arrows). No data on progression of dermoid cyst. * – intracranial dermoid cyst, Arrows – punctate fat-containing lesions.
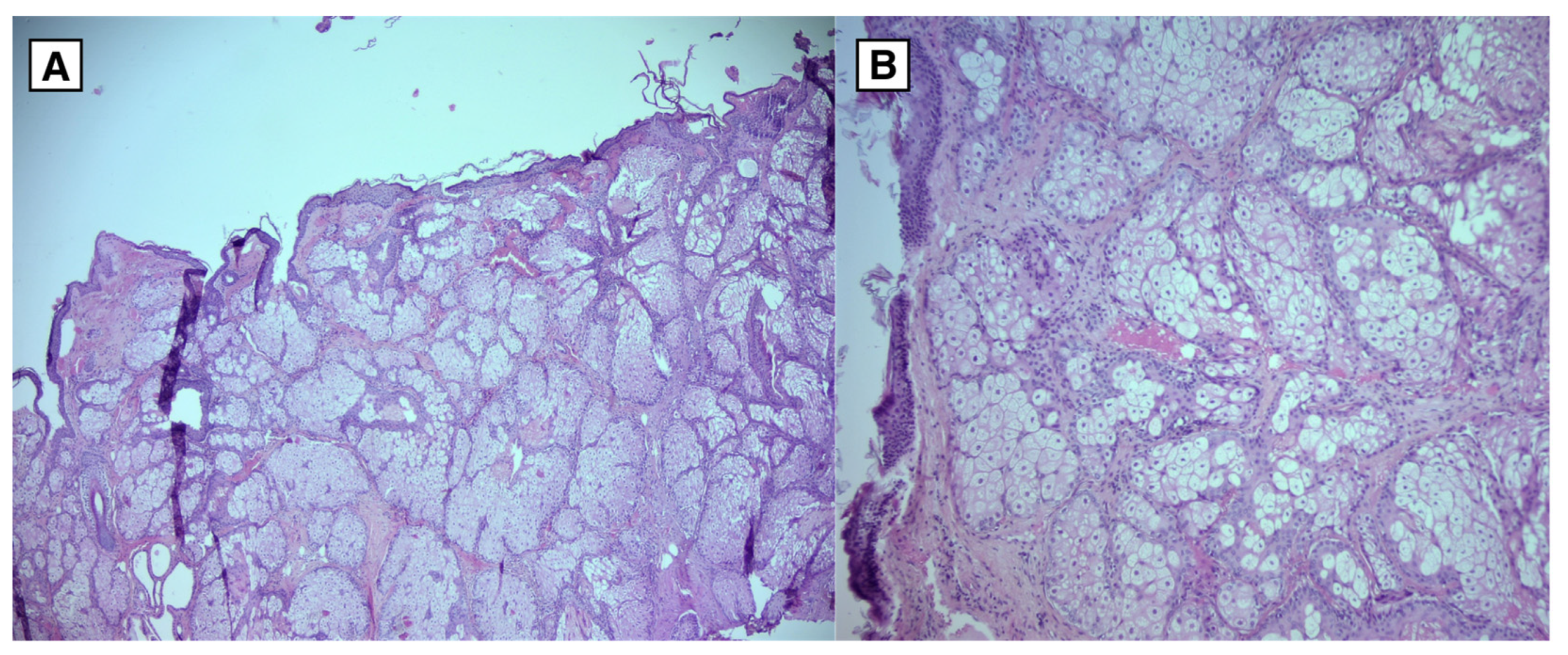
Figure 6.
Hematoxylin and Eosin stain from the dermoid cyst. Tissue samples lined by squamous epithelium with keratinization. Subepithelially abundant sebaceous gland foci and hair follicles. Tissue lacks cytological atypia. A: Microscopic magnification 4x (HPF). B: Microscopic magnification 10x (HPF).
Figure 6.
Hematoxylin and Eosin stain from the dermoid cyst. Tissue samples lined by squamous epithelium with keratinization. Subepithelially abundant sebaceous gland foci and hair follicles. Tissue lacks cytological atypia. A: Microscopic magnification 4x (HPF). B: Microscopic magnification 10x (HPF).
3. Discussion
Intracranial dermoid cysts, also called dermoid tumours, are congenital benign cystic formations. The incidence of intracranial dermoid cysts is reported to be 0.04–0.7% of all intracranial tumours [
3].
Intracranial dermoid cyst ruptures account for approximately 0.18% of all surgically treated CNS tumours, based on data from a 12-year study in a major clinic [
6]. Ruptures are most often described in adults (commonly 30–60 years) and rarely in children (commonly under 10 years). Men are slightly more affected than women [
5]. The age and gender of the patient align with these epidemiological trends.
Ruptures may occur spontaneously, during intracranial surgery, or they may present after trauma [
7]. Ruptures of brain dermoid cysts are uncommon, therefore, little is known about their pathogenetic mechanisms. Some authors suggest rapid cyst growth due to age-related hormonal changes or rapid expansion of small cysts against the resistance of their capsule [
8,
9]. Others dispute this theory, noting that the majority of ruptures happen between the ages of 30 and 50 when hormonal changes are no longer significant [
8,
9].
Clinical picture is variable due to the location of the cyst rupture. The most commonly noted symptoms include sudden, excruciating, or prolonged progressive headaches, mostly in younger patients (31.8%) [
10,
11,
12,
13,
14,
15,
16,
17], generalized or focal seizures in older individuals (29.5%) [
5,
15,
18,
19], impaired consciousness or awareness [
11,
20,
21], and focal symptoms of CNS lesions (16.3%) [
15]. In this case, the main complaints of the patient included a prolonged headache and vision impairment, which were followed by gradual improvement.
More than 30 similar publications on intracranial dermoid cysts have been published so far. Out of those, cases of dermoid cyst ruptures are described in selected publications. Illustrative materials and detailed radiological findings are offered even more seldom. In 16 of these publications, cases of anterior cranial fossa dermoid cyst ruptures with fat dissemination into the subarachnoid space have been described specifically. In these reports, the patient group enrolled 16 people, where 10 of them were men, and the age ranged between 21 to 60 years, with the highest incidence of these cyst ruptures in the 30–50–year–olds. According to the statistics, supratentorially located dermoid cysts are the most frequently reported, with anterior cranial fossa cysts accounting for 29.6% of cases [
4]. They are often located parasagittally on the basal portion of the anterior cranial fossa [
14].
When fat tissue enters the subarachnoid and ventricular systems, it results in obstruction and hydrocephalus. Some authors also highlight the mass effect of the cyst as a compressive factor [
22]. Liu J.K. et al. observed the development of hydrocephalus in 29% [
4] of all cases of brain dermoid cyst ruptures. Case reports indicate the formation of hydrocephalus both in the early days of the illness and in the late postoperative period [
20,
22,
23].
The fat-containing cyst materials directly irritate cerebral blood vessels, resulting in vasospasms, which can act as a trigger for transient brain ischemia or cerebral infarction [
6,
16].
Considering the close association of the dermoid cyst with the optic nerve and the anterior cerebral artery, a subtotal cyst resection was performed in this clinical case. Authors describe recurrences of dermoid cysts after subtotal resection as very rare [
20,
21,
22], which is also confirmed by this clinical case – over a two–year period, no recurrence of the dermoid cyst was observed.
In this case, the patient underwent a subtotal resection of the cyst. Among the literature, such a procedure is performed extremely rarely [
20,
21,
22]. This case report not only provides highly illustrative material of intracranial dermoid cyst rupture but also raises the knowledge of intracranial dermoid cyst rupture, adding to the very limited number of case reports available.
All literature sources indicate computed tomography (CT) and magnetic resonance imaging (MRI) as diagnostic methods of choice in cases of dermoid cyst ruptures in the anterior cranial fossa. Imaging characteristics relate to specific signal intensities of the cyst contents, lack of edema around the lesion site as well as clear visibility of defined cyst borders [
24]. The best diagnostic indicators suggesting dermoid cyst rupture are the dissemination of fat droplets in the subarachnoid space, brain sulci, and ventricles [
6].
Brain CT shows low-density lesions corresponding to the fatty content of the cyst fluid. Hypodense foci in the subarachnoid space and ventricular system indicate the dissemination of fat droplets after cyst rupture [
6,
22]. In CT examinations, the density of fat corresponds to negative Hounsfield units. However, the density value may be higher than typical for fat due to the influence of the dermoid cyst's contents [
16]. Clinical cases describe Hounsfield unit values of 87 and 97 at the site of the lesion [
10,
14]. When lipid content dominates at the lesion site, CT scans show homogeneous hypodense changes, while heterogeneity is determined by cyst inclusions such as hair derivatives, calcifications, and epidermal remnants [
22].
According to literature, peripheral calcification of the cyst capsule is detected in 20% of intracranial dermoid cysts during CT scans [
25]. The mass effect of the cyst is typically minor compared to the size itself [
24]. In the clinical cases described in available literature sources, contrast was not used during CT examinations.
In magnetic resonance imaging (MRI) examinations of intracranial dermoid cyst ruptures, a characteristic hyperintense lesion is observed on T1-weighted images due to fat content, while a heterogeneous lesion appears on T2-weighted images, which is related to various cyst inclusions (e.g., hair, calcifications, nails, and epidermal remnants) [
26,
27,
28,
29]. A high-intensity signal in the subarachnoid space and ventricular system on MRI indicates fat dissemination following dermoid cyst rupture [
22]. T1W1 is the best sequence for detecting fat droplets in the subarachnoid space in cases of cyst rupture. In FLAIR sequences, a ruptured dermoid cyst also appears hyperintense, while GRE sequence reveals blooming artifact in the cyst mass and surrounding area [
10]. The examination is performed with intravenous gadolinium contrast. A signal intensity similar to cerebrospinal fluid can be seen if the inner fat content of the cyst is fairly low [
16].
The blooming artifact is commonly observed at the periphery of fat droplets as a dark, narrow rim around the fat globule [
30]. This phenomenon arises from the interaction of fat and water with the resonance frequency signal, leading to it spreading beyond the boundaries of the structure. The hydrogen protons in fat and water create a resonance frequency difference, which can result in signal intensity loss in these proton pixels due to mutual cancellation. This effect produces the characteristic appearance of a dark rim around the fat globule and, in phase images, may generate significant phase shift contrasts. The role of the susceptibility gradient at the fat-tissue interface in this process is also noted [
31,
32].
In this case, typical radiological features were observed. As well as a blooming artifact indicating a fat-containing lesion in the subarachnoid space and ventricular system (
Figure 3). These low-signal foci in T2 and SWI sequences must be differentiated from acute subarachnoid hemorrhage or hemosiderin inclusions. To aid in detecting blood products in the brain, SWI sequence is used, as it is much more sensitive to hemosiderin inclusions, resulting in more weighted T2W series [
31,
33]. Residual calcifications of the dermoid may be seen along the front and left side of the postoperative lobe on a follow-up MRI one year post-surgery (
Figure 5).
The results of the MRI sequences revealed not only typical radiological features of dermoid cysts but also findings that need to be differentiated from other diseases. The hyperintensity on DWI with reduced diffusion mimicked an epidermoid cyst (
Figure 4); T2-FLAIR and T1W sequences showed blood deposits in the brain (
Figure 1 and
Figure 2).
In the available English-language literature, there are only few clinical case reports describing findings typical of epidermoid cysts in brain MRI DWI sequences that have been identified in cases of dermoid cysts. Johnson D.G. et al. present a clinical case where DWI examination revealed reduced diffusion [
21]. A high-intensity DWI signal with reduced diffusion has also been reported in the study by Aksoy F.G. et al. [
34]. Furthermore, the literature includes a reference to a similar finding described by Japanese researchers Shinoyama M. et al. [
35]. In this clinical case, the DWI sequence and ADC map demonstrated slight diffusion restriction and a minimally decreased ADC value. Such radiological findings are characteristic of epidermoid cysts. The epithelial cells of cyst walls secrete the protein keratin, which also contributes to the reduction in the diffusion observed on DWI. Dermoid cysts also contain keratin, and if the fat content within the cyst is low, it can mimic the radiologic features characteristic of epidermoid cysts. This can potentially lead to diagnostic errors in the preoperative period. [
34,
36]. Since these findings are rarely discussed, they represent some of the novel contributions of this article.
The keratin-rich nature of the lesion, which can result in diffusion restriction, may account for the observed signal characteristics. Furthermore, the unusual imaging appearance is thought to have been caused by the dermoid cyst's less dense fatty content (
Figure 4).
Histological examination of surgical material confirms the diagnosis, particularly in cases with atypical radiological findings. Histological samples of intracranial dermoid cysts reveal keratinised squamous epithelium containing sebaceous glands, sweat glands, hair follicles, and other ectodermal structures. In contrast, epidermoid cysts have a stratified squamous epithelium with no skin derivatives.
Summarizing the available information about the treatment of a ruptured dermoid cyst in the anterior cranial fossa, surgical cyst resection is the main choice, as well as the use of intravenous glucocorticoids. Removing the capsule and cyst contents is the aim of the procedure [
20]. Complete evacuation of fat droplets from the subarachnoid space and ventricular system, considering their diffuse dissemination across both hemispheres, is usually not possible [
20]. Authors also highlight limitations for total resection of dermoid cysts: proximity of the cyst capsule to surrounding neurovascular structures, which prevents separation of the cyst capsule without causing neurological damage [
20]. In the reviewed clinical cases, only one involved a subtotal resection of a dermoid cyst [
11]. A particularly technically challenging operation is described in the resection of a ruptured dermoid cyst due to the rupture of a cerebral artery aneurysm, where the cyst capsule was separated from the aneurysm's edge [
11].
In cases of chemical meningitis, high-dose glucocorticoids administered intravenously in a hospital setting have been described for treatment and prevention, with continuation of oral administration on an outpatient basis [
22].
Author Contributions
Conceptualization, A.B., K.V., R.T., V.R.K.; methodology, K.V., R.T.; software, A.B., E.V., J.D.; validation, S.S., A.B.; formal analysis, S.S., A.B.; investigation, K.V., R.T., S.S., A.B.; resources, K.V., R.T.; data curation, K.V., R.T., S.S.; writing—original draft preparation, K.V., R.T., S.S., A.B.; writing—review and editing, S.S., A.B.; visualization, V.R.K., A.B., J.D., E.V.; supervision, A.B., S.S., E.V.; project administration, A.B.; funding acquisition, A.B..