1. Introduction
Nematophagous fungi are a specialized group of organisms that naturally prey upon nematodes, offering a sustainable and environmentally friendly alternative to chemical anthelmintics in controlling parasitic nematode populations in livestock [
1,
2,
3]. Infection of livestock with parasitic gastrointestinal nematodes (GIN) is a significant concern in both agricultural and veterinary contexts, potentially leading to poor animal health and substantial economic losses [
4,
5]. These GIN infections can result in significant morbidity and mortality rates, especially in young or immunocompromised animals, thus posing a persistent challenge to livestock farmers globally [
6,
7]. The economic impact of GIN infections extends beyond direct productivity losses, including costs associated with veterinary care, anthelmintic treatments, and pasture management [
1,
8,
9]. Additionally, the contamination of pastures by infective larvae contributes to the reinfection cycle, further exacerbating the problem [
10,
11].
Chemical anthelmintics have been the primary method of controlling these infections; however, the growing issue of drug resistance among GIN populations has necessitated the development of alternative control strategies. Overuse and misuse of chemical anthelmintics have accelerated the development of drug-resistant nematodes, making traditional treatments less effective [
12,
13,
14]. Reports of multidrug-resistant nematodes have become increasingly common, highlighting the urgent need to explore sustainable, non-chemical methods for managing GIN infections. Anthelmintic resistance threatens the sustainability of livestock production, particularly in regions heavily reliant on intensive farming practices [
16,
17,
18]. Consequently, researchers and farmers alike are increasingly seeking alternative methods to control parasitic infections in a manner that aligns with sustainable agricultural practices [
19].
Among the various nematophagous fungi,
Duddingtonia flagrans has garnered significant attention due to its unique ability to produce chlamydospores—thick-walled, environmentally resistant spores that can survive harsh environmental conditions and remain viable in the gastrointestinal tract of livestock [
20,
21,
22,
23]. These spores are excreted in the feces, where they sporulate and form fungal traps that capture and kill nematode larvae in the fecal environment. By reducing the larval population in the pasture,
D. flagrans helps to interrupt the nematode life cycle and reduce reinfection rates in livestock [
24]. This biological control method offers a promising alternative to chemical interventions, helping to manage parasite burdens in a more sustainable and environmentally friendly way. The ability of
D. flagrans to reduce pasture contamination with infective GIN larvae without negatively impacting soil health or beneficial organisms makes it a valuable tool in integrated pest management systems [
25].
Several studies have demonstrated the efficacy of
D. flagrans in controlling nematodes in various livestock species, including cattle, sheep, goats, and horses [
3,
24,
25,
26]. The fungus has shown promise in reducing the need for chemical treatments, improving animal health, and contributing to sustainable livestock management practices [
25,
26,
27,
28,
29,
30]. The benefits of incorporating
D. flagrans into parasite control programs extend beyond direct improvements in animal health to include improved public perception of farming practices.
Despite its potential, there are still challenges in implementing this biocontrol agent on a large scale, including variability in field efficacy due to environmental conditions, the need for optimized delivery systems, cost, and limited awareness among farmers and veterinarians. Environmental factors, such as temperature, humidity, and UV radiation can affect the viability and effectiveness of
D. flagrans spores [
31,
32,
33], necessitating further research to optimize its use in diverse climatic conditions. Additionally, the development of practical and cost-effective delivery methods, such as incorporating fungal spores into feed pellets or minerals, is essential to facilitate the widespread adoption of this biocontrol strategy. Other considerations include ensuring the stability and shelf life of commercial products and determining appropriate dosing regimens to achieve consistent results in varying livestock systems [
34,
35,
36,
37].
This systematic review aims to consolidate existing research on the application of
D. flagrans in controlling GIN in livestock. The review evaluates the effectiveness of this fungal species, explores the various strategies employed for its administration, and discusses potential challenges and limitations. By providing a comprehensive synthesis of the current literature, this review seeks to highlight gaps in knowledge and identify future research directions for the effective use of
D. flagrans in livestock management. Conducting a systematic review using the Kitchenham framework [
35] is essential to ensure a rigorous and transparent process in synthesizing existing studies. The qualitative approach adopted in this review allows for a detailed examination of diverse research findings and the identification of patterns and trends that may not be apparent in individual studies.
A systematic review is particularly valuable in the context of D. flagrans because it addresses the growing body of research in a structured and methodical manner, ensuring that relevant studies are identified, critically appraised, and synthesized to inform practice and policy. The need for such a review is driven by the increasing interest in biological control methods and the challenges posed by drug-resistant nematodes. By systematically reviewing the literature, this study aims to provide a clear and evidence-based understanding of the current state of knowledge, identify areas where further research is needed, and support the development of best practices for integrating D. flagrans into parasite management programs. Ultimately, this review will contribute to the advancement of sustainable livestock production systems, ensuring long-term animal health and productivity while minimizing the environmental impact of parasite control measures.
2. Materials and Methods
This systematic review was conducted following the comprehensive guidelines proposed by Kitchenham and Charters for performing systematic literature reviews in software engineering [
35]. These guidelines were adapted appropriately to meet the specific needs of veterinary parasitology research. The methodology employed involved a series of systematic steps, including defining research questions, developing a robust search strategy, applying clearly defined inclusion and exclusion criteria, and extracting and synthesizing data from relevant studies to address the research questions. By following a structured and transparent approach, this review aims to ensure the reliability and reproducibility of the findings presented.
2.1. Research Questions
The review was guided by three primary research questions aimed at evaluating the application of D. flagrans as a biological control agent for GIN in livestock. These questions were formulated to cover the key aspects of efficacy, application methods, and potential challenges. The specific research questions are as follows:
- o
Efficacy: What is the effectiveness of D. flagrans in controlling GIN infections in livestock?
- o
Application Methods: What are the various strategies employed to administer D. flagrans to livestock?
- o
Challenges and Limitations: What are the potential challenges and limitations associated with the use of D. flagrans in livestock?
These research questions provided a clear framework for guiding the literature search and data synthesis process. They were designed to address both the practical and scientific aspects of using D. flagrans in livestock management.
2.2. Search Strategy
A comprehensive literature search was conducted to ensure that all relevant studies on the use of D. flagrans in controlling GIN were identified and included. The search spanned multiple scientific databases, including PubMed, Web of Science, and Google Scholar. The search covered publications from January 1995 to December 2024, ensuring that the most recent and relevant research was included in the review. The search terms used were carefully chosen to capture a wide range of relevant studies and included combinations of the following keywords:
The search strategy was designed to include various types of studies, such as field trials, laboratory experiments, and reviews that provided data on the efficacy, application methods, and challenges associated with D. flagrans. The objective was to capture a comprehensive range of literature that could address the research questions effectively.
2.3. Inclusion and Exclusion Criteria
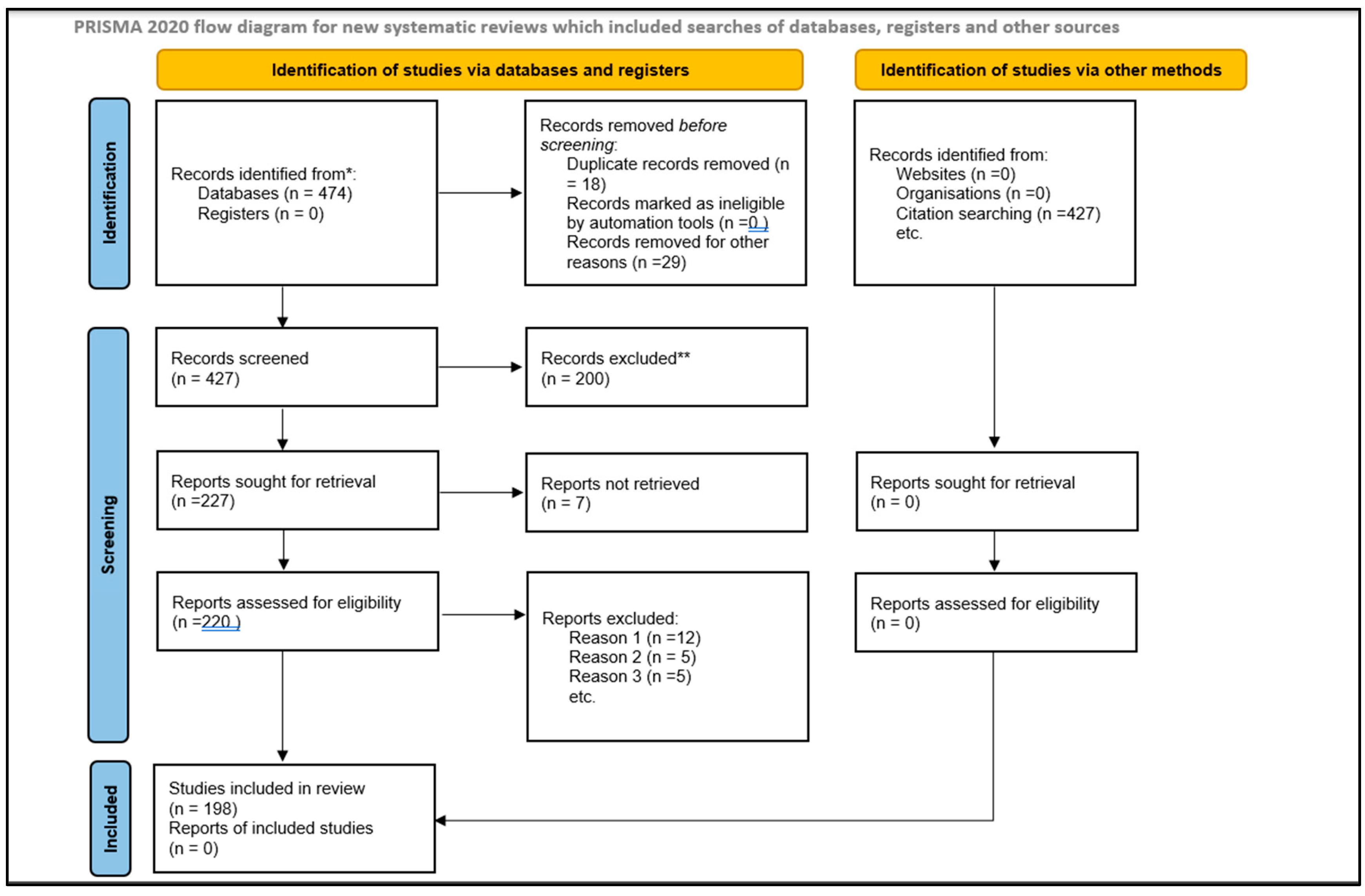
To ensure the relevance and quality of the studies included in the review, a set of inclusion and exclusion criteria was established. These criteria were applied consistently throughout the screening process to filter out irrelevant or low-quality studies.
Inclusion Criteria:
Studies evaluating the efficacy of D. flagrans in controlling GIN in livestock.
Research articles published in peer-reviewed journals.
Studies available in English.
Exclusion Criteria:
By applying these criteria, the review ensured that only high-quality and relevant studies were included in the final synthesis. The focus was on primary research articles that provided empirical data on the use of
D. flagrans in livestock systems. The complete PRISMA flow diagram is provided for reference (
Figure 1) [
36].
3. Data Extraction and Synthesis
Data from the selected studies were extracted using a standardized form to ensure consistency and accuracy. The data extraction process involved capturing key information from each study, including the study design, animal species involved, administration methods of D. flagrans, outcomes measured, and key findings. The standardized form ensured that all relevant aspects of each study were recorded systematically. The specific data points extracted from each study included:
Study Design: Whether the study was a field trial, laboratory experiment, or another type of study.
Animal Species: The species of livestock involved in the study (e.g., cattle, sheep, goats, horses).
Administration Methods: The methods used to administer D. flagrans to livestock (e.g., feed supplementation, direct application).
Outcomes Measured: The specific outcomes measured in the study, such as reduction in nematode egg counts over time, larval mortality rates, and overall efficacy.
Key Findings: The main findings of the study, including efficacy rates and any challenges or limitations encountered.
The extracted data were then synthesized qualitatively to address the research questions posed at the beginning of the review. The synthesis process involved identifying common themes, patterns, and trends across the studies. Particular attention was given to factors influencing the efficacy of D. flagrans, such as environmental conditions, delivery methods, and the duration of the fungal effect in the field. By identifying these factors, the review aimed to provide a comprehensive understanding of the potential role of D. flagrans in sustainable parasite management in livestock systems.
The findings of this systematic review will be presented in subsequent sections, providing a comprehensive overview of the current state of knowledge on D. flagrans and its practical implications for livestock health and management.
4. Results and Discussion
The review of research on D. flagrans as a biological control agent for GIN in livestock presents strong evidence of its efficacy, while also highlighting several challenges and knowledge gaps. The analysis of keyword occurrences, geographical distributions, thematic focus, and sentiment analysis offers valuable insights into how D. flagrans has been studied and perceived across different research contexts.
4.1. Efficacy of Duddingtonia flagrans in Controlling Gastrointestinal Nematodes
The analysis presented in the paper confirms that
D. flagrans is a highly effective biological control agent, with substantial research supporting its ability to reduce GIN in livestock [
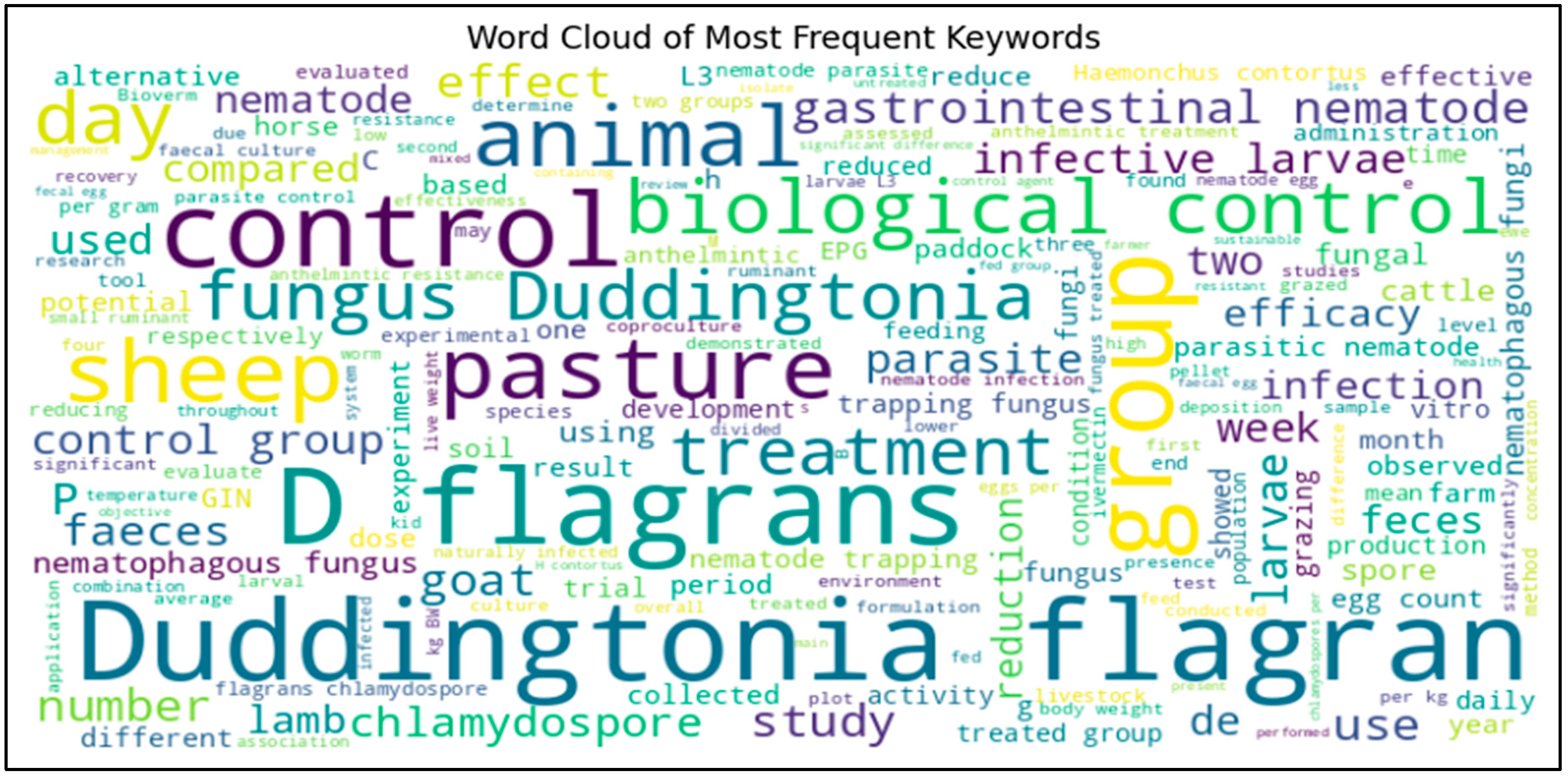
37]. The word cloud analysis highlighted key terms, such as
“Duddingtonia flagrans,” “biological control,” “gastrointestinal nematodes,” and
“pasture management,” reflecting the prominence of this fungus in parasite control research. Its mechanism of action, where fungal spores are ingested by animals, excreted in feces, and then germinate to trap and kill nematode larvae, is well-established. This approach directly disrupts the nematode life cycle at the environmental stage, significantly reducing the number of infective larvae on pasture (
Figure 2). The word cloud analysis provides a visual representation of the most frequently discussed topics, offering further insights into research priorities and gaps. The dominance of terms like
“biological control,” “gastrointestinal nematodes,” “pasture,” and “treatment” reinforces
D. flagrans’ role as a sustainable parasite control agent. The frequent appearance of “fungus,” “chlamydospore,” and “larvae” suggests that much of the research focuses on fungal mechanisms of action, particularly how spores germinate in feces and trap nematode larvae. Words such as “infection,” “reduction,” and “efficacy” indicate that quantitative efficacy assessments remain a central focus, with researchers prioritizing studies that measure GIN reduction. The presence of “resistance,” “climate,” and “sustainability” suggests growing interest in addressing long-term adaptation challenges and environmental viability.
However, one major missing element in the word cloud is the absence of terms related to economic feasibility, cost analysis, and farmer adoption challenges. While research strongly supports D. flagrans’ efficacy as a parasite control measure, few studies evaluate its affordability, accessibility, and ease of implementation in real-world farm settings. This represents a significant barrier to widespread adoption, as economic concerns often outweigh environmental motivations for farmers. Future research should incorporate cost-effectiveness analyses, adoption studies, and economic modeling to determine whether fungal-based control methods can compete with traditional chemical dewormers in commercial livestock production systems.
However, one potential reason for the variability in efficacy reported across different studies is environmental dependency.
Duddingtonia flagrans requires adequate moisture and temperature conditions for optimal sporulation and trapping efficiency. In arid or extreme climatic conditions, fungal germination and hyphal growth may be compromised, leading to lower-than-expected reductions in parasite loads [
37,
38,
39]. This suggests that while laboratory and controlled trials demonstrate strong efficacy, field applications need further validation across diverse agro-ecological zones. Future studies should explore ways to enhance fungal survival under less-than-ideal environmental conditions, such as developing encapsulated spore formulations or integrating fungal spores with soil amendments to improve persistence.
Another major benefit of
D. flagrans is its ability to reduce the need for anthelmintic drugs by offering a non-chemical alternative for controlling parasites. As highlighted in the network co-occurrence analysis (Enhanced Co-occurrence Keyword Analysis), terms such as
“sustainability,” “pasture rotation,” and “parasitic control” frequently appeared alongside
D. flagrans, indicating its role in integrated parasite management (IPM) programs (
Figure 3). By reducing the reliance on chemical dewormers,
D. flagrans provides a sustainable approach that aligns with organic livestock production systems. However, a key question remains: can producers rely on Df to keep re-infection at a minimum to minimize need for dewormers, or should it be used as part of an integrated strategy? The current literature suggests that
D. flagrans works best when combined with strategic anthelmintic use, rotational grazing, and genetic selection for parasite-resistant breeds. This highlights the need for future research on synergistic effects between
D. flagrans and other parasite control measures to develop a comprehensive, multi-pronged parasite management approach. While the efficacy of
D. flagrans is well-documented in both laboratory and field studies, its effectiveness is largely dependent on the method of administration [
40,
41,
42]. The mode of delivery plays a crucial role in ensuring adequate fungal spore ingestion and subsequent pasture contamination reduction. The network co-occurrence analysis (
Figure 3) provides deeper insights into the interrelationships between key research themes and methodologies used to study
D. flagrans. The network analysis identifies
D. flagrans as the primary node, strongly linked to parasitic control, sustainability, and pasture-based livestock management. This underscores its central role in biological pest control research, where it is positioned as a viable alternative to chemical dewormers. Given the increasing focus on sustainable agricultural practices,
D. flagrans is emerging as a core component of environmentally friendly livestock management strategies. Distinct clusters emerge in the network, illustrating the multidimensional nature of
D. flagrans research. One major cluster associates
D. flagrans with biological control, pasture rotation, and sustainable grazing management, suggesting a growing integration of fungal-based nematode control with holistic pasture management practices, emphasizing the need for ecosystem-based solutions rather than stand-alone interventions. Another cluster links
D. flagrans with natural enemies, nematophagous fungi, and predatory fungi, highlighting the interest in understanding multi-organism interactions in controlling nematode populations. This signals an opportunity for further studies on combining different fungal strains or microorganisms to enhance efficacy.
Thus, the next section evaluates the various strategies employed to administer D. flagrans, examining their practical feasibility, advantages, and limitations.
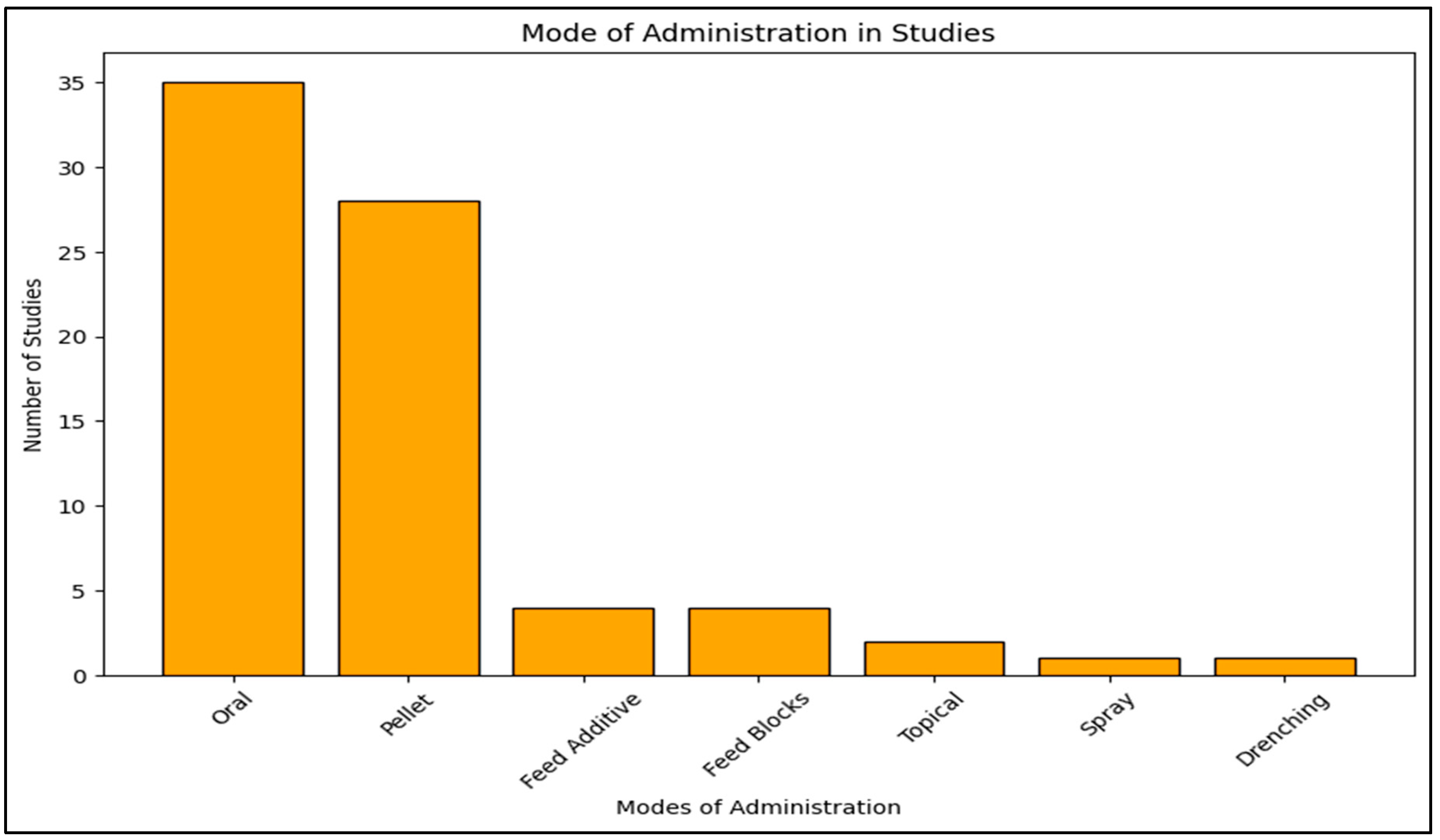
2. Application Methods of Duddingtonia flagrans and Their Effectiveness
The effectiveness of
D. flagrans is highly dependent on its mode of administration, ensuring that sufficient fungal spores reach livestock feces to exert control over nematode larvae [
42,
43]. The Mode of Administration Studies analysis revealed that oral administration, particularly through pellet supplementation and direct feed incorporation, is the most common delivery method. These approaches ensure that animals ingest a consistent number of spores, which are then distributed into feces where they can germinate and kill nematode larvae. The preference for oral administration can be attributed to its ease of implementation in farm settings and its ability to deliver consistent fungal loads.
However, while oral dosing offers practical advantages, it also presents long-term challenges. Unlike conventional anthelmintics, which often provide immediate parasite suppression,
D. flagrans requires prolonged administration over weeks or months, a factor that may deter adoption among farmers managing large herds. Additionally, the lack of immediate parasite reduction compared to chemical dewormers could be a major barrier, particularly for farmers seeking rapid results [
20,
23,
42,
43].
2.1. Bridging the Gap Between Lab Studies and Field Applications
Despite promising laboratory findings, there remains a disparity between experimental trials and real-world field applications. Several key methodological gaps need to be addressed to enhance the applicability and scalability of D. flagrans as a sustainable parasite control strategy.
One of the most critical gaps is the underutilization of computational modeling and simulation techniques. While most studies rely on direct observation and experimental trials, predictive modeling remains largely unexplored. Computational approaches could provide valuable insights into fungal persistence, pasture contamination reduction, and long-term sustainability, allowing researchers to simulate field conditions before deployment. By incorporating AI-driven modeling, researchers could refine application strategies based on climatic conditions, management practices, and soil microbiota interactions.
Another major research gap involves fungal formulation optimization. Current studies predominantly focus on feeding fungal spores to livestock, but alternative delivery formulations remain largely untested. Technologies such as controlled-release capsules, fungal-coated pellets, and microbial consortia could prolong fungal viability, ensuring that spores remain active in manure and pasture environments for extended periods. Future studies should explore formulation stability across varying environmental conditions, particularly in high-temperature and low-humidity settings, where fungal survival is challenging.
2.2. Long-Term Sustainability and Field Integration
Beyond formulation optimization, long-term sustainability studies are notably lacking. Most research assesses short-term parasite load reductions, typically spanning only a few months. However, sustainable parasite control requires multi-year field trials that monitor parasite population fluctuations over time. This is critical to ensure that D. flagrans remains effective without inadvertently selecting for resistant nematode populations. Future research should incorporate extended field studies, evaluating soil microbiota interactions, pasture composition shifts, and livestock health outcomes over multiple grazing seasons.
In addition to biological efficacy, farmer adoption and economic feasibility remain key concerns. Many existing studies emphasize scientific validation, but practical implementation challenges—including logistical barriers, farmer perceptions, and cost considerations—are often overlooked. Field-based studies should prioritize farmer-centric perspectives, exploring cost-sharing models, government incentives, and local fungal production feasibility to increase accessibility.
2.3. Refining Mode of Administration for Greater Efficiency
Experimental and survey methodologies have provided strong empirical validation of D. flagrans efficacy in reducing nematode loads. These approaches have offered quantifiable data on parasite suppression rates and fungal persistence, reinforcing confidence in D. flagrans as a reliable biocontrol agent. However, as previously noted, modeling and simulation remain underutilized, despite their potential to predict long-term outcomes, optimize fungal application rates, and identify effective deployment strategies across different climates.
Although oral administration remains dominant, alternative methods such as feed blocks, drenching, and spray formulations are notably underrepresented in the literature. The lower prevalence of these methods may be due to difficulties in ensuring even spore distribution in the animal’s digestive system or high production costs associated with formulation development [
25,
29,
32,
44,
45]. While drenching provides controlled administration, its frequent reapplication makes it impractical for large-scale operations. Similarly, spray formulations remain largely unexplored, likely due to concerns regarding fungal viability in varying environmental conditions. Future research should focus on alternative, cost-effective delivery systems that maximize fungal viability while ensuring consistent dosing.
Another crucial factor in administration is dose optimization. Studies indicate that an optimal spore concentration of 1×10⁶ to 1×10⁸ chlamydospores per gram of feed is necessary to maintain efficacy [
24,
29]. However, fungal persistence in manure depends on multiple variables, including temperature, humidity, and manure decomposition rates. Research should investigate bio-encapsulated formulations, which may enhance fungal survival in manure and extend long-term efficacy [
32].
While multiple administration strategies have been explored, D. flagrans effectiveness is influenced by various environmental and logistical challenges that may hinder widespread adoption. From climatic constraints that affect fungal persistence to economic feasibility concerns and farmer hesitancy, these barriers must be critically addressed to ensure successful integration into livestock parasite management strategies. For D. flagrans to transition from controlled research settings into real-world livestock operations, a multi-faceted approach is required. This includes improving delivery formulations, integrating computational modeling for predictive applications, conducting multi-year sustainability studies, and prioritizing farmer adoption research. Only through these advancements can D. flagrans fulfill its potential as a sustainable and scalable alternative to chemical anthelmintics.
3. Challenges and Limitations of Using Duddingtonia flagrans in Livestock Management
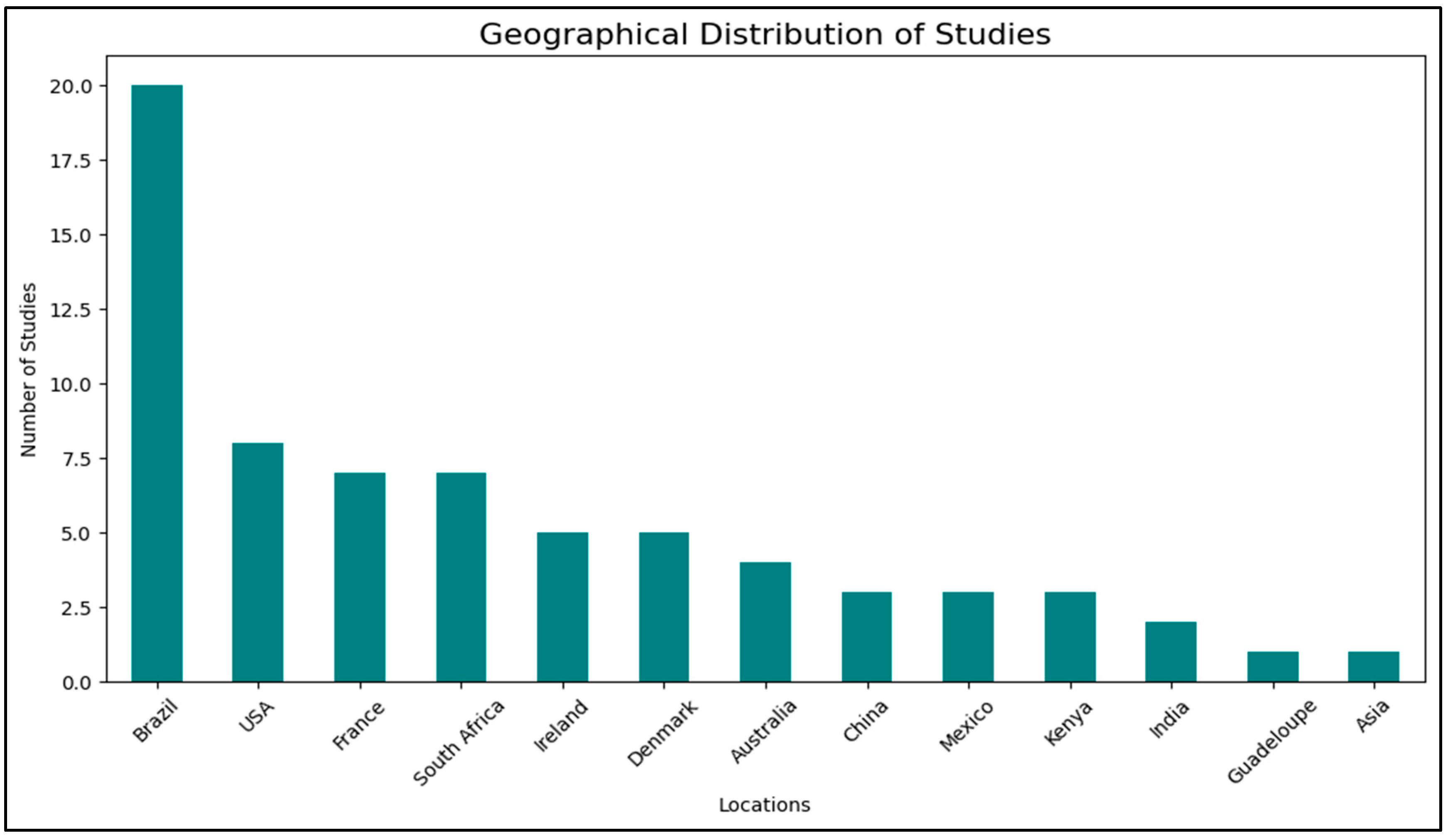
Despite its promise, several barriers to adoption limit the widespread use of
D. flagrans. Geographical distribution analysis (Geographical Studies) revealed a strong research focus in Brazil, the USA, France, and South Africa with , but significantly fewer studies in tropical and arid regions, where GIN infections are often more severe [
29,
30,
46,
47] (
Figure 5). This discrepancy suggests that while
D. flagrans is well-studied in temperate livestock systems, its performance in high-temperature, low-humidity environments remains underexplored.
Figure 5.
Graphical representation of geographical locations based on the published studies.
Figure 5.
Graphical representation of geographical locations based on the published studies.
Regional coverage analysis (Regional Coverage) also indicated a lack of studies in subtropical and arid environments, raising concerns about fungal adaptability to different climates [
46,
47]. More field trials are needed in tropical livestock systems, where parasite burdens are often higher and more persistent throughout the year (
Figure 6).
Figure 6.
Graphical coverage of studies based on regions.
Figure 6.
Graphical coverage of studies based on regions.
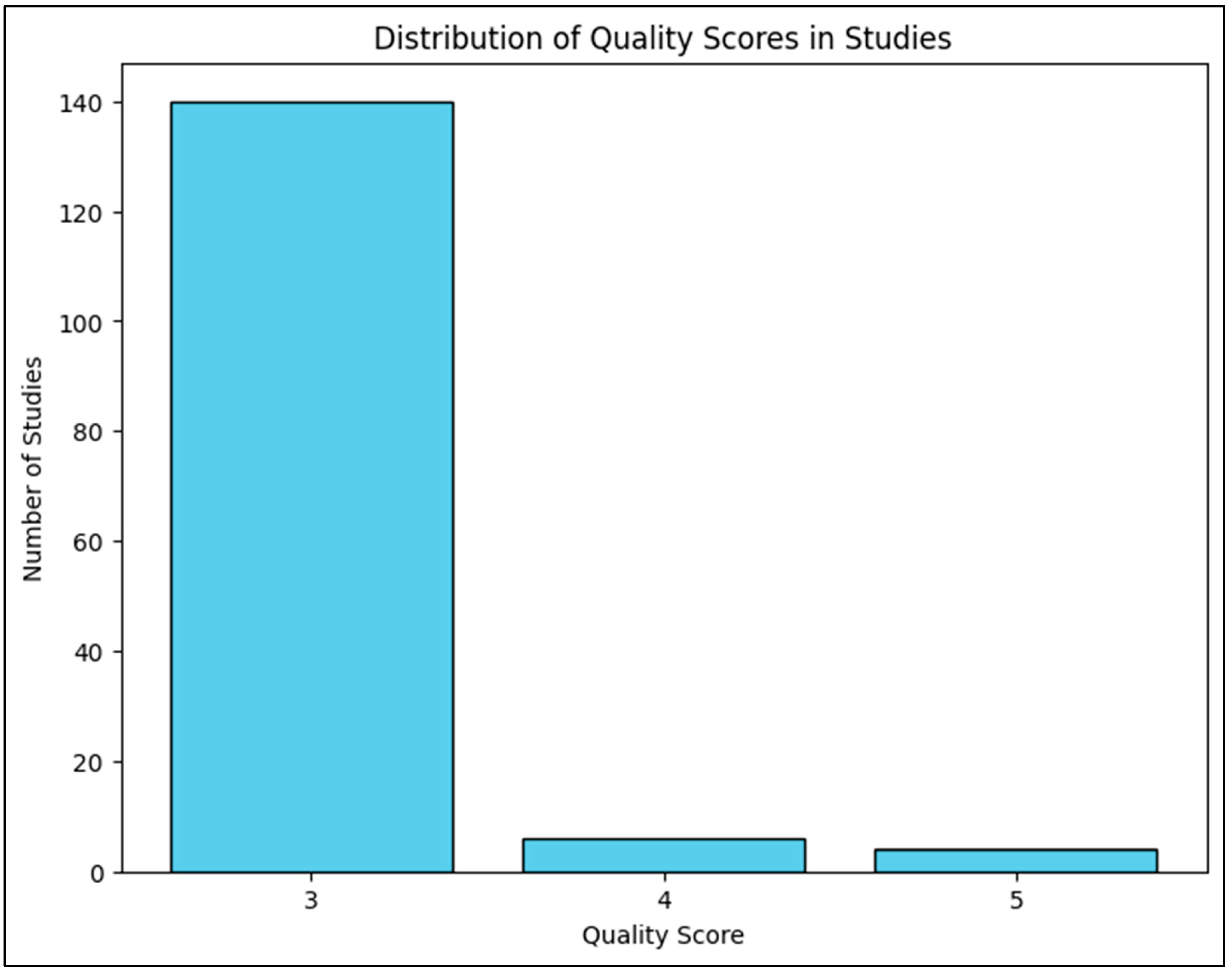
The Quality Score Analysis assesses the scientific rigor, reliability, and practical applicability of studies on
D. flagrans as a biological control agent. In this context, ’quality’ is measured on a scale from 1 to 5, where 1 represents low-quality studies with weak experimental design, poor reproducibility, and limited practical relevance. In contrast, 5 represents high-quality studies with robust methodologies, strong statistical validation, and real-world applicability. Scores in between reflect varying levels of experimental strength and practical feasibility, with 2 indicating minimal reliability, 3 representing moderate credibility with some limitations, and four denoting strong but not fully validated findings. While laboratory and controlled trials strongly support
D. flagrans, the need for large-scale farm trials to assess its real-world effectiveness and economic feasibility is paramount. The current lack of such trials presents a challenge in implementing
D. flagrans at the farm level, as farmers need evidence from field trials to justify adoption. Without practical validation, uncertainties remain regarding its affordability, ease of use, and long-term benefits in sustainable parasite management. Farmers may be reluctant to adopt fungal-based parasite control due to higher upfront costs compared to chemical dewormers [
25,
28,
29,
47]. Additionally, fungal formulations are not widely commercially available, which restricts their accessibility (
Figure 7).
Figure 7.
Graphical representation of number of D. flagrans studies with their quality index. X-axis represents quality index, Y-axis represents number of studies.
Figure 7.
Graphical representation of number of D. flagrans studies with their quality index. X-axis represents quality index, Y-axis represents number of studies.
An often-overlooked limitation is potential risk of resistance development among parasites. While D. flagrans targets nematode larvae rather than adult worms, its prolonged use may exert selection pressure, leading to the emergence of fungi-resistant nematode populations. Long-term monitoring programs should assess whether parasite populations develop adaptations that reduce the trapping efficiency of D. flagrans. The identification of key challenges highlights existing limitations in D. flagrans research and practical application. However, beyond these constraints, recent studies have also pointed to emerging research trends that could shape future innovations in fungal-based parasite control.
4. Patterns and Trends in Themes and Methodologies in Duddingtonia flagrans Research
The analysis of research on
Duddingtonia flagrans as a biological control agent for gastrointestinal nematodes (GINs) in livestock reveals distinct patterns and methodological trends that provide valuable insights into the evolving research landscape. The word cloud analysis highlights dominant topics such as
“Duddingtonia flagrans,” “biological control,” “gastrointestinal nematodes,” and
“pasture management,” indicating that most research efforts focus on evaluating
D. flagrans as an alternative parasite control strategy while integrating it into sustainable livestock production systems. This reflects a growing recognition of the need for non-chemical, ecologically viable parasite control solutions in light of anthelmintic resistance and environmental concerns (
Figure 1).
A key trend emerging in recent studies is the integration of
D. flagrans with sustainable livestock management practices. Researchers are increasingly exploring the combined use of biological control, pasture rotation, and selective breeding for parasite resistance to create comprehensive Integrated Parasite Management (IPM) strategies. This shift reflects an understanding that biological control alone is insufficient for long-term nematode suppression, and a multi-faceted approach is necessary [
5,
16,
27,
29]. The emphasis on sustainability is particularly evident in studies focusing on pasture management and reduced chemical dewormer use, as these strategies aim to preserve soil health, while mitigating the risk of anthelmintic resistance [
1,
11,
48].
However, despite the strong body of research supporting the efficacy of
D. flagrans, key gaps remain, particularly in optimizing fungal survival under diverse environmental conditions. Many studies focus on controlled experimental conditions, but fewer address the long-term persistence of fungal spores in real-world pasture systems. The success of
D. flagrans in field conditions depends on factors such as temperature, humidity, manure decomposition rates, and soil microbiota interactions [
38,
39,
49,
50]. Without robust field validation across different agroecological zones, it remains unclear how
D. flagrans will perform in highly variable environmental conditions. Future research should prioritize experimental field trials to explore how fungal persistence and efficacy vary under different climatic and grazing conditions.
Another critical gap is the limited economic feasibility assessments comparing
D. flagrans with conventional anthelmintics. While its biological efficacy is well-documented, there is little research evaluating its cost-effectiveness in large-scale livestock production systems. Factors such as the cost of fungal formulations, labor-intensive application methods, and the frequency of administration required for long-term control need further exploration [
51,
52,
53,
54]. Without economic validation, farmer adoption may remain limited, as cost considerations often outweigh environmental concerns in decision-making processes.
5. Emerging Themes: New Areas of Research Focus
Several emerging themes point to expanding research directions that seek to improve
D. flagrans’ practical applicability. Fungal formulation stability studies are beginning to explore ways to enhance spore viability and persistence in manure, addressing concerns over its short-lived efficacy in field conditions [
51,
55,
56]. There is increasing recognition that regional climate conditions influence fungal performance, prompting research on optimizing
D. flagrans for different environmental conditions [
31,
39,
57,
58]. Integration with rotational grazing systems has been observed in studies to examine
D. flagrans in free-ranging livestock systems, but more research is needed to establish best practices for integrating fungal control with rotational grazing schedules to maximize its impact on parasite populations [
32,
43,
59,
60].
6. Conclusions and Future Research
The research on Duddingtonia flagrans as a biological control agent for gastrointestinal nematodes (GINs) in livestock has demonstrated strong efficacy, sustainability benefits, and long-term potential. The existing body of literature confirms that D. flagrans effectively reduces parasite burdens, minimizes pasture contamination, and presents a viable alternative to chemical anthelmintics. Despite these advantages, the widespread adoption of D. flagrans remains hindered by several key challenges that must be addressed to bridge the gap between laboratory success and practical field application.
One of the most significant gaps in research pertains to its economic feasibility and cost-effectiveness compared to traditional anthelmintics. While numerous studies focus on fungal action, parasite reduction, and pasture management, less attention has been given to the financial viability of large-scale implementation. Farmers require cost-benefit analyses that compare D. flagrans with conventional dewormers, accounting for input costs, long-term savings, and economic trade-offs. Without clear financial incentives and affordability, the widespread adoption of fungal-based control methods remains uncertain. Future research must prioritize economic modeling and farmer perception studies to assess the practicality of integrating D. flagrans into commercial livestock production.
Beyond economic feasibility, another crucial factor influencing the large-scale viability of D. flagrans is its variable performance under different climatic conditions. Although laboratory and controlled studies indicate high efficacy, real-world field trials remain limited in scope and geographical distribution. The network analysis highlights a strong focus on fungal mechanisms and pasture management, but less research is dedicated to climate adaptation—a crucial factor influencing fungal survival, germination, and nematode trapping efficiency. In arid or extreme environments, fungal spores may degrade faster, leading to reduced efficacy and inconsistent parasite suppression. Conducting multi-location field trials in diverse climatic regions is essential to evaluate spore persistence, longevity, and optimal environmental conditions for effectiveness. Additionally, research should explore formulation improvements, such as bio-encapsulation and slow-release technologies, to enhance fungal stability under harsh environmental conditions.
While climate adaptation remains a key concern, an equally important but often overlooked factor is the integration of D. flagrans within broader sustainable livestock management practices. While many studies emphasize its individual efficacy, fewer investigations explore its synergies with rotational grazing, selective breeding for parasite resistance, and alternative biological controls. Integrating D. flagrans into comprehensive parasite management strategies could maximize its effectiveness, prolong its impact, and create a more resilient parasite control system. Research should focus on optimal grazing schedules, microbial consortia approaches, and holistic health monitoring systems to ensure sustained parasite suppression with minimal intervention.
Moreover, large-scale farmer adoption and extension studies are critical to bridging the gap between scientific validation and real-world implementation. Many livestock producers may be hesitant to adopt fungal-based deworming strategies due to a lack of awareness, uncertainty about efficacy, and perceived complexity in administration. Future research should incorporate on-farm trials, participatory research with farmers, and outreach programs to evaluate acceptance, usability, and barriers to adoption. Collaborative efforts involving veterinary professionals, extension officers, and researchers will be essential in educating farmers, optimizing delivery methods, and ensuring seamless integration into existing livestock systems.
Addressing these challenges requires a multi-pronged research approach, focusing on cost-effective formulations, fungal resilience, and its seamless integration into holistic parasite management systems. A multi-disciplinary approach—combining biological control innovations, predictive modeling, economic analysis, and farmer education—will be crucial in scaling up its application and ensuring long-term sustainability. By addressing these gaps, D. flagrans can become a cornerstone of sustainable livestock production, providing a long-term solution to anthelmintic resistance while minimizing environmental impacts.
Ultimately, translating laboratory success into widespread field adoption will require not only scientific advancements but also policy support, farmer engagement, and strategic extension initiatives. Ensuring that D. flagrans is cost-effective, climate-resilient, and farmer-friendly will solidify its role as a scalable and sustainable parasite control strategy. Ultimately, advancing research in these critical areas will contribute to more resilient, environmentally responsible, and economically viable livestock systems, paving the way for a future where biological control plays a central role in global parasite management efforts.
Author Contributions
Conceptualization, A.S.; methodology, A.S., O.S.; T.C and T.T.; validation, TT, A.S and N.W formal analysis, A.S., T.C. and O.S; investigation, A.S., J.B; O.S, T.T, J.M., and T.C.; data curation, A.S. and O.S; writing—original draft preparation, A.S.; writing—review and editing, J.B; O.S, T.T, J.M., and N.W; supervision, T.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
I would like to thank FVSU library resources for providing us with access to the databases. I would also like to thank my co-authors for their valuable insight during preparation of manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mendoza-de Gives, P. Soil-Borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens. 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-de Gives, P.; Braga, F.R.; Araújo, J.V. Nematophagous fungi, an extraordinary tool for controlling ruminant parasitic nematodes and other biotechnological applications. Biocontrol Science and Technology. 2022, 32, 777–93. [Google Scholar] [CrossRef]
- Paraud, C.; Pors, I.; Chartier, C. Efficiency of feeding Duddingtonia flagrans chlamydospores to control nematode parasites of first-season grazing goats in France. Veterinary research communications. 2007, 31, 305–15. [Google Scholar] [CrossRef] [PubMed]
- Moliszewska, E.B.; Nabrdalik, M.; Kudrys, P. Nematicidal potential of nematophagous fungi. In Fungal Biotechnology; CRC Press: 2022; pp. 409-434.
- Vande Velde, F.; Charlier, J.; Claerebout, E. Farmer behavior and gastrointestinal nematodes in ruminant livestock—uptake of sustainable control approaches. Frontiers in Veterinary Science. 2018, 5, 255. [Google Scholar] [CrossRef]
- Cabaret, J.; Mercier, M.; Mahieu, M.; Alexandre, G. Farmers’ Views and Tools Compared with Laboratory Evaluations of Parasites of Meat Goats in French West Indies. Animals. 2023, 13, 422. [Google Scholar] [CrossRef]
- Hou, B.; Yong, R.; Wuen, J.; Zhang, Y.; Buyin, B.; Subu, D.; Zha, H.; Li, H.; Hasi, S. Positivity rate investigation and anthelmintic resistance analysis of gastrointestinal nematodes in sheep and cattle in Ordos, China. Animals. 2022, 12, 891. [Google Scholar] [CrossRef]
- Kaplan RM, editor. Ruminant Parasitology, An Issue of Veterinary Clinics of North America: Food Animal Practice. Elsevier Health Sciences; 2020.
- Masur, E.; Tsakiris, A.; Bruno, K.; Theurer, M.; Gerb, S. Assessment of an Herbal Feed Additive on Reducing Gastrointestinal Nematodes in an Alpaca Operation. J Vet Med Health. 2022;6(151):2.
- Molento, M.B.; Buzatti, A.; Sprenger, L.K. Pasture larval count as a supporting method for parasite epidemiology, population dynamic and control in ruminants. Livestock Science. 2016, 192, 48–54. [Google Scholar] [CrossRef]
- Barger, I.A. The role of epidemiological knowledge and grazing management for helminth control in small ruminants. International journal for parasitology. 1999, 29, 41–7. [Google Scholar] [CrossRef]
- Bloemhoff, Y.; Danaher, M.; Forbes, A.; Morgan, E.; Mulcahy, G.; Power, C.; Sayers, R. Parasite control practices on pasture-based dairy farms in the Republic of Ireland. Veterinary parasitology. 2014, 204, 352–63. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P. Soil-Borne nematodes: Impact in agriculture and livestock and sustainable strategies of prevention and control with special reference to the use of nematode natural enemies. Pathogens. 2022, 11, 640. [Google Scholar] [CrossRef]
- Aboshady, H.M.; Mandonnet, N.; Johansson, A.M.; Jonas, E.; Bambou, J.C. Genomic variants from RNA-seq for goats resistant or susceptible to gastrointestinal nematode infection. Plos one. 2021, 16, e0248405. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Daemen, M.; Sahoo, G.; Luyten, W. Essential oils as novel anthelmintic drug candidates. Molecules. 2022, 27, 8327. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.W.; Van Wyk, J.A.; Hamie, J.C.; Byaruhanga, C.; Kenyon, F. Refugia-based strategies for parasite control in livestock. Veterinary Clinics: Food Animal Practice. 2020, 36, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, J.A. Refugia-overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance.
- Gill, J.H.; Redwin, J.M.; Van Wyk, J.A.; Lacey, E. Detection of resistance to ivermectin in Haemonchus contortus. International journal for parasitology. 1991, 21, 771–6. [Google Scholar] [CrossRef]
- Van Wyk, J.A.; Reynecke, D.P. Blueprint for an automated specific decision support system for countering anthelmintic resistance in Haemonchus spp. at farm level. Veterinary Parasitology. 2011, 177, 212–23. [Google Scholar] [CrossRef]
- Braga, F.R.; Araújo, J.V.; Silva, A.R.; Carvalho, R.O.; Araujo, J.M.; Ferreira, S.R.; Benjamin, L.A. Predatory activity of the nematophagous fungus Duddingtonia flagrans on horse cyathostomin infective larvae. Tropical animal health and production. 2010, 42, 1161–5. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V, Azimonti G, de Lourdes Bastos M, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López-Alonso M, López Puente S, Marcon F. Safety and efficacy of BioWorma®(Duddingtonia flagrans NCIMB 30336) as a feed additive for all grazing animals. EFSA Journal. 2020, 18, e06208.
- Wernet, V.; Wäckerle, J.; Fischer, R. The STRIPAK component SipC is involved in morphology and cell-fate determination in the nematode-trapping fungus Duddingtonia flagrans. Genetics. 2022, 220, iyab153. [Google Scholar] [CrossRef]
- Aguilar, J.A.; De Gives, P.M.; López-Arellano, M.E.; Hernández, E.L. Evaluation of multinutritional pellets containing Duddingtonia flagrans chlamydospore for the control of ovine haemonchosis. Annals of the New York Academy of Sciences. 2008, 1149, 161–3. [Google Scholar] [CrossRef]
- Dias, A.S.; Araujo, J.V.; Campos, A.K.; Braga, F.R.; Fonseca, T.A. Application of a formulation of the nematophagous fungus Duddingtonia flagrans in the control of cattle gastrointestinal nematodiosis. World Journal of Microbiology and Biotechnology. 2007, 23, 1245–52. [Google Scholar] [CrossRef]
- Junco, M.; Iglesias, L.E.; Sagüés, F.; Zegbi, S.; Guerrero, I.; Saumell, C.A. A review of the use of Duddingtonia flagrans as a biological controller of strongylid nematodes in horses. Parasitology Research. 2023, 122, 357–68. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Zegbi, S.; Sagües, F.; Iglesias, L.; Guerrero, I.; Saumell, C. Trapping Behaviour of Duddingtonia flagrans against gastrointestinal nematodes of cattle under year-round grazing conditions. Pathogens. 2023, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Torres-Acosta, J.F. Non chemical control of helminths in ruminants: adapting solutions for changing worms in a changing world. Veterinary parasitology. 2011, 180, 144–54. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Miller, J.E. Sustainable approaches to parasite control in ruminant livestock. Veterinary Clinics of North America: Food Animal Practice. 2020, 36, 89–107. [Google Scholar] [CrossRef]
- Canhão-Dias, M.; Paz-Silva, A.; de Carvalho, L.M. The efficacy of predatory fungi on the control of gastrointestinal parasites in domestic and wild animals—A systematic review. Veterinary Parasitology. 2020, 283, 109173. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Olmedo-Juárez, A.; Mendoza-de Gives, P. Control and prevention of nematodiasis in small ruminants: background, challenges and outlook in Mexico. Revista mexicana de ciencias pecuarias. 2021, 12. [Google Scholar]
- Buske, R.; Santurio, J.M.; de Oliveira, C.V.; Bianchini, L.A.; da Silva, J.H.; de la Rue, M.L. In vitro influence of temperature on the biological control activity of the fungus Duddingtonia flagrans against Haemonchus contortus in sheep. Parasitology research. 2013, 112, 473–8. [Google Scholar] [CrossRef]
- Vieira, Í.S.; de Castro Oliveira, I.; Campos, A.K.; de Araújo, J.V. In vitro biological control of bovine parasitic nematodes by Arthrobotrys cladodes, Duddingtonia flagrans and Pochonia chlamydosporia under different temperature conditions. Journal of Helminthology. 2020, 94, e194. [Google Scholar] [CrossRef]
- Floate, K. D. , & Coghlin, P. Lack of effect of the nematophagous fungus Duddingtonia flagrans on the development of the dung beetle, Aphodius constans. Biological Control.
- Chandrawathani, P.; Jamnah, O.; Adnan, M.; Waller, P.J.; Larsen, M.; Gillespie, A.T. Field studies on the biological control of nematode parasites of sheep in the tropics, using the microfungus Duddingtonia flagrans. Veterinary Parasitology. 2004, 120, 177–87. [Google Scholar] [CrossRef]
- Fontenot, M.E.; Miller, J.E.; Peña, M.T.; Larsen, M.; Gillespie, A. Efficiency of feeding Duddingtonia flagrans chlamydospores to grazing ewes on reducing availability of parasitic nematode larvae on pasture. Veterinary Parasitology. 2003, 118, 203–13. [Google Scholar] [CrossRef]
- Mendoza-de Gives, P.; López-Arellano, M.E.; Aguilar-Marcelino, L.; Olazarán-Jenkins, S.; Reyes-Guerrero, D.; Ramírez-Várgas, G.; Vega-Murillo, V.E. The nematophagous fungus Duddingtonia flagrans reduces the gastrointestinal parasitic nematode larvae population in faeces of orally treated calves maintained under tropical conditions—Dose/response assessment. Veterinary parasitology. 2018 Nov 15;263:66-72.
- Sagüés, M.F.; Fusé, L.A.; Fernández, A.S.; Iglesias, L.E.; Moreno, F.C.; Saumell, C.A. Efficacy of an energy block containing Duddingtonia flagrans in the control of gastrointestinal nematodes of sheep.
- Kitchenham, B.; Brereton, P. A systematic review of systematic review process research in software engineering. Information and software technology. 2013, 55, 2049–75. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. bmj. 2021, 372. [Google Scholar]
- Fernández, A.S.; Larsen, M.; Nansen, P.; Grønvold, J.; Henriksen, S.A.; Bjørn, H.; Wolstrup, J. The efficacy of two isolates of the nematode-trapping fungus Duddingtonia flagrans against Dictyocaulus viviparus larvae in faeces. Veterinary parasitology. 1999, 85, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Paraud, C.; Hoste, H.; Lefrileux, Y.; Pommaret, A.; Paolini, V.; Pors, I.; Chartier, C. Administration of Duddingtonia flagrans chlamydospores to goats to control gastro-intestinal nematodes: dose trials. Veterinary Research. 2005;36(2):157-66.
- Larsen, M.; Nansen, P.; Grøndahl, C.; Thamsborg, S.M.; Grønvold, J.; Wolstrup, J.; Henriksen, S.A.; Monrad, J. The capacity of the fungus Duddingtonia flagrans to prevent strongyle infections in foals on pasture. Parasitology. 1996, 113, 1–6. [Google Scholar] [CrossRef]
- Paraud, C.; Pors, I.; Chartier, C. Efficiency of feeding Duddingtonia flagrans chlamydospores to control nematode parasites of first-season grazing goats in France. Veterinary research communications. 2007, 31, 305–15. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Roque, F.L.; Lima, B.A.; Silva Filho, G.M.; Oliveira, C.S.; Sousa, L.C.; Silva, A.L.; Lima, E.F.; Feitosa, T.F.; Braga, F.R.; Araújo, J.V. Control of sheep gastrointestinal nematodes on pasture in the tropical semiarid region of Brazil, using Bioverm®(Duddingtonia flagrans). Tropical Animal Health and Production. 2022, 54, 179. [Google Scholar] [CrossRef]
- Preston, E.M. The Use of Duddingtonia Flagrans Aged in Mineral for Gastrointestinal Parasitic Nematode Control in Small Ruminants.
- Baiak, B.H.; Gasparina, J.M.; Ianke, L.; de Sousa, K.T.; Deniz, M.; Pereira, L.M.; Araújo, J.V.; da Rocha, R.A.; Dittrich, J.R. Predatory activity of nematophagus fungus Duddingtonia flagrans in infective larvae after gastrointestinal transit: Biological control in pasture areas and in vitro. Journal of Helminthology. 2021, 95, e31. [Google Scholar] [CrossRef]
- Sanyal, P.K.; Sarkar, A.K.; Patel, N.K.; Mandal, S.C.; Pal, S. Formulation of a strategy for the application of Duddingtonia flagrans to control caprine parasitic gastroenteritis. Journal of helminthology. 2008, 82, 169–74. [Google Scholar] [CrossRef]
- Voinot M, Cazapal-Monteiro C, Hernández JÁ, Palomero AM, Arroyo FL, Sanchis J, Pedreira J, Sánchez-Andrade R, Paz-Silva A, Arias MS. Integrating the control of helminths in dairy cattle: Deworming, rotational grazing and nutritional pellets with parasiticide fungi. Veterinary parasitology. 2020, 278, 109038.
- Molento, M. B. , Kloster, F. S., Yoshitani, U., & Sprenger, L. K. Biological control using the fungi Duddingtonia flagrans against cyathostomins of horses. Veterinary Parasitology.
- Blair, J. , & Biddle, A. Stimulating Duddingtonia flagrans chlamydospore production through dehydration. Parasitology Research 2020, 119(1), 123–128. [Google Scholar]
- Faedo, M.; Krecek, R.C. Possible application of a nematophagous fungus as a biological control agent of parasitic nematodes on commercial sheep farms in South Africa. Journal of the South African Veterinary Association. 2002, 73, 31–5. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D. F. , Céspedes-Gutiérrez, E., Cubides-Cárdenas, J. A., & Gómez-Álvarez, M. I. Survival of the nematophagous fungus Duddingtonia flagrans to in vitro segments of sheep gastrointestinal tract. Experimental Parasitology 2021, 231, 108172. [Google Scholar]
- Braga, F. R. , Ferraz, C. M., Araújo, J. V., & Silva, A. R. Bioverm® (Duddingtonia flagrans) compared to the association of Duddingtonia flagrans and Pochonia chlamydosporia for the biological control of cattle nematodes in southeast Brazil. 3 Biotech 2023, 13, Article 62. [Google Scholar] [CrossRef]
- Feitosa, T. F. , Vilela, V. L. R., Athayde, A. C. R., & Silva, M. E. Control of sheep gastrointestinal nematodes using the combination of Duddingtonia flagrans and Levamisole Hydrochloride 5%. Revista Brasileira de Parasitologia Veterinária, 27(1), 1‐10, 2018. [Google Scholar]
- Sanyal, P. K. , Chauhan, J. B., & Mukhopadhyaya, P. N. N. Implications of fungicidal effects of benzimidazole compounds on Duddingtonia flagrans in integrated nematode parasite management in livestock. Veterinary Research Communications 2004, 28(5), 375–385. [Google Scholar] [CrossRef]
- Fausto, G. C. , Vieira, Í. S., Freitas, S. G., Carvalho, L. M., Oliveira, I. C., Silva, E. N., Campos, A. K., & Araújo, J. V. Formulation of the nematophagous fungus Duddingtonia flagrans in the control of equine gastrointestinal parasitic nematodes. Veterinary Parasitology 2021, 295, 109458. [Google Scholar]
- Braga, F. R. , Ferraz, C. M., Araújo, J. V., & Silva, A. R. Bioverm® (Duddingtonia flagrans) compared to the association of Duddingtonia flagrans and Pochonia chlamydosporia for the biological control of cattle nematodes in southeast Brazil. 3 Biotech 2023, 13, Article 62. [Google Scholar]
- Buske, R. , Santurio, J. M., de Oliveira, C. V., Bianchini, L. A., da Silva, J. H. S., & de la Rue, M. L. In vitro influence of temperature on the biological control activity of the fungus Duddingtonia flagrans against Haemonchus contortus in sheep. Parasitology Research.
- Braga, F.R.; Araujo, J.V.; Silva, A.R.; Araujo, J.M.; Carvalho, R.O.; Tavela, A.O.; Campos, A.K.; Carvalho, G.R. Biological control of horse cyathostomin (Nematoda: Cyathostominae) using the nematophagous fungus Duddingtonia flagrans in tropical southeastern Brazil. Veterinary Parasitology. 2009, 163, 335–40. [Google Scholar] [CrossRef]
- Eysker, M.; Bakker, N.; Kooyman, F.N.; Olthuis, S.O.; Ploeger, H.W. Effect of biological control through the daily application of spores of Duddingtonia flagrans in lambs kept under an evasive grazing system in the Netherlands. Veterinary parasitology. 2006, 140, 312–20. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).