Introduction
Attention deficit hyperactivity disorder (ADHD) is a neuropsychiatric condition characterized by symptoms of inattention, hyperactivity, and impulsivity. ADHD affects between 5-7% of the child population and up to 4% of adults worldwide, making it one of the most diagnosed neuropsychiatric disorders (Thomas et al., 2015). Methylphenidate (MPH) is one of the most commonly prescribed drugs in childhood and adolescence, with millions of people in chronic treatment (Caye et al., 2019). Although its origin has traditionally been attributed to dysfunctions in the dopaminergic and noradrenergic systems, recent research suggests that neuroinflammation and activation of the renin-angiotensin-aldosterone system (RAAS) could play significant roles in its pathophysiology (Oades et al., 2010; Miller & Haroon, 2019).
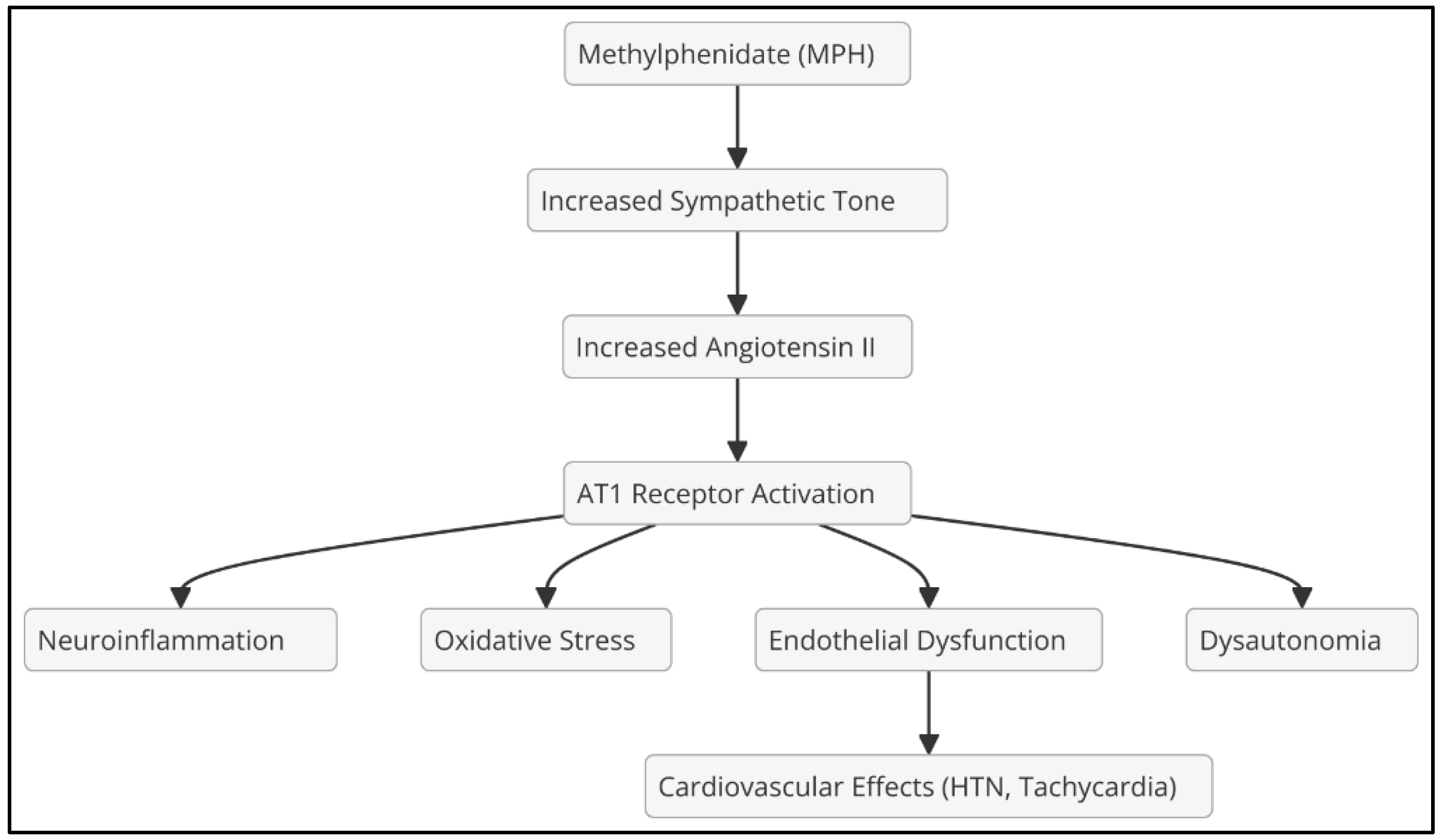
MPH increases the release of catecholamines (dopamine and norepinephrine) by inhibiting the dopamine transporter (DAT) and the norepinephrine transporter (NET). However, it can also activate RAAS, raising angiotensin II (Ang II) levels due to increased sympathetic tone (Watts et al., 2013), increasing blood pressure and AT1R receptor activity, which promotes vasoconstriction, oxidative stress, and endothelial dysfunction (Brown et al., 2020); alter serotonergic neurotransmission, which could amplify the impact of Ang II on the CNS (
Figure 1).
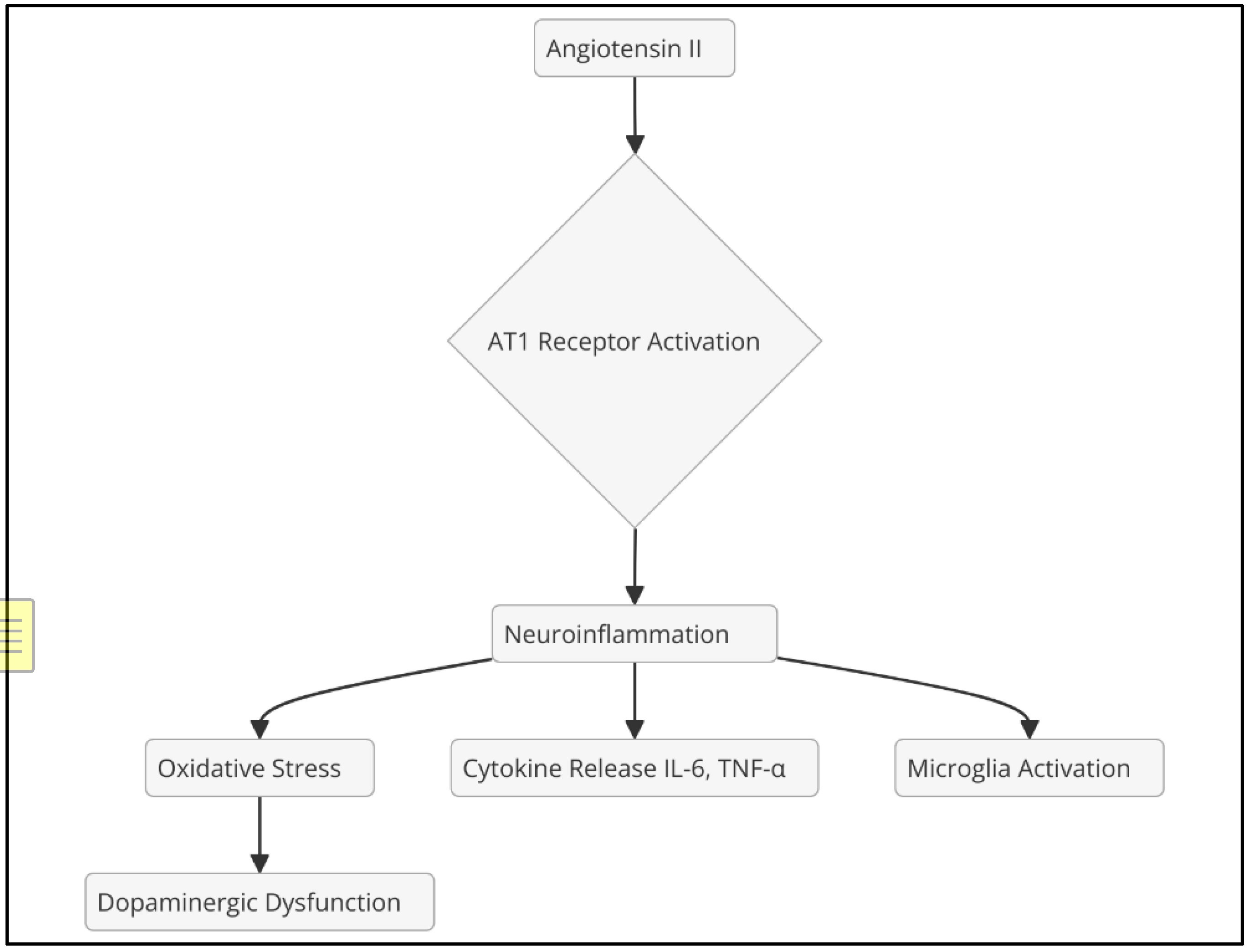
Ang II, the main effector of RAAS, exerts its effects predominantly through the AT1 receptor, promoting vasoconstriction, aldosterone release, and sympathetic activation. In addition, activation of the AT1 receptor in the central nervous system (CNS) can induce neuroinflammation by activating microglia and releasing proinflammatory cytokines (Kehoe et al., 2016) (
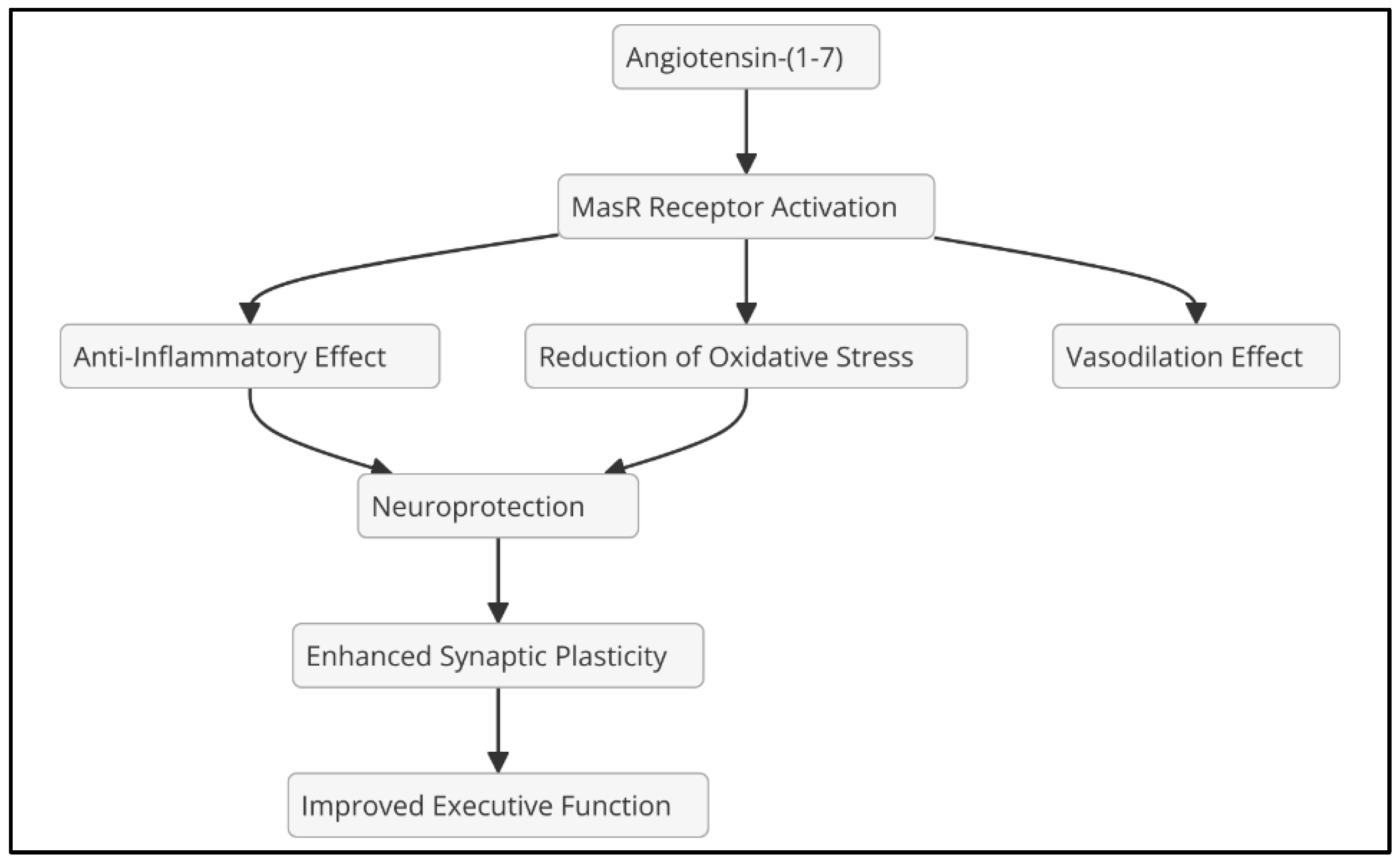
Figure 2). In contrast, angiotensin-(1-7), formed from Ang II by the action of angiotensin-converting enzyme 2 (ACE2), exerts opposite effects, including vasodilation and anti-inflammatory properties, primarily through the MasR receptor (Santos et al., 2018).
Blocking the AT1 receptor through the use of angiotensin II receptor antagonists (ARBs) could reduce neuroinflammation and improve executive function in patients with ADHD, especially those with chronic inflammation and psychostimulant treatment.
Evidence of Neuroinflammation in ADHD
Elevated Inflammatory Biomarkers
Studies have identified elevated levels of inflammatory biomarkers in ADHD patients, including increased peripheral blood C-reactive protein (CRP), correlated with inattention and impulsivity (Oades et al., 2010), and interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α) related to dopaminergic dysfunction and alterations in the prefrontal cortex (Miller & Haroon, 2019).
Microglial Activation and Oxidative Stress
Neuroimaging studies with PET have shown microglial hyperactivation in the frontal lobe of adults with ADHD (Corona, 2020). Ang II-induced activation of NADPH oxidase: Generates oxidative stress, affecting neuronal plasticity in the prefrontal cortex (Wright et al., 2013).
Relationship Between RAAS, Angiotensin II and ADHD
Ang II is a key neuromodulator in autonomic regulation, neuroinflammation and neuronal plasticity. Its excess, mediated by the activation of the AT1 receptor, can produce deleterious effects in the CNS. Through the activation of microglia and the release of proinflammatory cytokines (Kehoe et al., 2016) an increase in neuroinflammation occurs. In addition, there would be a decrease in dopamine in the prefrontal cortex, affecting executive function and attention (Santos et al., 2018). The increase in Ang II leads to hyperactivity of the sympathetic system, exacerbating dysautonomia in patients with ADHD (Wright et al., 2013).
AT1 Receptor Antagonists (ARBs) as Neuroprotectors
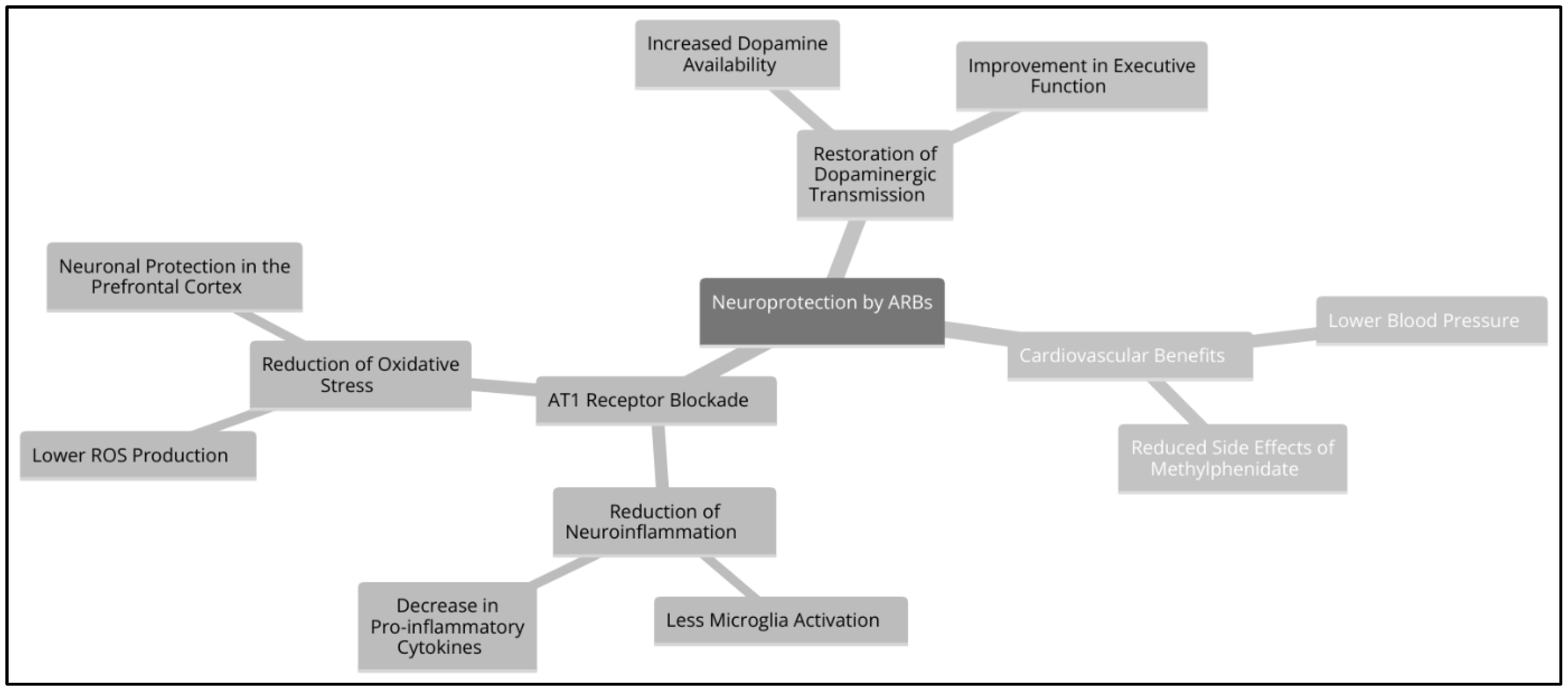
AT1 receptor antagonists (ARA-II or sartans, such as Losartan, Valsartan, and Candesartan) block AT1R activation, which could prevent damage from excess Ang II caused by methylphenidate (MPH) and chronic inflammation. Its action reduces neuroinflammation and microglial activation, which decreases the release of proinflammatory cytokines such as IL-6, TNF-α and IL-1β (Saavedra, 2012). In addition, they lower blood pressure and sympathetic tone, counteracting the hypertensive effects of MPH and promoting the balance of the renin-angiotensin-aldosterone system (RAAS). This mechanism allows Ang II to be converted to Ang-(1-7), which activates the MasR receptor and generates neuroprotective effects (Benicky et al., 2011). They also prevent endothelial dysfunction and dysautonomia, protecting the blood-brain barrier and promoting better autonomic regulation.
If AT1R receptors are blocked by an ARA-II, the detrimental effects of chronic Ang II surging would be redirected to alternative routes that promote protective effects. The conversion of Ang II into Ang-(1-7) by the action of ACE2 favours the activation of the MasR receptor, which generates anti-inflammatory and vasodilatory effects. Likewise, the transformation of Ang II into Ang III and Ang IV activates the AT2R receptor, which counteracts neuroinflammation and promotes neuroprotection. Added to this is the reduction of oxidative stress through a lower activation of NADPH oxidase, which decreases the production of reactive oxygen species (ROS) and reduces neuronal damage (Kehoe et al., 2016). Since MPH and chronic inflammation induce an increase in Ang II, blocking AT1R by ARA-II could prevent neuroinflammation, dysautonomia, and endothelial dysfunction, preserving central nervous system homeostasis.
Therapeutic Potential of ARBs in ADHD
Reduction of Neuroinflammation
Neuroinflammation is a key pathological mechanism in multiple neuropsychiatric disorders, including ADHD. Activation of the AT1 receptor by angiotensin II (Ang II) has been identified to promote a proinflammatory state in the central nervous system (CNS) by inducing activation of microglia, the main immune cell in the brain. This activation leads to the release of proinflammatory cytokines such as interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), and interleukin-1β (IL-1β), promoting a neurotoxic environment that affects neuronal connectivity and synaptic plasticity (Kehoe et al., 2016).
The use of AT1 receptor antagonists (ARA-II) interrupts this inflammatory cascade by preventing microglial hyperactivation, reducing cytokine production, and attenuating neuroinflammation. In addition, ARA-IIs decrease the expression of NADPH oxidase, a key enzyme in the generation of reactive oxygen species (ROS), which reduces oxidative stress and protects neuronal integrity in key areas such as the prefrontal cortex and basal ganglia (Wright et al., 2013). This reduction in oxidative stress is crucial, as persistent oxidative damage in the CNS can affect executive function and attentional capacity, core symptoms in ADHD.
Additionally, preclinical studies have shown that angiotensin II type 1 receptor antagonists (ARBs) can modulate the permeability of the blood-brain barrier, reducing the infiltration of peripheral immune cells into the brain. A study conducted by Rodríguez-Pérez et al. (2013) in a rat model with angiotensin II-induced hypertension found that treatment with losartan, an ARA-II, preserved the integrity of the blood-brain barrier and decreased immune cell infiltration into brain tissue. The authors suggest that this effect is due to the reduction of the expression of cell adhesion molecules and the decrease in microglial activation. This suggests an additional mechanism of neuroinflammatory protection, which could prevent the progression of neuronal damage associated with chronic inflammation in ADHD patients.
Restoration of Dopaminergic Neurotransmission
The dopaminergic system is one of the main ones affected by ADHD, and its dysfunction has been associated with deficits in attention, motivation, and impulse control. Chronic activation of the AT1R receptor in the CNS has been linked to a decrease in dopamine synthesis and release in the prefrontal cortex and basal ganglia, areas essential for executive function and behavioural regulation (Santos et al., 2018).
One of the most relevant effects of ARBs is their ability to promote the availability of dopamine in the CNS by mitigating the inhibitory effects of Ang II on dopaminergic activity. Inhibition of the AT1R receptor with ARA-II has been shown to increase the expression of tyrosine hydroxylase (Aschrafi et al., 2019), the key enzyme in dopamine synthesis, thereby improving the levels of this neurotransmitter in the synapse.
In addition, chronic inflammation has been implicated in reducing the density of dopaminergic receptors D1 and D2 in the prefrontal cortex, which compromises the efficacy of dopaminergic neurotransmission and hinders behavioural regulation (Wright et al., 2013). Treatment with ARA-II could reverse this dysregulation by decreasing neuroinflammation and oxidative stress, promoting a more balanced neurochemical environment, and facilitating the action of dopamine in the CNS.
In addition, in animal models, it has been observed that ARBs can improve synaptic plasticity and cognitive function through the modulation of neuroprotective pathways, such as the activation of the MasR receptor by angiotensin-(1-7), which favours the formation and consolidation of new neural connections essential for learning and memory.
Potential Reduction of Methylphenidate Side Effects
Methylphenidate (MPH) is one of the most commonly used drugs for the treatment of ADHD. However, its prolonged use is associated with adverse cardiovascular effects, such as hypertension, tachycardia, and increased sympathetic tone. These effects can be problematic, especially in patients with a predisposition to cardiovascular disease or in those who require chronic treatment with stimulants.
MPH acts by increasing the activity of the sympathetic system, which raises Ang II levels and promotes the activation of the AT1R receptor, exacerbating vasoconstriction and oxidative stress (Miller & Haroon, 2019). This overactivity of RAAS may contribute to the development of dysautonomia and endothelial dysfunction, which aggravates cardiovascular risk in these patients.
Blocking AT1R with ARA-II might mitigate these side effects by reducing sympathetic activation and improving vascular function. It has been shown that ARBs can lower blood pressure and improve vasodilation by promoting the conversion of Ang II to Ang-(1-7), which activates the MasR receptor with cardioprotective effects. This mechanism could be particularly beneficial in patients who experience cardiovascular adverse effects due to prolonged use of stimulants.
In addition to cardiovascular effects, MPH-induced oxidative stress and AT1R activation may compromise mitochondrial function in dopaminergic neurons, which has been associated with increased neuronal vulnerability and possible neurotoxicity in prolonged treatments (Oades et al., 2010). By reducing oxidative stress and modulating inflammation in the CNS, ARBs could protect dopaminergic neurons and improve the tolerability of psychostimulant treatment (
Figure 3).
Finally, because ADHD has a strong neurobiological basis but also involves metabolic and cardiovascular factors, the use of ARBs could represent a comprehensive therapeutic strategy, not only optimizing dopaminergic neurotransmission and reducing neuroinflammation but also minimizing the adverse effects of conventional treatment and improving the patient's overall health.
Recommendations for Future Studies
It is essential to conduct controlled clinical trials to evaluate the impact of ARB-II treatment in ADHD patients receiving methylphenidate, with the aim of determining its neuroprotective potential and its influence on the modulation of dopaminergic neurotransmission. In parallel, studies in preclinical models would allow to delve deeper into the effects of AT1 receptor blockade on neurotransmission and neuroinflammatory activity in the CNS, providing a solid mechanistic basis for its clinical application. In addition, the identification and analysis of biomarkers of inflammation and oxidative stress, such as IL-6, TNF-α and CRP, could serve as predictors of response to ARB-II treatment, facilitating the personalization of therapy in patients with ADHD. Finally, the implementation of functional neuroimaging studies using fMRI and PET would allow the evaluation of changes in brain connectivity and neural network activity after the administration of ARA-II, providing additional evidence of its effects on the regulation of RAAS and its impact on executive function and neuronal plasticity.
Limitations
Although this review presents a compelling hypothesis regarding the role of the renin-angiotensin system (RAS), neuroinflammation, and AT1 receptor blockade as a potential therapeutic strategy for Attention-Deficit/Hyperactivity Disorder (ADHD), several limitations must be considered.
While preclinical studies suggest that angiotensin II receptor blockers (ARBs) have neuroprotective effects by reducing neuroinflammation, oxidative stress, and dopamine dysregulation, there are no large-scale clinical trials specifically evaluating their effects in ADHD patients. Most data on ARBs come from cardiovascular and neurodegenerative research, making it uncertain how well these findings translate to ADHD pathophysiology.
Furthermore, ADHD is a heterogeneous disorder with multiple contributing factors, including genetic, environmental, and neurobiological influences. While neuroinflammation and RAS activation appear to play a role, ADHD also involves dopaminergic, noradrenergic, and serotonergic dysfunctions, which may not be directly addressed by ARB therapy.
On the other hand, ARBs are primarily used for hypertension and have not been widely studied in younger populations or individuals without cardiovascular conditions. Possible side effects, such as hypotension, fatigue, and electrolyte imbalances, may limit their long-term use in ADHD patients. The interaction between ARBs and stimulant medications (e.g., methylphenidate) remains unclear and requires further investigation.
Identifying subpopulations of ADHD patients who exhibit high levels of neuroinflammation (e.g., elevated CRP, IL-6, TNF-α) would allow for personalized treatment approaches. Without reliable biomarkers, it is difficult to determine which ADHD patients might benefit most from ARB therapy.
Future Directions
To further validate this hypothesis, the following areas of research should be prioritized:
Clinical Trials Evaluating ARBs in ADHD
Randomized controlled trials (RCTs) should assess the cognitive and behavioural effects of ARBs in ADHD patients, particularly in those with elevated inflammatory markers. These trials should compare ARBs alone and in combination with standard ADHD treatments (e.g., stimulants, non-stimulants) to determine synergistic or antagonistic effects.
Neuroimaging Studies on RAS and ADHD
Functional MRI (fMRI) and PET imaging should be used to investigate how AT1 receptor blockade affects brain connectivity, dopaminergic function, and neuroinflammation in ADHD patients. Imaging could help identify neural circuits that are most affected by AT1 receptor activation and determine whether ARB therapy restores regular activity in these regions.
Preclinical Research on ARBs and Dopaminergic Neurotransmission
Animal models of ADHD should be used to evaluate the effects of ARB treatment on dopamine release, synaptic plasticity, and neuroinflammation in the prefrontal cortex and the potential of ARBs to prevent or reverse stimulant-induced neuroinflammatory changes.
Identification of Biomarkers for Personalized Treatment
Future studies should aim to identify biomarkers (e.g., CRP, IL-6, TNF-α, oxidative stress markers) that predict which ADHD patients are most likely to respond to ARB therapy. This would allow for targeted treatment approaches rather than a one-size-fits-all strategy.
Comparison with Other Anti-Inflammatory Strategies
Future research should compare the efficacy of ARBs with other neuroinflammation-targeting therapies, including nonsteroidal anti-inflammatory drugs (NSAIDs), monoclonal antibodies (e.g., IL-6 inhibitors), omega-3 fatty acids, and lifestyle interventions.
Conclusions
ADHD has traditionally been considered a condition derived from dysfunctions in the dopaminergic and noradrenergic systems. However, growing evidence suggests that neuroinflammation and RAAS activation may play a crucial role in its pathophysiology. In particular, Ang II overexpression and AT1 receptor activation appear to be involved in processes that exacerbate neuroinflammation, oxidative stress, and endothelial dysfunction, negatively affecting dopaminergic neurotransmission and autonomic regulation in the central nervous system. ARBs emerge as a possible neuroprotective strategy, as blocking them could redirect the conversion of Ang II towards the production of Ang-(1-7), a peptide with anti-inflammatory and neuroprotective properties through activation of the MasR receptor. In addition, the use of ARBs could counteract the adverse effects of chronic inflammation and MPH-induced sympathetic hyperactivity, thereby reducing the impact of neuroinflammation and promoting better RAAS homeostasis in the brain (
Figure 4).
If this hypothesis is validated through preclinical studies and controlled clinical trials, AT1 receptor blockade could represent a significant therapeutic advance in the treatment of ADHD, offering an alternative or complement to conventional approaches with psychostimulants. The evaluation of inflammatory biomarkers, functional neuroimaging studies and animal models would determine more precisely the effects of ARBs on dopaminergic neurotransmission, neuroinflammation and executive function in these patients. Given the impact of ADHD globally and its high prevalence in children and adults, this line of research could not only improve the understanding of the underlying mechanisms of the disease but also open up new opportunities for the development of more effective treatments with fewer side effects.
By addressing the above-mentioned limitations and conducting targeted clinical and preclinical research, the potential role of AT1 receptor blockade in ADHD treatment can be better understood, paving the way for personalized and neuroprotective treatment strategies.
References
- Aschrafi, A., Berndt, A., Kowalak, J. A., Gale, J. R., Gioio, A. E., & Kaplan, B. B. (2019). Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine β-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. Journal of neurochemistry, 150(6), 666–677. [CrossRef]
- Caye, A., Swanson, J. M., Coghill, D., & Rohde, L. A. (2019). Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Molecular Psychiatry, 24(3), 390–408. [CrossRef]
- Corona J. C. (2020). Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants (Basel, Switzerland), 9(11), 1039. [CrossRef]
- Kehoe, P. G., Wong, S., Al Mulhim, N., Palmer, L. E., & Miners, J. S. (2016). Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimer’s Research & Therapy, 8, 50.
- Miller, A. H., & Haroon, E. (2019). The immunology of behavior: Implications for psychiatry. Annual Review of Clinical Psychology, 15, 293-316.
- Oades, R. D., Dauvermann, M. R., Schimmelmann, B. G., & Schwarz, M. J. (2010). Attention-deficit hyperactivity disorder (ADHD) and inflammation: An overview. Psychiatry Research, 177(1-2), 6-11.
- Rodríguez-Pérez, A. I., Labandeira-García, J. L., & Garrido-Gil, P. (2013). Losartan reduces the permeability of the blood-brain barrier and the infiltration of immune cells in the brain of hypertensive rats. Journal of Neuroinflammation, 10(1), 114.
- Santos, R. A. S., Ferreira, A. J., & Simões e Silva, A. C. (2018). Recent advances in the angiotensin-converting enzyme 2–angiotensin (1-7)–Mas axis. Experimental Physiology, 93(5), 519-527.
- Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. Pediatrics, 135(4), e994–e1001. [CrossRef]
- Wright, J. W., Harding, J. W., & Hanesworth, J. M. (2013). A role for the brain RAS in Alzheimer’s and Parkinson’s diseases. Frontiers in Neuroscience, 7, 65.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).