Submitted:
28 January 2025
Posted:
28 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study population
2.2. Clinicopathological variables
2.3. Statistical analysis
3. Results
3.1. Overall clinicopathologic characteristics
3.2. Overall survival
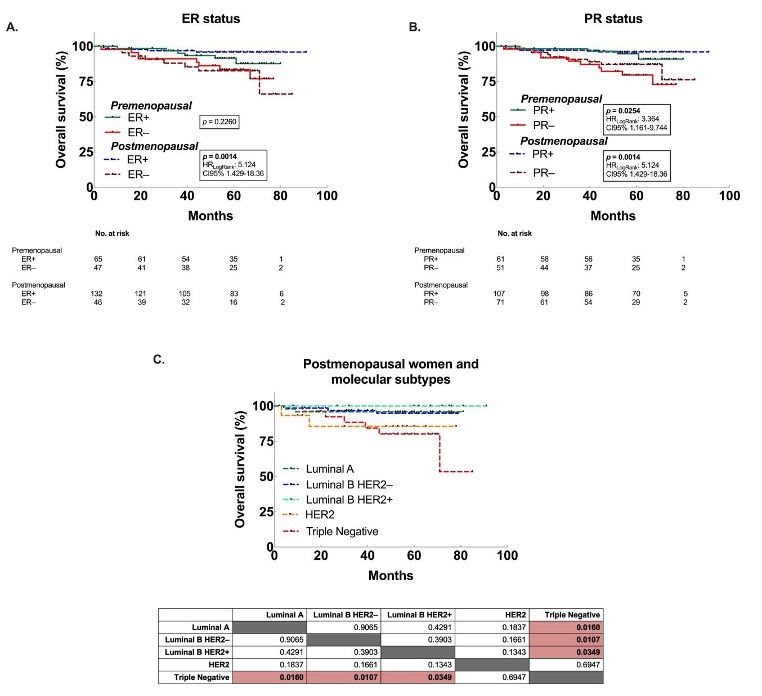
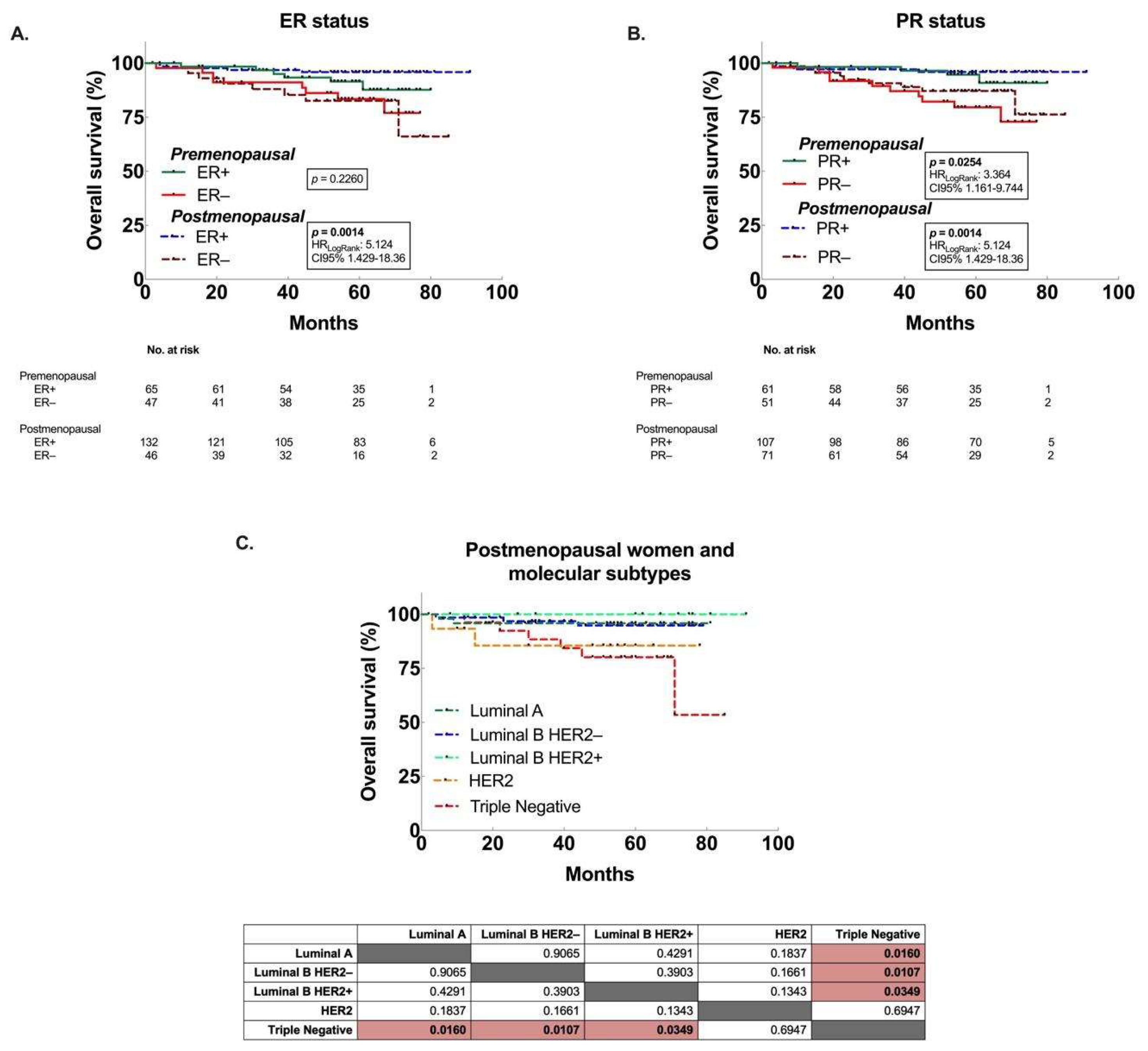
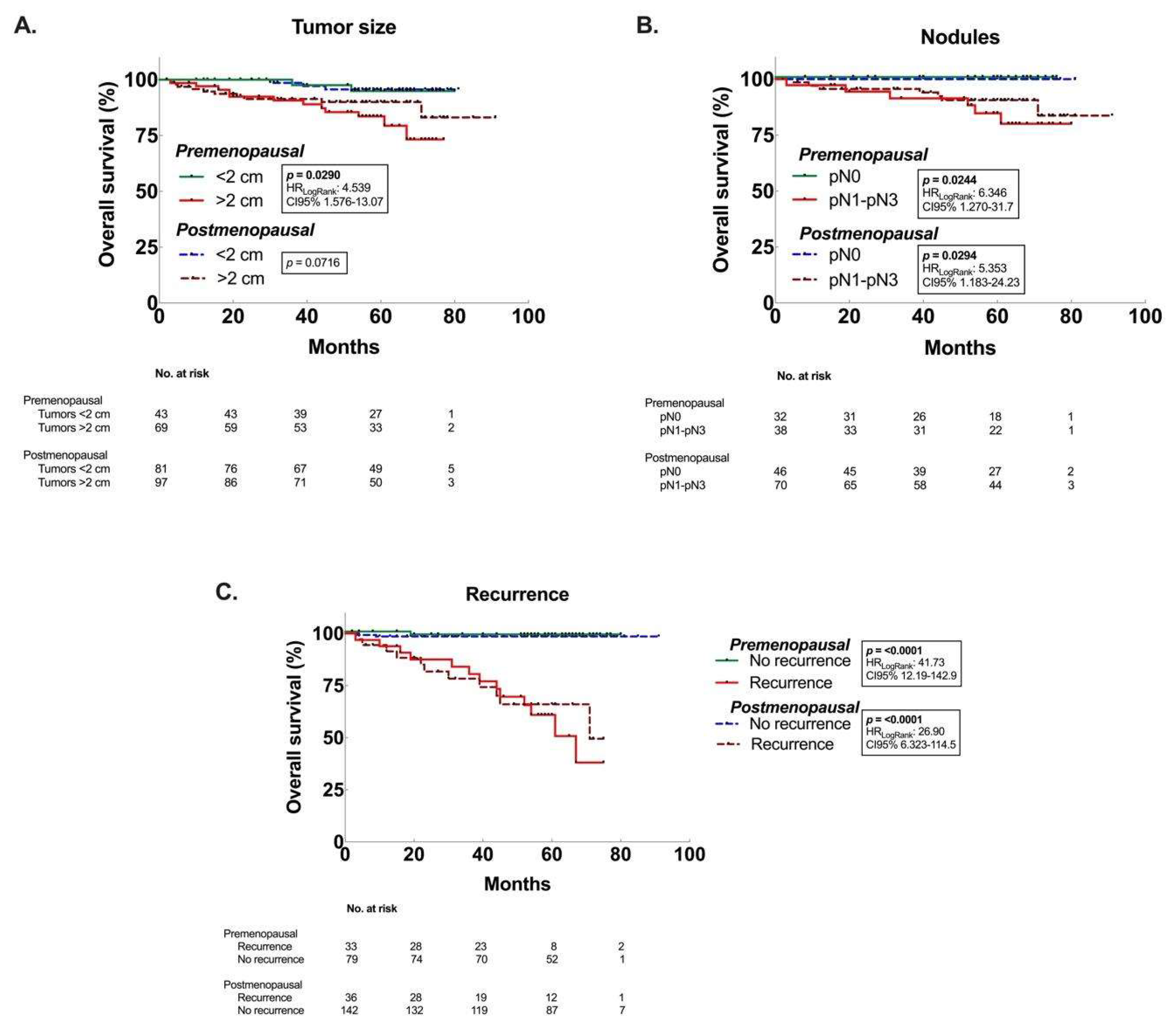
3.3. Decreased OS associated to premenopausal women with larger tumors, and postmenopausal women with ER– and TN tumors.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Conflicts of Interest
References
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021. [CrossRef]
- Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134-50. [CrossRef]
- Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocrine-Related Cancer. 2010;17(4):R245-R62. [CrossRef]
- Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers. 2021;13(17):4287. [CrossRef]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74. [CrossRef]
- Cárdenas-Sánchez J, Valle-Solís AA, Arce-Salinas C, Bargalló-Rocha JE, Bautista-Piña V, Cervantes-Sánchez MG, et al. Consenso Mexicano sobre diagnóstico y tratamiento del cáncer mamario. 9 ed. Colima, México. 2021. 233 p.
- Justo N, Wilking N, Jönsson B, Luciani S, Cazap E. A Review of Breast Cancer Care and Outcomes in Latin America. The Oncologist. 2013;18(3):248-56. [CrossRef]
- Reynoso-Noverón N, Villarreal-Garza C, Soto-Perez-De-Celis E, Arce-Salinas C, Matus-Santos J, Ramírez-Ugalde MT, et al. Clinical and Epidemiological Profile of Breast Cancer in Mexico: Results of the Seguro Popular. Journal of Global Oncology. 2017;3(6):757-64. [CrossRef]
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391-401. [CrossRef]
- Flores-Diaz D, Arce C, Flores-Luna L, Reynoso-Noveron N, Lara-Medina F, Matus JA, et al. Impact of invasive lobular carcinoma on long-term outcomes in Mexican breast cancer patients. Breast Cancer Res Treat. 2019;176(1):243-9. [CrossRef]
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M RJ, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2018, National Cancer Institute. Bethesda, MD, , based on November 2020 SEER data submission, posted to the SEER web site, April 2021. SEER Cancer Statistics Review, 1975-2018. National Cancer Institute; 2021.
- Rojas-Lima E, Gamboa-Loira B, Cebrián ME, Rothenberg SJ, López-Carrillo L. A cumulative index of exposure to endogenous estrogens and breast cancer by molecular subtypes in northern Mexican women. Breast Cancer Research and Treatment. 2020;180(3):791-800. [CrossRef]
- Hortobagyi GN, Edge SB, Giuliano A. New and Important Changes in the TNM Staging System for Breast Cancer. Am Soc Clin Oncol Educ Book. 2018;38:457-67. [CrossRef]
- Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11(3):359-77. [CrossRef]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403-10. [CrossRef]
- Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med. 2020;144(5):545-63. [CrossRef]
- Lao C, Elwood M, Kuper-Hommel M, Campbell I, Lawrenson R. Impact of menopausal status on risk of metastatic recurrence of breast cancer. Menopause. 2021;28(10):1085-92. [CrossRef]
- Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol. 2008;66(1):65-74. [CrossRef]
- Nasim Z, Girtain C, Gupta V, Patel I, Hossain MA. Breast Cancer Incidence and Behavior in Younger Patients: A Study From the Surveillance, Epidemiology and End Results Database. World Journal of Oncology. 2020;11(3):88-97. [CrossRef]
- Dubsky PC, Gnant MF, Taucher S, Roka S, Kandioler D, Pichler-Gebhard B, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3(1):65-72. [CrossRef]
- Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8). [CrossRef]
- Barnes BB, Steindorf K, Hein R, Flesch-Janys D, Chang-Claude J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011;35(4):345-52. [CrossRef]
- Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5(3):153-65. [CrossRef]
- Mahmood H, Faheem M, Mahmood S, Sadiq M, Irfan J. Impact of age, tumor size, lymph node metastasis, stage, receptor status and menopausal status on overall survival of breast cancer patients in Pakistan. Asian Pac J Cancer Prev. 2015;16(3):1019-24. [CrossRef]
| Variable | Premenopausal n/% |
Postmenopausal n/% |
p value† |
|---|---|---|---|
| No. of patients | 113/38.7 | 179/61.3 | |
| Age, year, mean (SD) | 42.9 ±5.9 | 60.2 ±9.6 | <0.0001 |
| <30 | 4/1.4 | - | - |
| 31-40 | 34/11.6 | 1/0.3 | - |
| 41-50 | 64/21.9 | 30/10.3 | - |
| 51-60 | 11/3.8 | 66/22.6 | - |
| >61 | - | 82/28.1 | |
| BMI⊥ | 28.3 ±5.4 | 29.7 ±5.6 | 0.5943 |
| <18.4 | 2/0.7 | 1/0.4 | - |
| 18.5-24.9 | 26/9.5 | 34/12.4 | - |
| 25-29.9 | 43/15.6 | 62/22.5 | - |
| 30-34.9 | 23/8.4 | 45/16.4 | - |
| 35-39.9 | 9/3.3 | 14/5.1 | - |
| >40 | 4/1.5 | 12/4.4 | - |
| Age at menarche, year, mean (SD) | 12.4 ±1.6 | 13.0 ±1.5 | 0.0665 |
| <10 | 9/4.6 | 3/1.5 | |
| 11-12 | 50/25.5 | 74/37.8 | - |
| >13 | 25/12.8 | 35/17.9 | - |
| Age at 1st gestation, year, mean (SD) | 22.0 ±5.3 | 22.2 ±5.9 | 0.8607 |
| <19 | 22/16.8 | 28/21.4 | - |
| 20-34 | 36/27.5 | 38/29.0 | - |
| >35 | 3/2.3 | 4/3.1 | - |
| Parity | - | - | 0.0015 |
| Nullipara | 18/7.9 | 26/11.5 | - |
| 1-2 | 25/11.0 | 27/11.9 | - |
| 3-4 | 39/17.2 | 38/16.7 | - |
| >5 | 10/4.4 | 44/19.4 | - |
| No. gestations | 3.2 ±1.3 | 4.4 ±2.7 | - |
| Breastfeeding, months, mean (SD) | 6.6 ±5.6 | 6.3 ±6.5 | 0.1820 |
| <6 | 24/13.1 | 46/25.1 | - |
| >6 | 50/27.3 | 63/34.4 | - |
| Age at menopause, year, mean (SD) | - | 47.2 ±5.8 | - |
| <40 | - | 18/16.1 | - |
| 41-54 | - | 86/76.8 | - |
| >55 | - | 8/7.1 | - |
| EIλ, year, mean (SD) | 28.0 ±6.5 | 30.8 ±5.6 | 0.0020 |
| Low | 30/16.0 | 16/8.6 | - |
| Medium | 39/20.9 | 55/29.4 | - |
| High | 14/7.5 | 33/17.6 | - |
| Tumor size, cm, mean (SD) | 3.7 ±3.1 | 3.3 ±2.9 | 0.2255 |
| ≤2 | 43/14.7 | 81/27.7 | - |
| >2 | 70/24.0 | 98/33.6 | - |
| pT | - | - | 0.1868 |
| pT1 | 41/14.0 | 77/26.4 | - |
| pT2 | 37/12.7 | 61/20.9 | - |
| pT3 | 15/5.1 | 11/3.8 | - |
| pT4 | 20/6.8 | 30/10.3 | - |
| No. Nodules w/ Mets | 2.7 ±4.7 | 2.7 ±3.7 | 0.8536 |
| pN0 | 32/17.2 | 46/24.7 | - |
| pN1 | 21/11.3 | 41/22.0 | - |
| pN2 | 12/6.5 | 21/11.3 | - |
| pN3 | 5/2.7 | 8/4.3 | - |
| Metastasis | - | - | 0.8191 |
| M0 | 110/37.7 | 175/59.9 | - |
| M1 | 3/1.0 | 4/1.4 | - |
| Histologic grade | - | - | 0.6485 |
| 1 | 24/8.3 | 38/13.1 | - |
| 2 | 58/20.1 | 98/33.9 | - |
| 3 | 31/10.7 | 40/13.8 | - |
| Hormone receptors | - | - | 0.0245 |
| Positive (ER+/PR+) | 55/22.3 | 106/42.9 | - |
| Negative (ER-/PR-) | 42/17.0 | 44/17.8 | - |
| Estrogen receptor | - | - | 0.0028 |
| Positive | 65/22.3 | 133/45.5 | - |
| Negative | 48/16.4 | 46/15.8 | - |
| Progesterone receptor | - | - | 0.2842 |
| Positive | 61/20.9 | 108/37.0 | - |
| Negative | 52/17.8 | 71/24.3 | - |
| Molecular subtype | - | - | 0.0397 |
| Luminal A | 28/9.9 | 50/17.6 | - |
| Luminal B | 36/12.7 | 84/29.6 | - |
| HER2 | 12/4.2 | 16/5.6 | - |
| Triple negative | 30/10.6 | 28/9.9 | - |
| Overall Survival (mo.) | 53.6 ±19.4 | 52.3 ±20.7 | 0.5928‡ (ratio 0.5354) |
| Diseased* (mo.) | 31.4 ±19.4 | 28.9 ±18.9 | 0.2768‡ (ratio 1.090) |
| Recurrence (mo.) | 22.1 ±13.4 | 29.6 ±19.0 | 0.0003‡ (ratio 3.660) |
| Premenopausal, OS | Postmenopausal, OS | |||
|---|---|---|---|---|
| Variable | p value1 | HR (95%CI) | p value | HR (95%CI) |
| Premenopausal | - | - | 0.1926 | 1.64 (0.76 - 3.55) |
| Postmenopausal | 0.1926 | 0.61 (0.28 - 1.32) | - | - |
| Tumor size | ||||
| >2 cm | 0.0290 | 4.54 (1.58 - 13.07) | 0.0716 | 3.08 (1.04 - 9.14) |
| <2 cm | 0.22 (0.08 - 0.63) | 0.33 (0.11 - 0.96) | ||
| Nodules | ||||
| pN0 | 0.0244 | 0.16 (0.03 - 0.79) | 0.0294 | 0.19 (0.04 - 0.85) |
| pN1-pN3 | 6.35 (1.27 - 31.7) | 5.35 (1.18 - 24.23) | ||
| ER | ||||
| Positive | 0.2260 | 0.53 (0.18 - 1.53) | 0.0014 | 0.2 (0.05 - 0.7) |
| Negative | 1.9 (0.66 - 5.51) | 5.12 (1.43 - 18.36) | ||
| PR | ||||
| Positive | 0.0254 | 0.29 (0.1 - 0.84) | 0.0189 | 0.27 (0.09 - 0.83) |
| Negative | 3.45 (1.2 - 9.98) | 3.71 (1.21 - 11.39) | ||
| Molecular subtype | ||||
| Triple Negative | 0.1599 | 2.1 (0.64 - 6.89) | 0.0021 | 4.72 (1.05 - 21.28) |
| All subtypes | 0.48 (0.15 - 1.57) | 0.21 (0.05 - 0.96) | ||
| Recurrence | ||||
| Yes | <0.0001 | 41.73 (12.19 - 142.9) | <0.0001 | 26.9 (6.32 - 114.5) |
| No | 0.02 (0.01 - 0.08) | 0.04 (0.01 - 0.16) | ||
| 1p value calculated withMantel-Cox test (Log-Rank); OS: overall survival; HR: hazard ratio; CI: confidence interval.ER: estrogen receptors; PR: progesterone receptors. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





