3. Discussion
In the first step of our experiments we evaluated the indirect influence of ECS on the anxiety-like effects in mice using EPM test. For this purpose, we used selected ligands of CB1, CB1/CB2 and CB2 receptors to assess which type of CB receptors is mainly involved in the anxiety-related responses in mice.
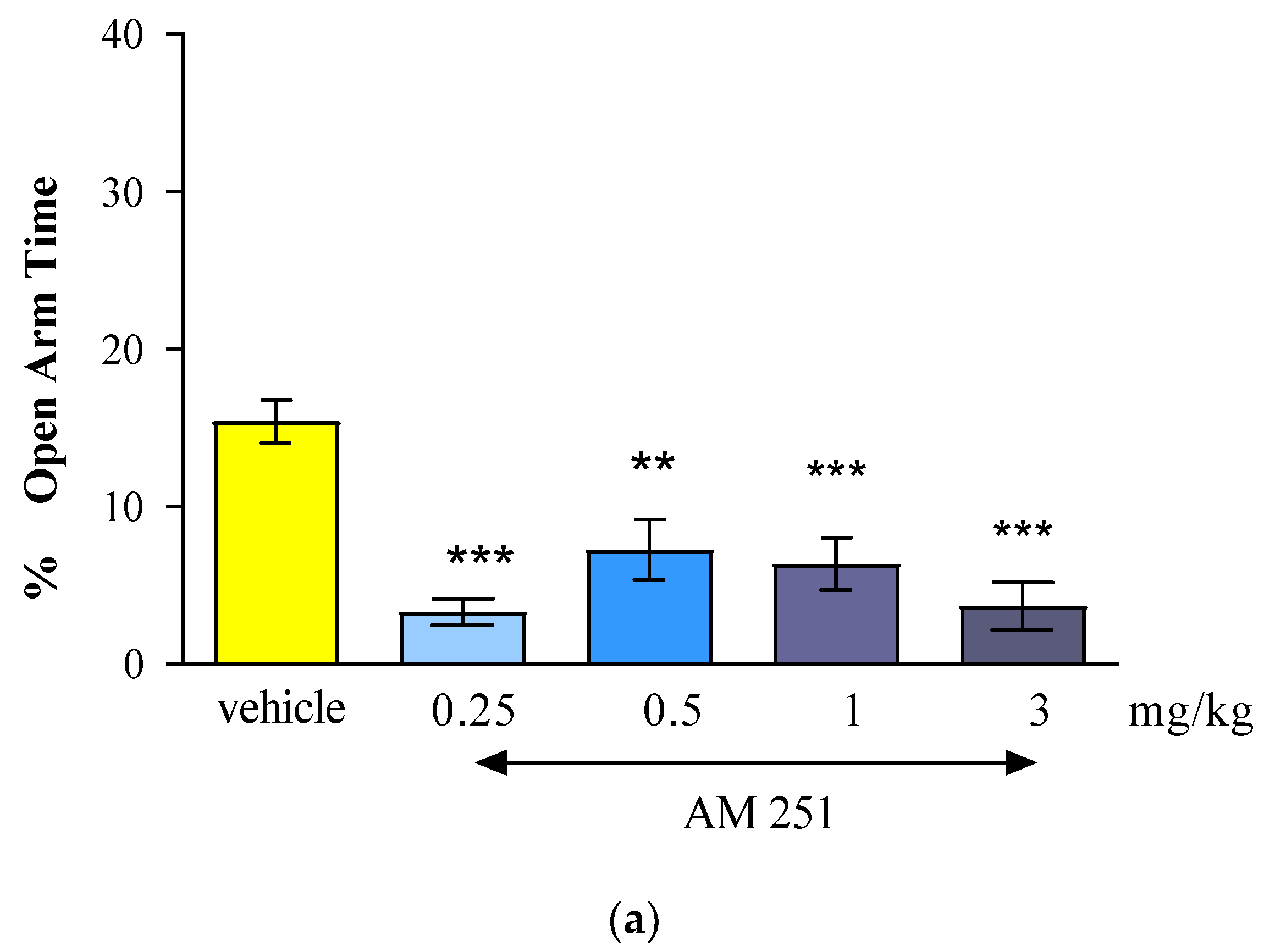
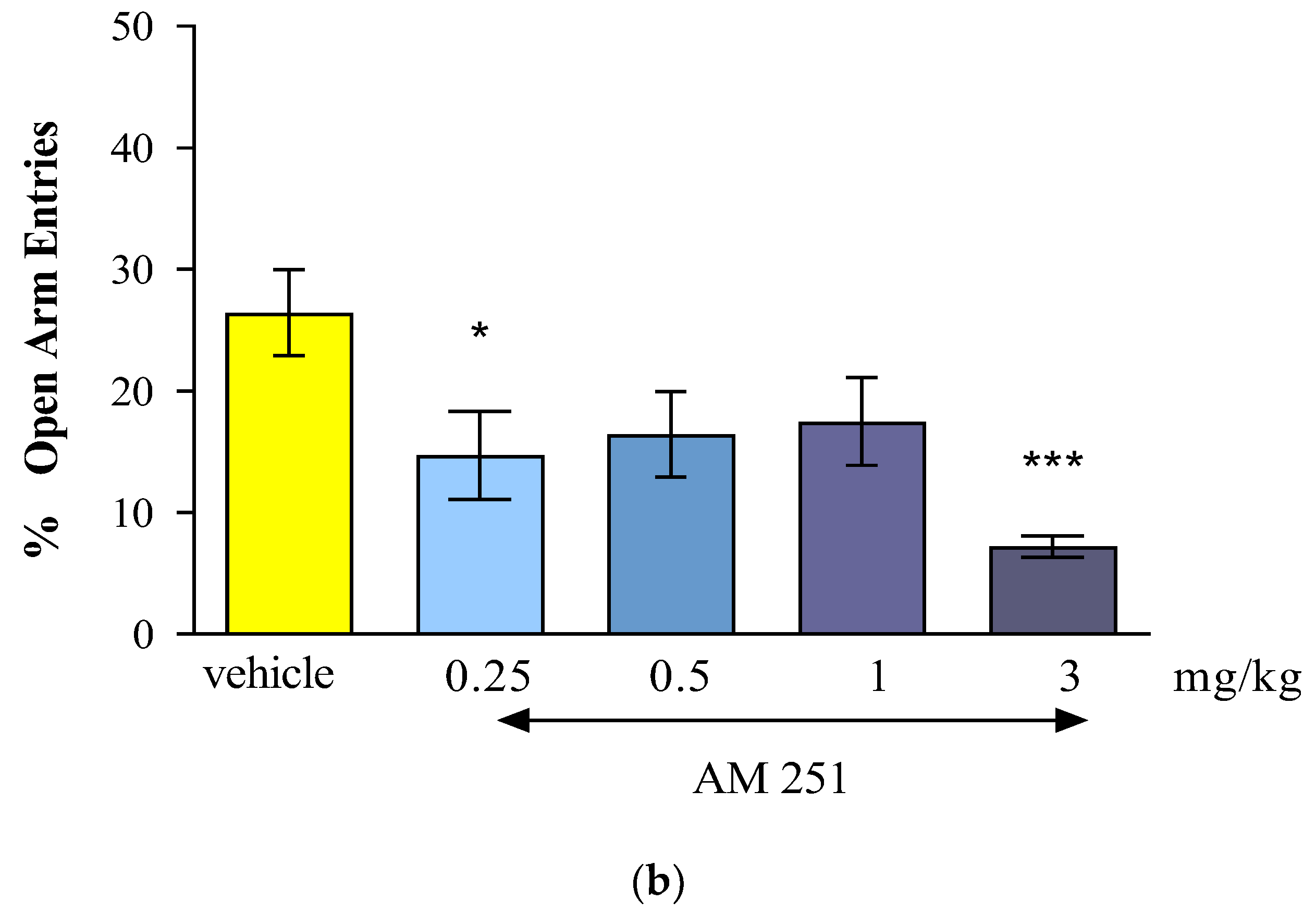
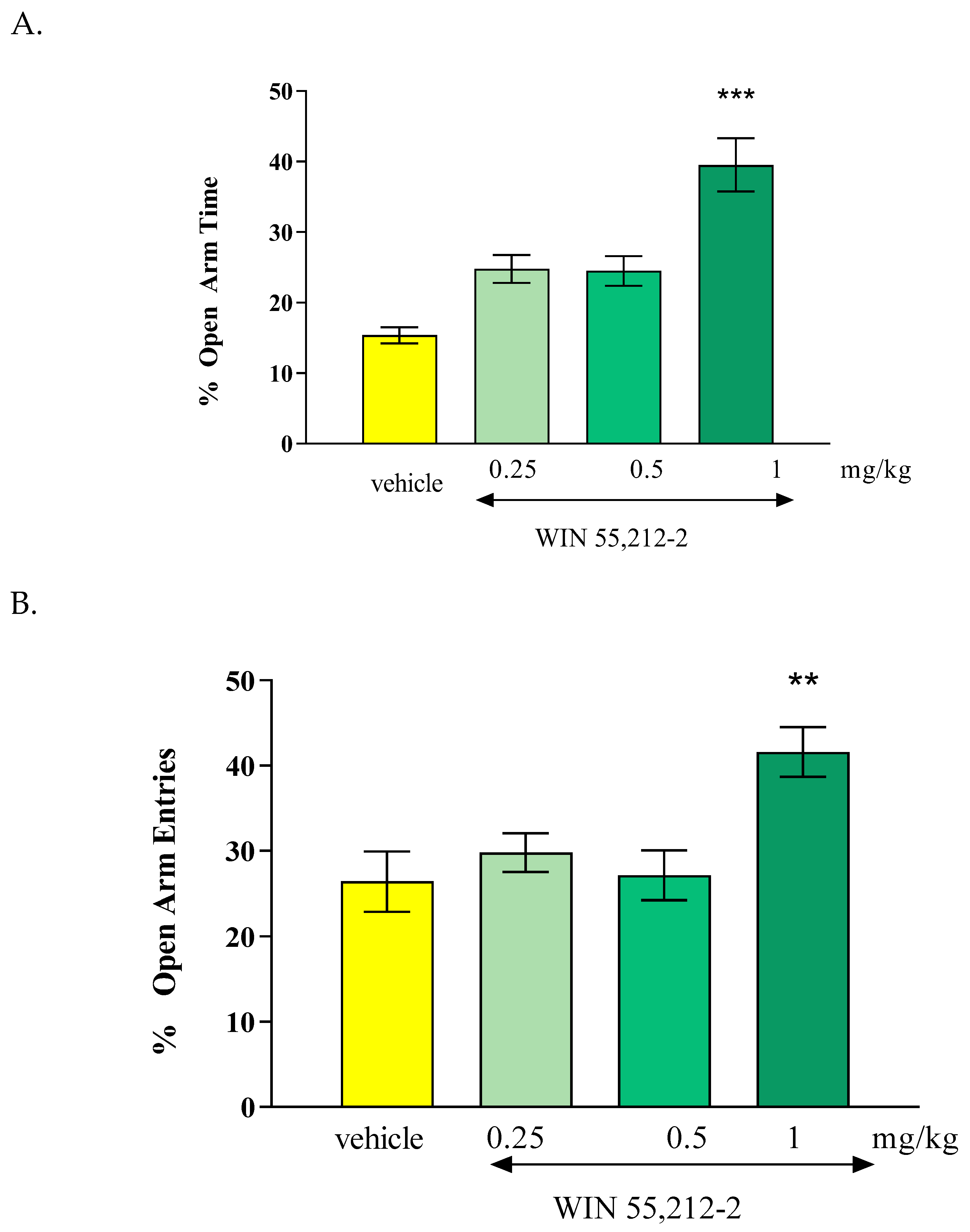
The results of our presented research showed that the CB1 receptor agonist - oleamide administered at doses of 2.5 mg/kg, 10 mg/kg and 20 mg/kg had an anxiogenic effect. Interestingly, the CB1 receptor antagonist - AM 251 administered at the lowest dose of 0.25 mg/kg, had also an anxiogenic effect, while the administration of higher doses of AM-251 (0.5 and 1 mg/kg) had an anxiolytic effect. Moreover, the acute injection of mixed CB1/CB2 receptor agonist - WIN55,212-2 at a dose of 1 mg/kg had an anxiolytic observed in the EPM test in mice.
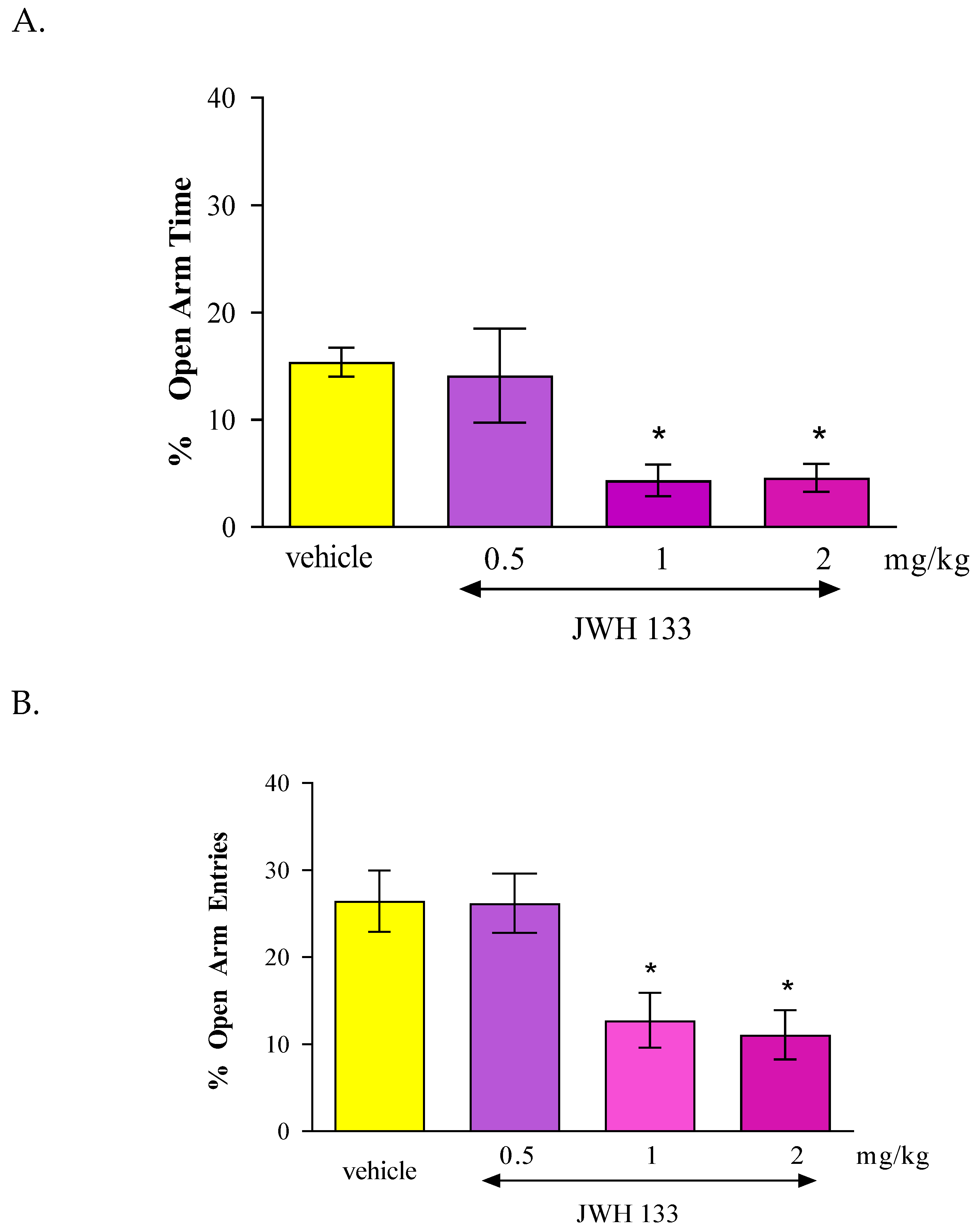
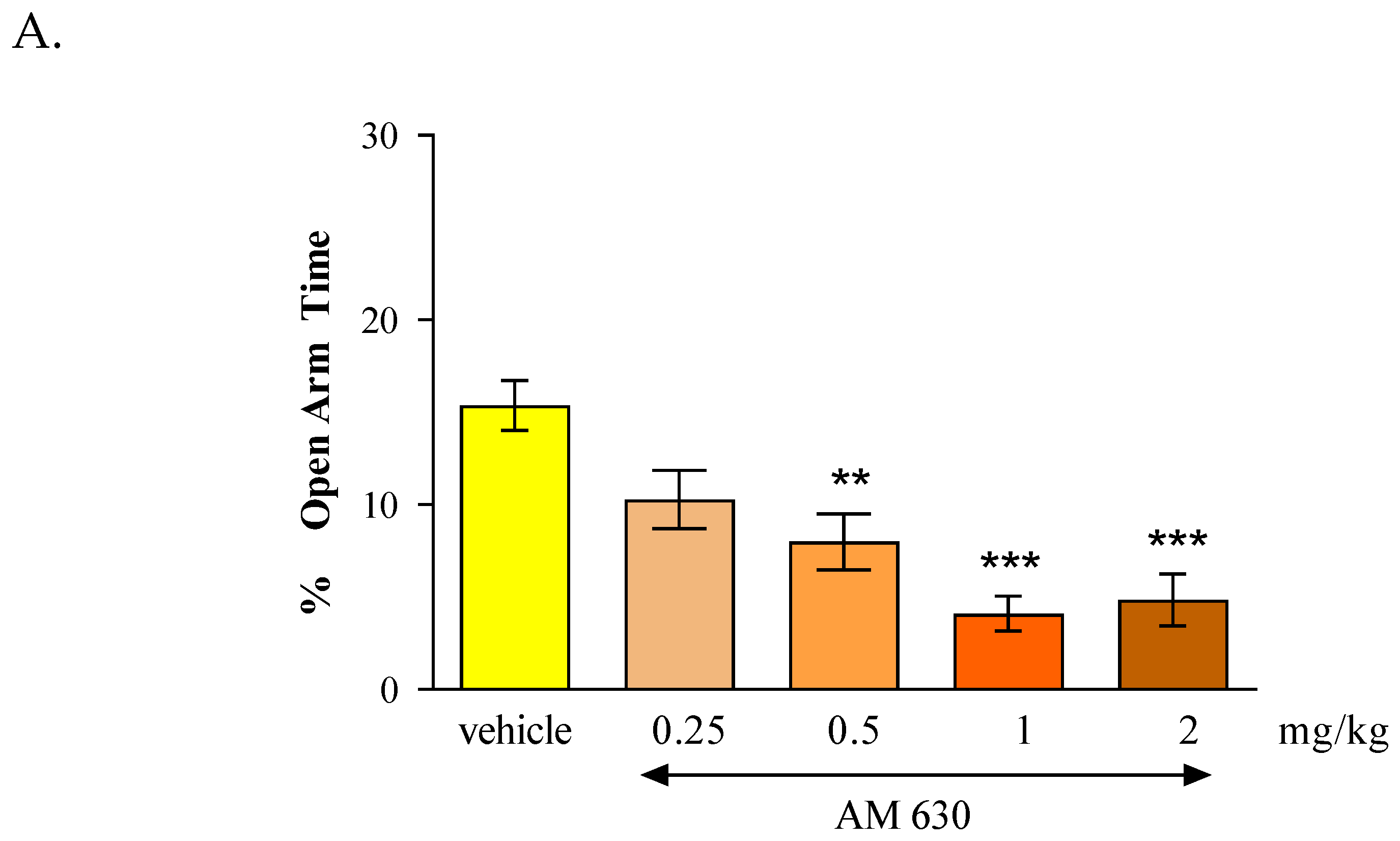
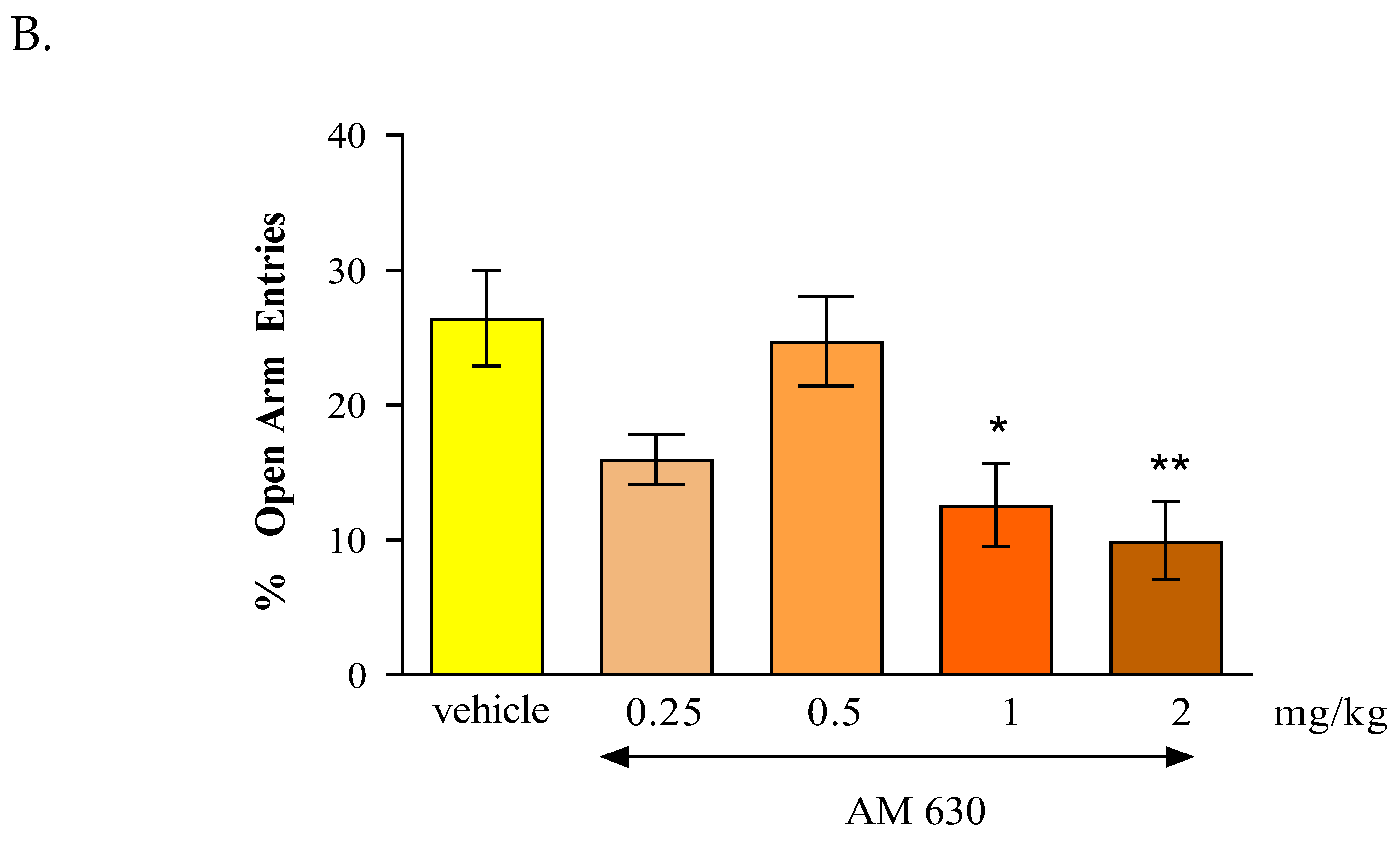
In the case of CB2 receptor involvement in anxiety-related behavior in mice, we revealed that an acute administration of CB2 receptor agonist JWH 133 and also the CB2 receptor antagonist AM 630 had similar influence on the parameters measured in the EPM test. It was shown that the CB2 receptor agonist JWH 133 injected at doses of 1 and 2 mg/kg exhibited anxiogenic effects and an identical effect was observed after an acute injection of the CB2 receptor antagonist AM 630 injected at doses of 0.5, 1 and 2 mg/kg.
Our results should be discussed in the context of other available data which indicate that cannabinoids compounds can significantly modulate various behaviors and emotions, both in terms of mood and anxiety in animals and humans [
23]. However, these reports are not unequivocal. According to some scientists, CB1 receptor agonists exhibit anxiogenic or anxiolytic effects in rodents, depending on the administered dose - low doses induce anxiolytic effects while higher doses - anxiogenic effects [
16,
17,
18,
23]. It has been also reported that intracerebral administration of CB1 receptor agonists causes anxiolytic and antidepressant effects [
23,
24,
25,
26]. For example CB1 receptor agonist - THC, shows anxiolytic effects in humans; thus, this compound can be also anxiogenic in rodents, depending on the dose and context [
27]. Low doses of THC can reduce anxiety-like behavior in the EPM test, where rodents are tested for anxiety based on their reluctance to enter open spaces. Higher doses of THC are often associated with increased anxiety or paranoia [
28,
29,
30]. What is of interest, in experimental animal models, THC, administered at low doses, mimics the anxiolytic effects of diazepam [
31,
32]. Moreover, in addition to their rewarding effects, cannabinoids can also induce aversive effects, especially anxiety and panic attacks, especially after very high doses of the drugs [
32,
33,
34]. It has also been shown that they can induce conditioned aversion, especially when animals are placed in an environment that they associate with previous drug administration [
35]. In turn, administration of CBD, a non-psychoactive cannabinoid, had gained significant attention for its potential anxiolytic properties. Unlike THC, CBD is generally considered to reduce symptoms of anxiety and its effects have been widely explored in both human and animal studies. For example, CBD has been found to reduce anxiety in commonly used models for assessing anxiety in rodents, e.g., the light/dark box (LDB) test, the EPM test, and the open field test (OFT) [
36,
37,
38,
39]. However, there is also contradiction in behavioral outcomes of CBD treatment with some studies reporting anxiogenic-like effects in rodents [
40,
41].
In the context of our results, interesting data described the influence of another CB1 receptor agonist - oleamide in various physical and mental functions in the body. The impact of that compound on the central nervous system (CNS) is especially of interest, especially pointing out on the memory and learning, sleep, mood and anxiety [
42,
43]. Some studies suggested that oleamide may have positive effects on cognition and depressive-related behavior; as such this cannabinoid compound shows calming impact and may decrease anxiety level in animal models [
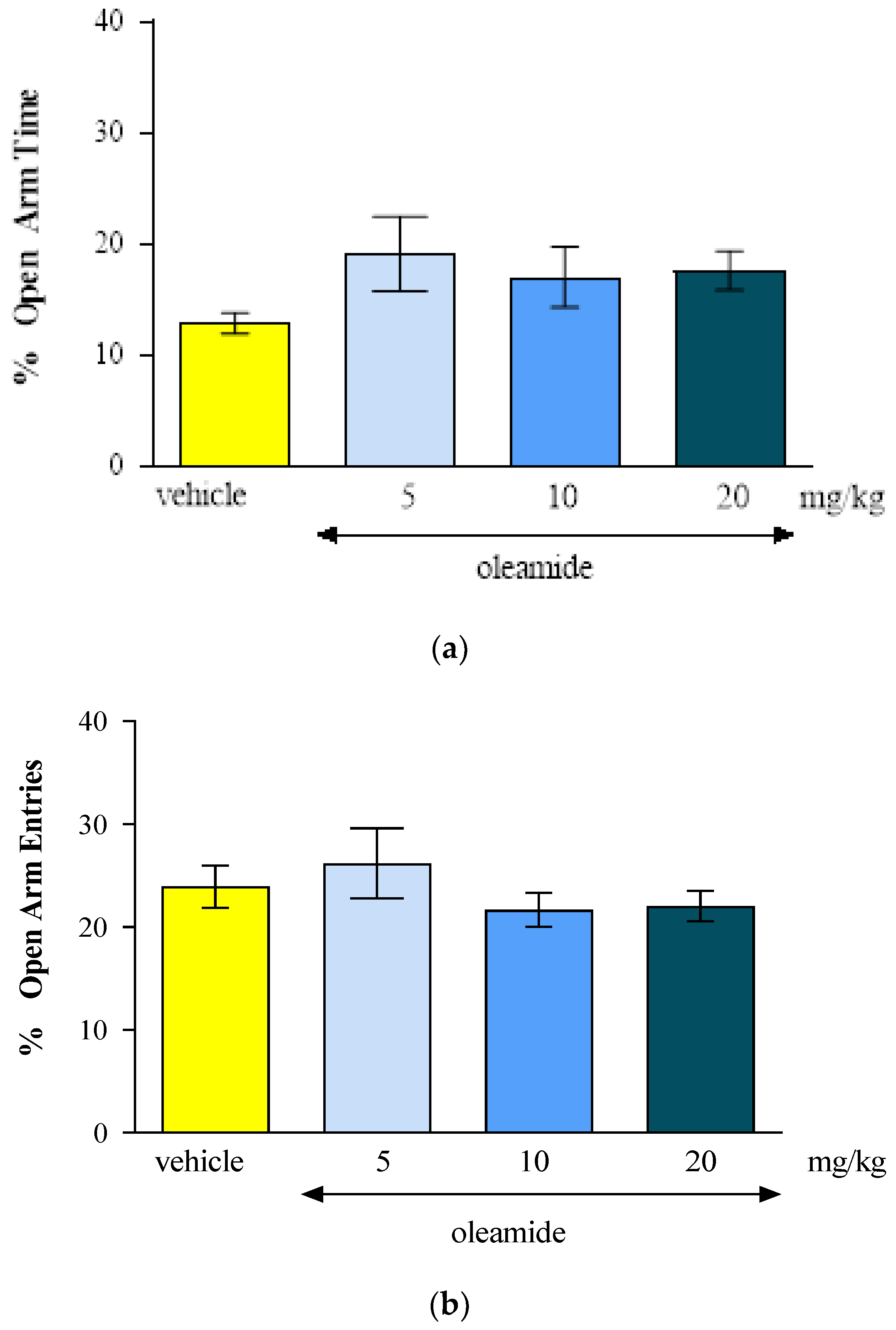
44]. Unfortunately, in our studies, oleamide at the doses used (5-20 mg/kg) did not affect the level of anxiety in mice in the EPM test, however, a tendency to increase in specific parameters of EPM (the percentage of the time and of the number entries spent into open arms of EPM) was maintained, which may indicate and confirm a potential anxiolytic effect of oleamide.
CB1 receptor activation (due to their localization in the CNS and the increase in endocannabinoids level in brain) is often associated with reduced anxiety. As a result of this localization (mainly in the brain structures involved in emotional control including basolateral amygdala, cortical regions and the hippocampus), CB1 activation might have a complex pattern of influence upon neurotransmitters known to modulate anxiety. Thus, the anxiolytic effects of oleamide is probably connected with the interaction with CB receptors (mainly CB1) and interaction with specific various neurotransmitters and systems. Oleamide interacts with gamma aminobutyric acid (GABA)-ergic and serotoninergic systems, enhancing these signaling pathways. Following that, these stimulations lead to anxiolytic effect which may be similar to effects of other CB1 receptor agonist described above [
42,
43,
44].
Therefore, CB1 receptor antagonists may potentially have opposite effect, increasing anxiety-related responses in animal models. Many scientific publications report that single injections of CB1 receptor antagonists – rimonabant and AM 251, cause an increase in aversive behaviors associated with generalized anxiety disorders [
17,
18,
45,
46,
47,
48]. Rimonabant was used successfully in the treatment of obesity, but it also showed a number of side effects, such as anxiogenic effects, decreased mood and induced pro-suicidal behaviors. There are also scientific studies indicating the lack of effect of rimonabant on anxiety-like behaviors assessed in the EPM test in mice within the range of the tested doses [
23,
49].
In case of AM 251, an analogue of rimonabant that is a potent and selective CB1 receptor antagonist but which, unlike rimonabant, has no activity at the novel receptor, the literature data have shown that this compound had also anxiogenic-like profile. In maze-naïve mice, the lower dose of AM-251 (1.5 mg/kg) significantly reduced percentage of open-arm time and increased grooming while the higher dose (3.0 mg/kg) additionally reduced percentage of open-arm entries and total head-dipping, and increased closed-arm returns in the EPM test [
50,
51]. These results are in accordance with the results obtained from our study. We also revealed that AM 251 tends to increase anxiety-like behaviors in mice in the EPM test. However, there are also scientific reports indicating the anxiogenic effect of AM 251, observed in wild-type mice, as well as the lack of any effect on mice lacking the gene encoding the CB1 receptor [
52]. Moreover, AM 251 has the potential to reverse the sedative effect induced by diazepam in rats in the EPM test [
53].
In turn, another CB1 receptor antagonist AM 281 did not influence anxiety reaction in the dark/light box (LDB) test when given alone [
54]. Similarly, other CB1 receptor antagonist, AM 4113, has no effect on anxiety reactions. It has also been shown that AM 4113, to a much lesser extent than AM 251, affects the activation of neurons in brain structures key to anxiety reactions, i.e., the amygdala [
55]. It is hypothesized that this effect is related to the fact that AM 251 also exhibits activity as a reversible agonist of CB1 receptors, whereas AM 4113 only exhibits antagonist effects at this receptor.
To clearly assess the role of CB receptors in anxiety-related processes, in subsequent experiments the next compound we tested was the mixed CB1/CB2 receptor agonist - WIN 55,212-2. We revealed that the administration of this cannabinoid compound at a dose of 1 mg/kg produced an anxiolytic effect, assessed in the EPM test in mice. Lower doses do not affect anxiety-like behaviors. These results are only partially consistent with the results of other researchers because in some animal studies, WIN 55,212-2 might cause anxiety or other negative psychological effects while other studies suggest potential anxiolytic effects, depending on the dosage, context, individual differences and circumstances of experience. The researchers described that a dose of 0.25 mg/kg caused an anxiolytic effect, while a high dose of 1.25 mg/kg showed an anxiogenic effect [
56,
57]. After the higher dose, WIN 55,212-2 (5 mg/kg) induced the anxiogenic-like effect accompanied by motor inhibition in the LDB test [
54]. These results are consistent with the effects of experiments conducted using other mixed CB receptor agonists: HU-210 and CP 55940. These cannabinoid compounds when administered at high doses, also intensify anxiety reactions in mice [
17,
25,
26,
45,
46]. What is of interest, both effects of WIN 55, 212-2, i.e., the anxiogenic-like and the sedative one were attenuated by a CB1 receptor antagonist, AM 281, suggesting mainly involvement of CB1 receptors [
54].
More complicated and unclear is the role of CB2 receptors in emotions because the CB2 receptor is mostly expressed in peripheral tissues, including immune system. Most evidence suggests that cannabinoid ligands acting on CB2 receptors could have less direct impact on mood and behavior compared to those targeting CB1 receptors, but they may modulate anxiety-related responses through immune system effects, e.g., modulating inflammation and potentially affecting neuroinflammation, which may contribute to anxiety disorders [
5,
6,
7]. We have found some scientific reports that described the influence of CB2 receptor ligands on emotions, mood and anxiety reactions. However, these data are so often contradictory. The available literature data results have shown their antidepressant, anxiolytic, anxiogenic effects or they show no effect on anxiety reactions at all [
58,
59]. The diversity of the results obtained may be due to many factors, including the strain of animals used in the experiments, the experimental conditions, the type of procedure used to assess the results [
60], the drugs previously used, differences in the doses used, and the initial level of anxiety [
61].
In our experiments, we revealed that both an acute administration of CB2 receptor agonist (JWH 133 at the doses of 1 and 2 mg/kg) and antagonist (AM 630 at the doses of 0.5-2 mg/kg) had anxiogenic effects in the EPM test in mice. These results are partially consistent with the results available in the scientific literature. In some studies, CB2 receptor ligands may be used to explore their potential anxiolytic or anxiogenic effects through animal models. Acute administration of JWH 133 (0.5-2 mg/kg, ip) failed to produce any effect observed in two animal anxiety models (LDB and EPM). In turn, acute administration of AM 630 (1-3 mg/kg, ip) increased anxiety and additionally this effect of AM 630 (3 mg/kg) was blocked by pre-treatment with JWH 133 (2 mg/kg). Chronic administration of JWH 133 (0.5-2 mg/kg, ip, twice a day) for a total of 7 day treatment increased anxiety-like behavior whereas chronic AM 630 treatment (1-3 mg/kg, ip, twice a day) produced a significant anxiolytic effect observed in LDB and EPM tests [
58].
The contrasting responses to anxiety-like behaviors observed following chronic and acute administration of AM 630 and JWH 133 confirm the complicate but key role of CB2 receptors in the regulation of anxiety-like behaviors. The distribution of CB2 receptors in brain areas associated with stress and anxiety responses also suggests the involvement of these receptors in the modulation of emotional behaviors. Scientific publications report that additional biochemical studies of the cerebral cortex and amygdala were conducted to determine the mechanisms underlying the above-described behavioral changes. It was proven that the anxiolytic effect induced by chronic administration of AM 630 is associated with increased expression of the gene encoding CB2 in both the cerebral cortex and the amygdala, as well as with reduced expression of the CB2 receptor protein in the cerebral cortex. Interestingly, the anxiolytic effect of JWH 133 was accompanied by opposite changes in CB2 receptors. Chronic administration of JWH 133 decreased expression of the CB2 receptor gene in the amygdala, and increased expression of the CB2 receptor protein in the cortex. What is of interest, in experiments conducted using transgenic mice with increased expression of CB2 receptors in the CNS, a phenotype resistant to a single exposure to aversive factors used in the LDB and EPM procedures was observed [
62,
63]. The latest findings confirming the role of CB2 receptors in the regulation of anxiety and mood disorders also include studies showing that mice lacking the gene encoding the CB2 receptor (CB2 - / -) show increased susceptibility to stressful stimuli, assessed in the LBD, EPM and tail suspension test (TST) procedures [
62,
63,
64].
In order to further suggest neurophysiological mechanisms underlying the above-described effects, as we already mentioned, the GABAergic system is considered to be a key element in the regulation of emotional states. Pentameric GABA-A receptors are formed by the assembly of various subunits containing α1, α2, α3 or α5 with β and γ2 subunits. Literature data indicate that GABA-A receptors containing α2 and γ2 subunits mediate the anxiolytic effects of benzodiazepines [
65]. Both subunits are located in the limbic system and cerebral cortex [
66,
67,
68]. It has been proven that genetic changes in CB2 receptors also lead to changes in GABA-A receptors. Transgenic mice with increased expression of CB2 receptors also show increased GABA-Aα2 and GABA-Aγ2 gene expression in the hippocampus and amygdala [
58]. Scientific reports indicated the inhibition of GABA-ergic neurotransmission in the cerebral cortex and hippocampus after the administration of the CB2 receptor agonist – JWH 133. These effects can be blocked by prior administration of AM 630. This result supports the thesis of the involvement of CB2 receptors in the effects exerted by JWH 133 on GABA-ergic transmission and thus the possibility of modulation of GABA-ergic neurotransmission via CB2 receptors [
58].
Summarizing the above described results, also taking into account the results of our work, it is not possible to clearly determine the influence of CB1 and/or CB2 receptor ligands on anxiety-related behavior, because they are dependent on many factors, as mentioned, on the dose, the test used or the route of administration. The mechanisms of these phenomena also require explanation, although the indirect influence of CB receptor activation on the activity of other neurotransmitter systems, including GABA and monoaminergic ones, can be taken into account. Further studies are therefore necessary to explain these effects, their type and potential mechanisms. Additionally, a direct modulation of ECS via ligands of CB receptors could be not effective in treating emotional disorders and is always connected with many adverse effects.
Alternative and promising approach to avoid the adverse effects of direct CB receptor ligands has been focused to evaluate the effects of indirect activation of ECS system, by inhibiting the process of endocannabinoids degradation by the FAAH and MAGL enzymes. Thus, to increase the knowledge in this context, in the second set of our experiments we evaluated the influence of indirect modulation of ECS function on the anxiety-like responses in mice in the EPM.
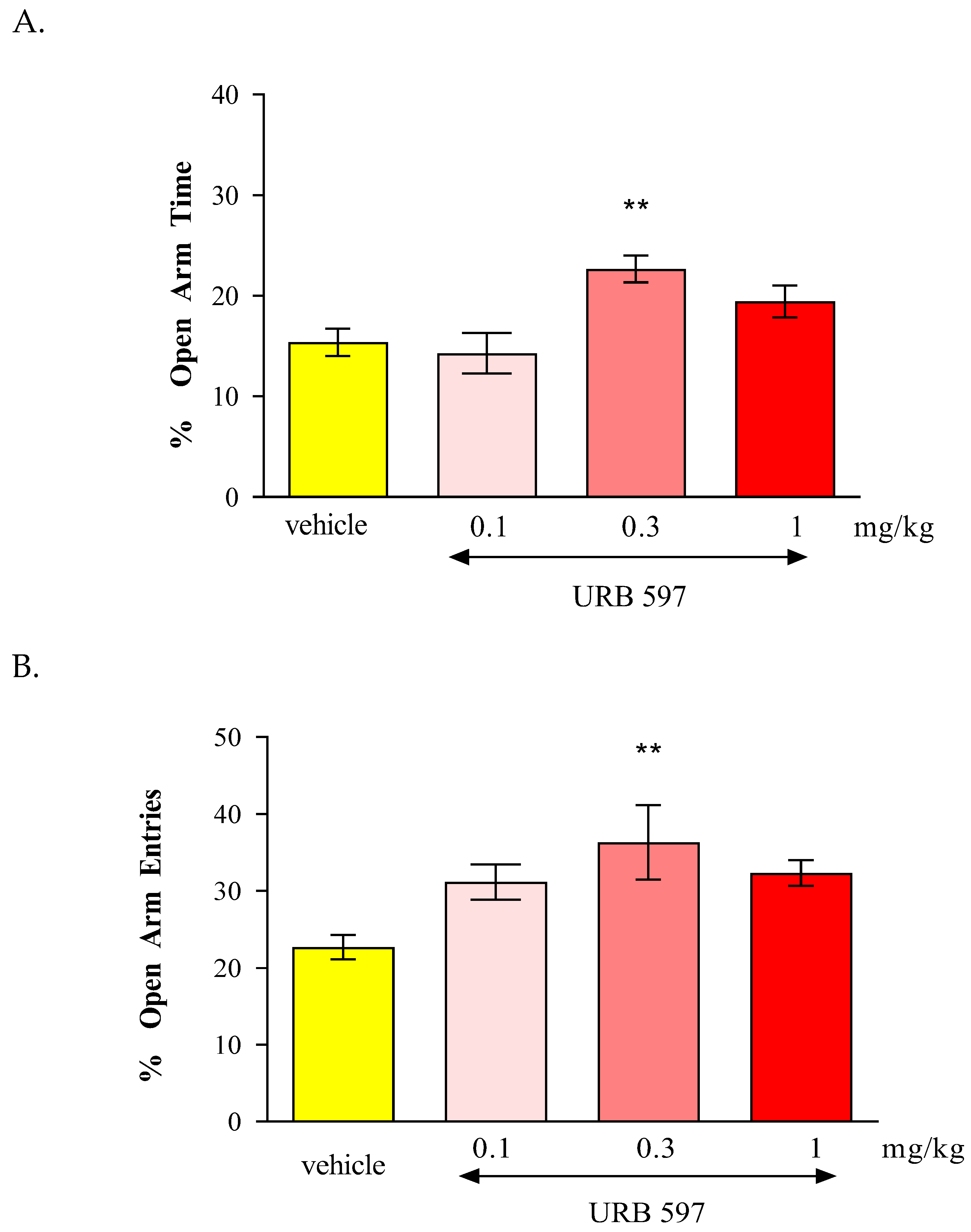
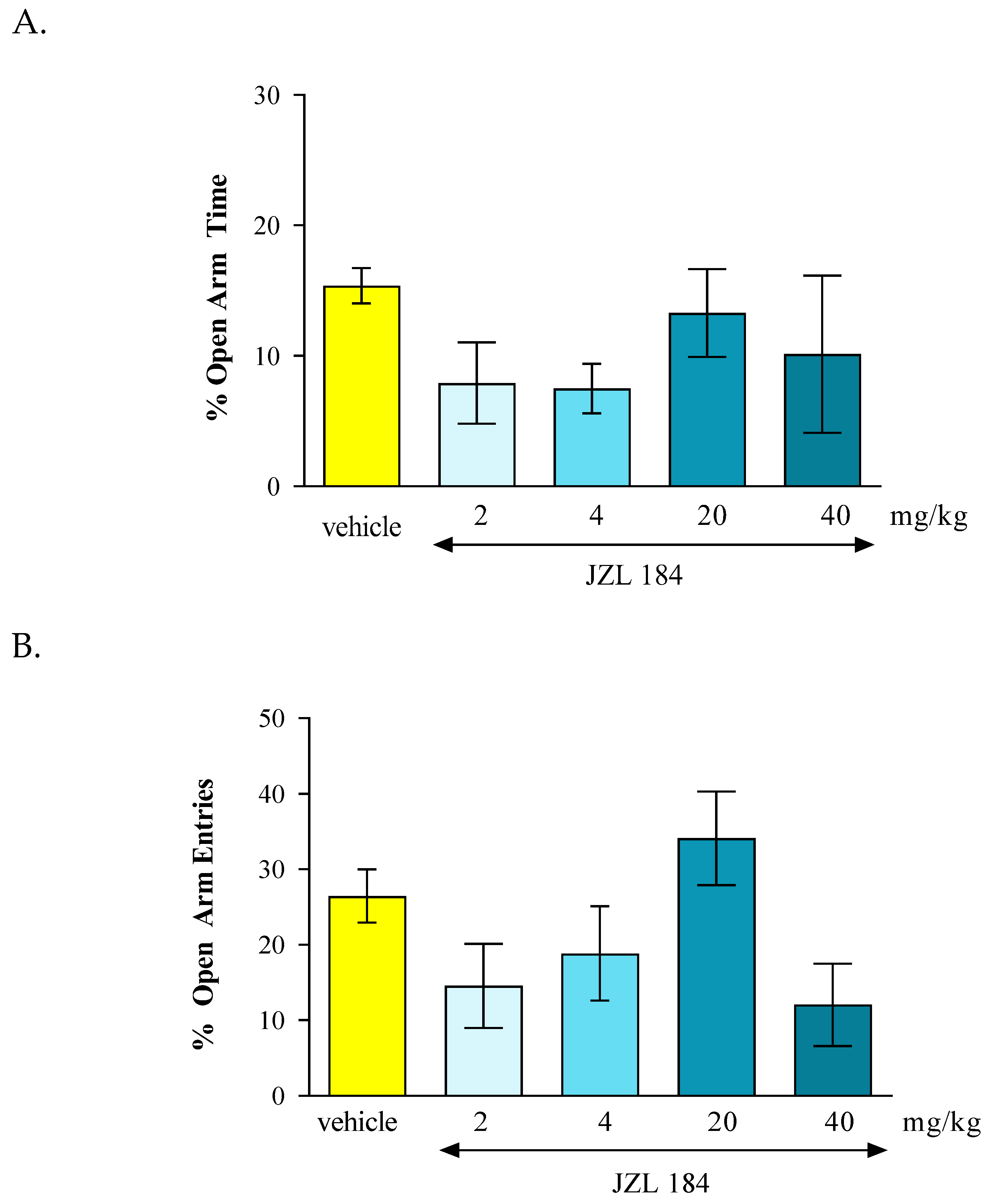
In the conducted experiments, after an acute administration of URB 597 (0.3 mg/kg), a FAAH inhibitor, we observed dose-dependent anxiolytic effects in the EPM test in mice. Lower doses may cause reduction in anxiety, while higher doses may produce sedation or other behavioral changes that might confound results. In our studies we observed anxiolytic effect of URB 597 only at the dose of 0.3 mg/kg. In turn, after an acute administration of JZL 184 (2 mg/kg, 4 mg/kg, 20 mg/kg and 40 mg/kg), a MAGL inhibitor, and no statistically significant change was observed in the amount of time spent in the open arms of the EPM, as well as the number of entries into these arms.
Our results should be discussed with other available literature data. Some studies suggest that inhibition of FAAH or/and MAGL could lead to enhanced endocannabinoid signaling and thus elevation of AEA or 2-AG could be represent potential targets for the development of new classes of pharmacotherapeutics to treat anxiety-related disorders [
69,
70,
71]. However, cited results are not clear and our results of the experiments in the presented work are not quite consistent with the literature data.
In our experiments we used URB 597, a selective inhibitor of FAAH, which is responsible for the breakdown endocannabinoids in the brain, particularly AEA. We can supposed that URB 597 by inhibiting FAAH and increasing AEA levels activated CB1 receptors, the mostly implicated in regulation of fear and anxiety responses, especially those located in brain regions like the hippocampus, amygdala and prefrontal cortex. Our results are consistent with other scientific reports. For instance, literature data indicated that URB 597 and its analog URB 532 produced anxiolytic-like effects in several animal models, including LDB and EPM test. These effects were blocked by the administration of CB1 receptor antagonists, rimonabant [
70]. Moreover, both compounds increased AEA level in the brain without modifying those of the second endogenous cannabinoid, 2-AG. It is therefore likely that their pharmacological actions, which are sensitive to the CB1 antagonist rimonabant, are primarily due to AEA accumulation. However, other FAAH inhibitor, e.g., ST 4070 also produced anxiolytic effect after intragastric administration in CD1 mice in the EPM test and LDB test [
16,
72] and increased the level of AEA and two AEA analogs N-palmitoylethanolamine oleoylethanolamide, whose biological effects are independent of CB1 receptors [
16,
70,
72,
73,
74]. Therefore, we should not exclude other mechanisms related to the action of FAAH inhibitors.
Additionally, it has been proven that URB 597 shows exceptional selectivity towards FAAH, while showing no affinity towards other ECS elements. Thanks to this, administration of URB 597 selectively inhibits FAAH activity and significantly increases the level of AEA in the brain. Due to this property, it does not cause a number of adverse classic effects accompanying the administration of direct CB receptor ligands, i.e., catalepsy or hypothermia, which are symptoms of cannabinoid poisoning in a rodent [
70,
75,
76,
77]. The results of the presented experiments suggest that AEA participates in the modulation of emotional states and indicate the inhibition of FAAH as an innovative approach to anti-anxiety therapy.
However, not only AEA, but also another major endocannabinoid 2-AG could activate CB1 receptors in the brain, particularly in regions involved in emotional processing, such as the amygdala and prefrontal cortex and thus can promote feelings of relaxation and reduce anxiety. Unfortunately, the effects of 2-AG on anxiety have been difficult to ascertain, due to its rapid in vivo metabolism. MAGL is the primary enzyme responsible for 85% of 2-AG catabolism and MAGL inhibitors prevent the breakdown of 2-AG, leading to increased 2-AG levels and could have a potential anxiolytic effects. However, it should be noted that other enzymes, such as ABHD6 and ABHD12, contribute to 2-AG degradation, thus, they have distinct intracellular distributions [
71]. As such, it is possible that the inhibition of these minor 2-AG catabolic enzymes may also affect anxiety-related behavior in ways that differ from MAGL inhibition.
In our experiments, we assessed the effect of inhibition of MAGL on the anxiety-related responses in mice observed in the EPM test. We used JZL 184 that is a potent and selective MAGL inhibitor. JZL184 [
29] significantly inhibits MAGL in vivo and is highly selective (300-fold higher for MAGL than for FAAH), with long-lasting effects (> 24 h). [
29,
78].
Unfortunately, in our studies, we did not demonstrate any effect of JZL on anxiety-related behavior in mice. However, there is some literature data describing the anxiolytic potential of MAGL inhibitors, including JZL 184 [
78,
79,
80,
81]. Sciolino et al. [
81] has been reported that chronic administration of JZL184 (8 mg/kg per day for six days) produced anxiolytic-like effects in the EPM under high, but not low, levels of environmental aversiveness. These anxiolytic effects of JZL 184 were prevented by the CB1 inverse agonist rimonabant. The amygdala seems to be critical to this response because the local infusion of an MAGL inhibitor into this structure recapitulates the effects of systemic MAGL blockade [
79]. In turn, Ivy et al. [
82] revealed that systemic administration of JZL 184 (4, 8 and 16 mg/kg, ip) caused dose-dependent significant anxiolytic-like effects in rats, as demonstrated in the EPM test and marble burying tests but surprisingly, the effect of JZL184 was prevented by co-administration of the CB2 inverse agonist AM630, but not rimonabant. Additionally, many other effects induced by 2-AG, including reduction in GABA transmission, are independent of mechanisms related to CB1 receptors. It has been revealed that these effects are mainly induced by CB2 receptors, as they are not blocked by the CB1 receptor antagonist - LY 320135, but by the CB2 receptor antagonist - AM 630 [
83].
The contradictory results presented in the experiments discussed above may result from significant differences between the test procedures used, the experimental conditions and the species of animals used in these studies. The anxiolytic effects of JZL-184 may be context-dependent, meaning they are influenced by environmental factors, such as stress levels or the presence of stressors. Additionally, further investigations are necessary to assessment the molecular mechanism of endocannabinoids mobilization and its connection with long-term anxiety responses.