1. Introduction
1.1. Breast Cancer
Breast cancer is the most common type of cancer globally, with more than 2.2 million cases reported in 2023, and the most frequent malignancy among women, making it the leading cause of cancer-related death in females (5.8% of all cancer deaths; 14.6% of cancer-related deaths in women). Moreover, 1 in every 12 women will develop this type of tumour during their lifetime [
1,
2,
3,
4].
Breast cancer typically occurs between the ages of 35 and 80, with the highest incidence observed in the 45–65 age group, coinciding with hormonal changes associated with peri- and postmenopause. Incidence increases with age; this explains why the number of breast cancer cases has risen in recent years, as life expectancy has also increased [
4].
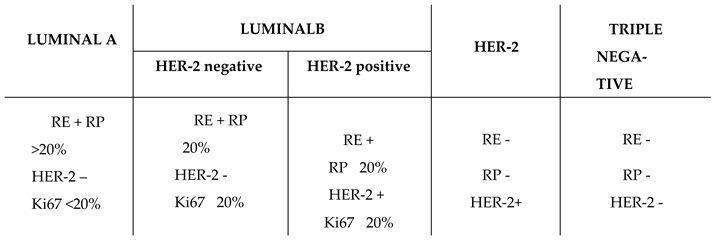
This type of tumour is categorised into four distinct types based on the molecular markers expressed by neoplastic cells, as outlined in
Table 1 [
5].
Luminal A and Luminal B subtypes are characterised by positive hormone receptor expression. The Luminal A subtype shows estrogen and progesterone receptor expression greater than 20%, no HER-2 expression, and a low proliferation index (Ki67 < 20%). The Luminal B subtype can be further divided into two subgroups: those expressing HER-2 and those that do not. Both subgroups exhibit estrogen receptor positivity, progesterone receptor expression ≤ 20%, and a high proliferation index (Ki67 ≥ 20%).
HER-2 subtype lacks any type of hormonal receptor but expresses HER-2.
Finally, the basal-like or triple-negative subtype does not express hormonal receptors or HER-2.
1.2. Therapeutic Options
Currently, various therapeutic options are available for breast cancer, as summarised in
Table 2. Breast cancer treatment must be individualised and is based on three main approaches: surgery, radiotherapy, and medical treatment (or systemic therapy).
Breast cancer surgery should aim to be breast-conserving (lumpectomy) whenever possible, provided that the tumour can be excised with clear margins. This approach yields the best cosmetic outcomes. However, when mastectomy is indicated, patients should be offered the option of breast reconstruction [
6].
Radiotherapy is recommended after all lumpectomies and for mastectomies involving tumours larger than 5 cm, cases of local extension regardless of nodal status, or when there are four or more affected axillary nodes [
6].
Systemic therapy includes chemotherapy, hormone therapy, immunotherapy, and targeted therapies.
Chemotherapy is determined based on tumour stage, prognostic factors, tumour molecular subtype, as well as the patient’s age and comorbidities. Chemotherapy regimens that have demonstrated the greatest survival benefit include anthracycline- and/or taxane-based protocols. Chemotherapy is recommended to begin no later than six weeks after surgery [
6].
Besides, hormone therapy is indicated for any patient with invasive breast cancer exhibiting hormone receptor positivity above 10%. For premenopausal women, treatment should always involve Tamoxifen (a selective estrogen receptor modulator), whereas postmenopausal women may be treated with aromatase inhibitors (Letrozole, Anastrozole, or Exemestane) or Tamoxifen. Hormone therapy should be administered after chemotherapy rather than concurrently with it [
6].
Also, targeted therapy is offered to patients whose tumours overexpress HER-2. Treatment involves Trastuzumab, a humanised monoclonal antibody that selectively blocks the HER-2 receptor, thereby inhibiting neoplastic cell proliferation. This therapy is administered alongside chemotherapy [
6].
Lastly, cyclin-dependent kinase 4 and 6 inhibitors are included within the group of targeted therapies. This review focuses on this specific class of drugs.
1.3. Cyclin-Dependent Kinase 4 and 6 Inhibitors
Cyclin-dependent kinases are proteins involved in tumour cell growth and are implicated in resistance to hormone therapy.
Numerous molecular studies suggest that the CDK4/6 pathway may be hyperactivated in breast cancers with positive hormone receptors. Preclinical trials have demonstrated that these drugs act synergistically with endocrine therapy in the treatment of metastatic breast cancer and may even serve as a therapeutic alternative for tumours resistant to endocrine therapy. However, the first CDK inhibitors tested in various studies exhibited unacceptable toxicity profiles (leading to treatment discontinuation) and limited clinical benefit [
7,
8].
Subsequently, in 2012, Pfizer introduced Palbociclib, a highly selective inhibitor of cyclin-dependent kinases 4 and 6. The PALOMA-1 study (a randomised, phase II clinical trial) confirmed its safety and clinical benefit, leading to its approval by the FDA (Food and Drug Administration) [
8].
Currently, the drugs used to block this pathway are Ribociclib, Palbociclib, and Abemaciclib, typically administered in combination with hormone therapy [
9].
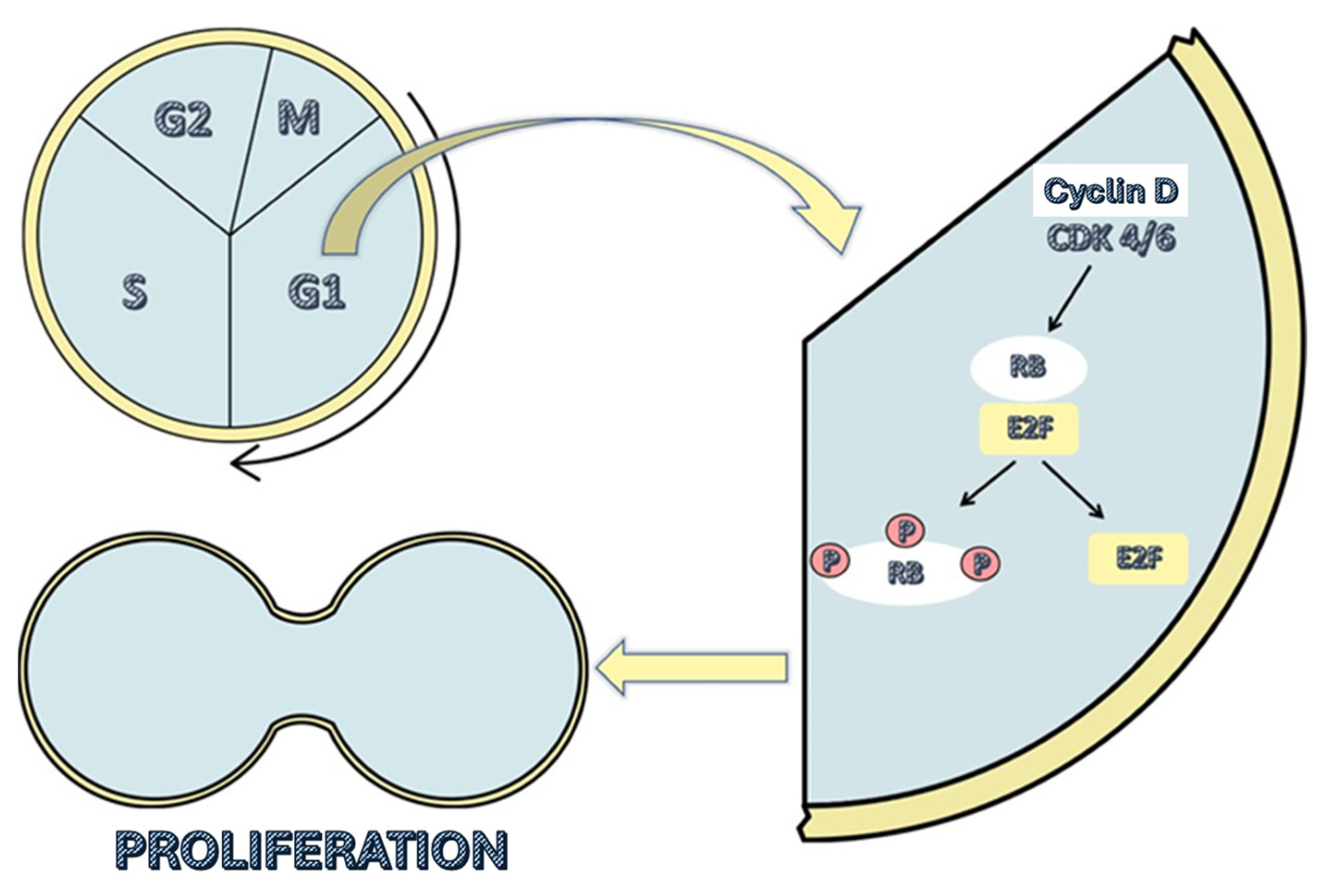
These drugs are administered orally, and their function is to inhibit cyclin-dependent kinases 4 and 6 (CDK4 and CDK6, respectively). These kinases promote the transition from the G1 phase of the cell cycle to the S phase by causing the release of the E2F transcription factor. The transcriptional function of E2F is inhibited by the retinoblastoma suppressor protein (RB), as it directly binds to the activation domain of E2F when it is not phosphorylated, thereby blocking it. At the beginning of the G1 phase, RB is dephosphorylated and thus blocks the activation of E2F. At this point in the cell cycle, the presence of growth factors increases the levels of cyclin D, which bind to CDK4/6, forming a complex that combines with a third protein (p21 or p27), resulting in an enzyme that phosphorylates the RB protein. This phosphorylation releases the E2F transcription factor, allowing it to function (
Figure 1) [
7].
By inhibiting CDK4/6, these drugs prevent RB phosphorylation and E2F release, halting the cell’s progression from the G1 phase to the S phase of the cell cycle. This ultimately results in cellular senescence and apoptosis [
10,
11,
12].
2. Justification and Aims
The potential to establish cyclin-dependent kinases 4 and 6 (CDK4/6) as a novel therapeutic target, in conjunction with hormone therapy, for advanced or metastatic breast cancer characterised by positive hormone receptors and HER-2 negative could result in lower toxicity compared to conventional chemotherapy. This is because these drugs selectively inhibit the hyperactivated pathway in this tumour type. Given the high incidence, morbidity, and mortality associated with this condition, there is a clear need to conduct a systematic review of the available scientific evidence.
The primary aim of this article is to assess the efficacy and safety of this pharmacological group for the aforementioned therapeutic indication.
3. Materials and Methods
This systematic review was conducted by searching the PubMed, Cochrane, and Web of Science databases using the following algorithm (based on MeSH terms):
“((((ALL=(breast neoplasm)) AND ALL=(neoplasm metastasis)) AND ALL=(cyclin-dependent kinase inhibitor proteins)) OR ALL=(cyclin-dependent kinase 6)) OR ALL=(cyclin-dependent kinase 4)”
Inclusion criteria were as follows:
Articles available in full text and free of charge.
Results published from 2017 to April 2022 (last 5 years).
Clinical trials (randomised or non-randomised).
Studies involving treatments with cyclin-dependent kinase 4 and 6 inhibitors (Palbociclib, Ribociclib, Abemaciclib, Dalpiciclib).
Studies that used these drugs in humans.
The remaining inclusion criteria, following the PICO strategy, are summarised in
Table 3.
Exclusion criteria were defined as follows:
Any other type of study.
Application of cyclin-dependent kinase 4 and 6 inhibitors to other types of neoplasms.
Studies involving patients with breast cancer with negative hormone receptors and/or HER-2 positive.
Studies involving patients with early-stage breast cancer.
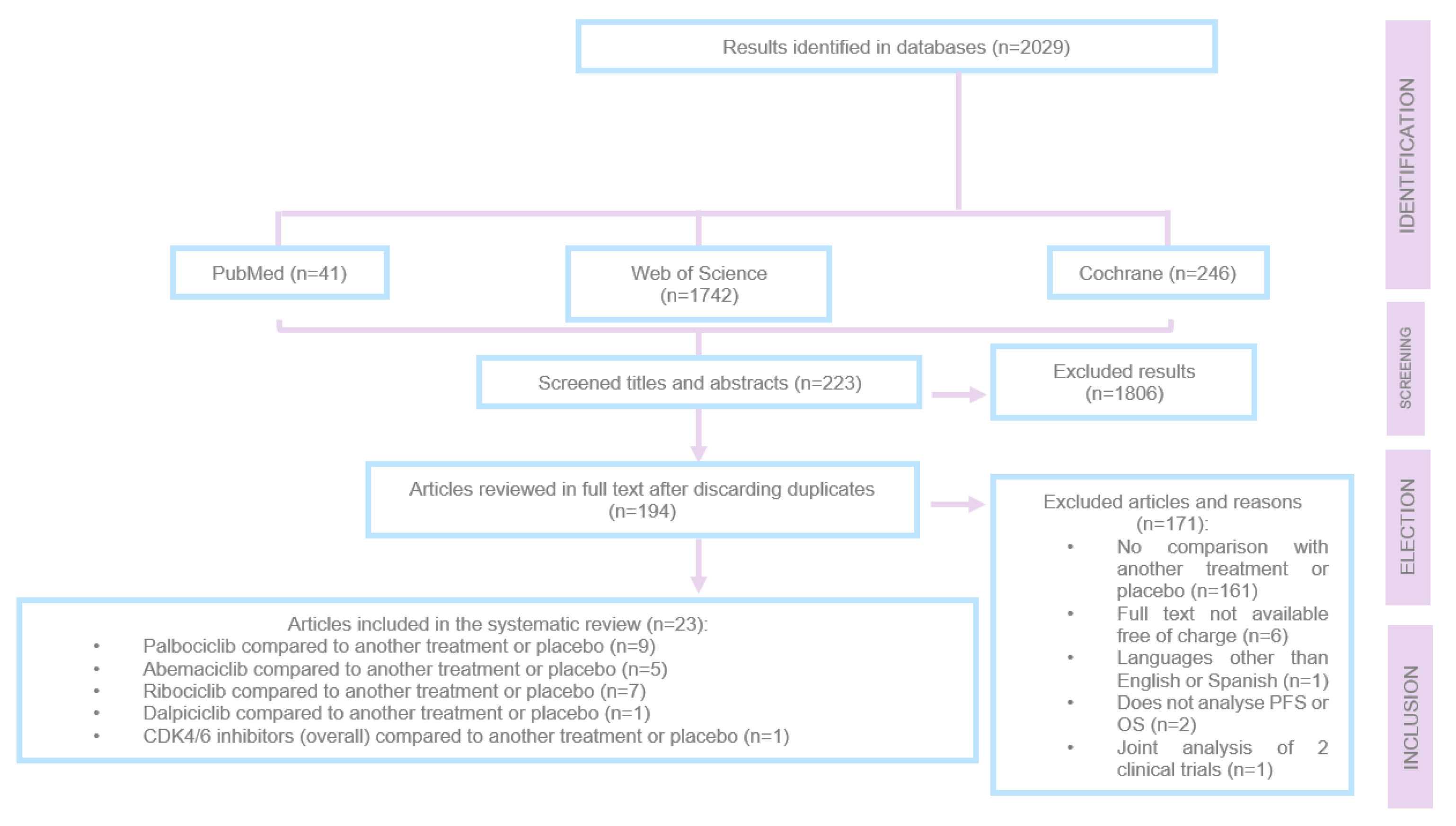
The article selection process is summarised in
Figure 2. During this process, a total of 2,029 articles were screened, of which 1,806 were excluded at the initial stage after evaluating titles and abstracts because they did not address advanced or metastatic breast cancer with positive hormone receptors and HER-2 negative.
After reviewing 223 articles in full, 171 were excluded for the following reasons:
They did not compare treatment with another treatment or placebo.
They were not available in full text and free of charge.
They were written in a language other than English or Spanish.
They did not analyse PFS or OS.
They analysed data from two different clinical trials together.
Of the 194 preselected articles, 23 were ultimately included in the review.
4. Results
The results are showed in efficacy and safety for each treatment.
4.1. Efficacy
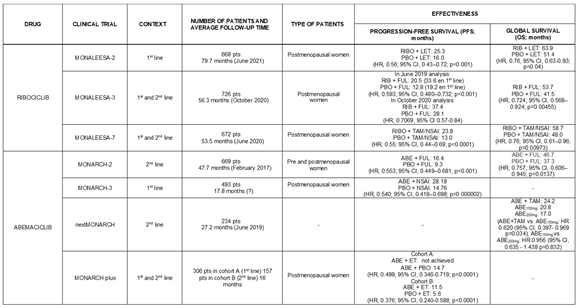
4.1.1. Ribociclib
Ribociclib is one of the cyclin-dependent kinase 4 and 6 inhibitors approved by the FDA for the treatment of metastatic or advanced breast cancer with positive hormone receptors and HER-2 negative.
The MONALEESA-7 study is a randomised clinical trial (1:1) that included pre- or perimenopausal patients who received Ribociclib or placebo, alongside Goserelin and a non-steroidal aromatase inhibitor (NSAI) or Tamoxifen. Participants were followed for a median of 53.4 months (cut-off date: June 29, 2020). In this study, patients were divided into two age groups: <40 years and ≥40 years. In patients <40 years in the Ribociclib treatment group, median overall survival (OS) was 51.3 months, compared to 40.5 months in the placebo group (HR: 0.65; 95% CI [0.43–0.98]), representing a 10.8-month increase. In patients ≥40 years, the median OS was 58.8 months in the Ribociclib group, compared to 51.7 months in the placebo group, though this difference was not statistically significant (HR: 0.81; 95% CI [0.62–1.07]) [
13,
14].
Moreover, MONALEESA-2 study is a phase III, randomised clinical trial (1:1) that included 668 postmenopausal women with advanced breast cancer and positive hormone receptors and HER-2 negative. They received first-line treatment with Ribociclib and Letrozole or Letrozole combined with placebo. At the cut-off date (June 10, 2021), the median follow-up was 79.9 months. By this time, 400 deaths had occurred: 54.2% (181 patients) in the Ribociclib group and 65.6% (219 patients) in the placebo group. At this stage, the combination of Ribociclib and Letrozole showed an increase in median OS compared to the placebo group (63.9 months vs 51.4 months, respectively; HR: 0.76, 95% CI [0.63–0.93], p=0.04), resulting in a 12.5-month increase in median OS. Additionally, at 6 years of treatment, the median survival was 44.2% for the Ribociclib group compared to 32.0% for the placebo group. The combination treatment also delayed the need for chemotherapy (chemotherapy-free survival), with patients receiving Ribociclib showing a median of 50.6 months vs 38.9 months in the placebo group (HR: 0.74; 95% CI [0.61–0.91]), gaining an average of 11.7 months of chemotherapy-free survival [
15,
16].
Finally, MONALEESA-3 study is a phase III, randomised (2:1), placebo-controlled clinical trial that included postmenopausal patients with advanced breast cancer and positive hormone receptors and HER-2 negative. This study investigated the combination of Ribociclib and Fulvestrant in both first- and second-line treatments. The median follow-up was 39.4 months (cut-off date: June 3, 2019). The combination of Ribociclib and Fulvestrant demonstrated an increase in median overall survival compared to the Fulvestrant and placebo group (median OS in the Ribociclib group not achieved vs 40 months in the placebo group; HR: 0.724, 95% CI [0.568–0.924]; p=0.00455). This benefit was consistent across all subgroups, including those receiving the treatment in the first line (median OS in the Ribociclib group not achieved vs 45.1 months in the placebo group; HR: 0.700; 95% CI [0.530–1.004]), with no significant difference in this latter subgroup. On the other hand, the median progression-free survival (PFS) in the subgroup treated with Ribociclib and Fulvestrant in the first line was 33.6 months, compared to 19.2 months in the placebo group (HR: 0.546; 95% CI [0.415–0.718]). Patients treated with the combination of Ribociclib and Fulvestrant took longer, on average, to require chemotherapy or die compared to those treated with Fulvestrant and placebo (39.8 vs 29.4 months, respectively; HR: 0.670; 95% CI [0.542–0.830]) [
17].
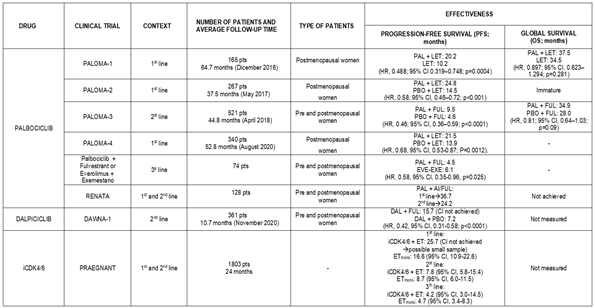
4.1.2. Palbociclib
Palbociclib is another drug belonging to the class of selective cyclin-dependent kinase 4 and 6 inhibitors.
Its activity was studied in PALOMA-1: a phase II, randomised (1:1), open-label clinical trial that included 165 postmenopausal women with advanced breast cancer and positive hormone receptors and HER-2 negative [
18]. One arm of the study received Palbociclib (125 mg/24 hours) and Letrozole (2.5 mg/24 hours), while the other received only Letrozole (same dose). The median follow-up time was 64.7 months (cut-off date: December 30, 2016). In the group treated with Palbociclib combined with endocrine therapy, the median OS was 37.5 months (95% CI [31.4–47.8]) compared to 34.5 months in the Letrozole-only group (95% CI [27.4–42.6]) (HR: 0.897; 95% CI [0.623–1.294]; p=0.281), although this difference was not statistically significant [
18].
Another study, PALOMA-2, is a phase III, double-blind, randomised (2:1) clinical trial that included 666 postmenopausal women with advanced breast cancer, positive estrogen receptors, and HER-2 negative who had not received any prior treatment. One arm (444 women) received treatment with Palbociclib (125 mg/24 hours, with a one-week break every 3 weeks of treatment) and Letrozole (2.5 mg/24 hours), while the other arm (222 patients) received endocrine therapy with placebo. By the cut-off date (May 31, 2017), the median follow-up was 37.6 months in the combined treatment group and 37.3 months in the placebo group. The combination of Abemaciclib and Letrozole showed an increase in PFS compared to the placebo arm, with a median of 27.6 vs 14.5 months, respectively (HR: 0.563; 95% CI [0.461–0.687]; p<0.0001). Similarly, the group that received the therapy combining Abemaciclib and Letrozole took longer to require another systemic therapy: 38.8 months (95% CI [34.4–not achieved]) compared to 28.8 months (95% CI [25.7–33.5]) in the group treated with Letrozole and placebo [
19,
20].
Another study that evaluated the addition of Palbociclib to endocrine therapy is PALOMA-3: a phase III, randomised (2:1), double-blind, placebo-controlled clinical trial, which included 521 patients (regardless of their menopausal status) with advanced breast cancer, positive hormone receptors, and HER-2 negative. One arm received treatment with Palbociclib (125 mg/24 hours with one week off for every 21 days of treatment) and Fulvestrant (500 mg), while the other arm received Fulvestrant (same dose) combined with placebo. They were followed for a median of 44.8 months (cut-off date: 13 April 2018). By this time, overall survival (OS) was 34.9 months (95% CI [28.8–40.0]) in the group receiving Palbociclib and Fulvestrant, compared to 28 months (95% CI [23.6–34.6]) in the placebo group. The 3-year median survival rate was 50% (95% CI [44–55]) in the group treated with Palbociclib and Fulvestrant, compared to 41% (95% CI [33–48) in the placebo arm. It is worth noting that 311 patients had visceral metastasis, for which the OS was 27.6 months (95% CI [24.4–31.2]) in the Palbociclib and Fulvestrant arm, compared to 24.7 months (95% CI [20.8–31.8]) in the placebo group. On the other hand, 210 patients did not have visceral metastatic disease, and in this group, the OS was 46.9 months (95% CI [39.3–not achieved]) for those treated with the combination therapy, compared to 35.4 months (95% CI [24.6–not achieved]) for those treated with placebo and Fulvestrant [
21].
In the same line, PALOMA-4 is a phase III, double-blind, randomised (1:1) clinical trial that included postmenopausal Asian women who had not received prior systemic treatment and presented with advanced breast cancer, positive hormone receptors, and HER-2 negative. The patients (total number: 340) received Palbociclib (125 mg/24 hours orally, with one week off for every 3 weeks of treatment) and Letrozole (2.5 mg/24 hours orally, without interruption) or placebo combined with Letrozole. They were followed for a median of 52.8 months (cut-off date: 31 August 2020). In this study, the median progression-free survival (PFS) was 21.5 months for the group that received Palbociclib and Letrozole, compared to 13.9 months in the placebo group (HR: 0.68; 95% CI [0.53–0.87], p=0.0012) [
22].
Another study evaluating the addition of Palbociclib to endocrine therapy is RENATA, a prospective study that included 128 Argentine women with breast cancer, positive hormone receptors, and HER-2 negative; 20% of them were premenopausal, and 44% had visceral metastasis. The objective of this study was to analyse the use of Palbociclib combined with endocrine therapy in the real-world population. In most patients (63.9%), the CDK4/6 inhibitor was combined with aromatase inhibitors, while it was combined with Fulvestrant in the remaining patients. The median PFS was 29.6 months (95% CI [19.5–38.8]) when used as first-line treatment and 24.2 months (95% CI [12.0–32.7]) when used as second-line or later therapy. Furthermore, PFS was greater when Palbociclib was combined with Fulvestrant (32.7 months; 95% CI [9.3–33.4]) compared to when it was combined with aromatase inhibitors (29.6 months; 95% CI [18.1–43.6]). After 36 months of follow-up, 7.2% of the patients who received first-line treatment had died, whereas 26% of those who received second-line treatment had died. The OS after treatment with Palbociclib was 15.6 months (95% CI [4.8–26.3]) [
23].
The final study to be analysed regarding the addition of Palbociclib to standard hormone therapy for breast cancer is the one conducted by Orlandi et al, who carried out a retrospective study that included 74 women with metastatic breast cancer, 48 of whom received treatment with Palbociclib and Fulvestrant, and 26 were treated with Everolimus and Exemestane as second-line treatment. All patients had received at least one or two lines of prior endocrine therapy. The median PFS was significantly higher in the patients who received Everolimus and Exemestane compared to those treated with Palbociclib and Fulvestrant (6.1 vs 4.5 months; HR: 0.58; 95% CI [0.35–0.96]; p=0.025) [
24].
4.1.3. Abemaciclib
Abemaciclib is the third cyclin-dependent kinase 4 and 6 inhibitor approved by the FDA for the treatment of advanced or metastatic breast cancer with positive hormone receptors and HER-2 negative.
The MONARCH-2 study is a global phase III, randomised (2:1), double-blind clinical trial that included 669 pre-, peri- (with ovarian suppression), and postmenopausal women with advanced breast cancer, positive hormone receptors, and HER-2 negative, who were resistant to endocrine therapy. One arm received treatment with Abemaciclib (150 mg/12 hours) and Fulvestrant (500 mg), while the other arm received Fulvestrant (500 mg) and placebo. The median survival was 46.7 months for the group receiving Abemaciclib and Fulvestrant, compared to 37.3 months for the placebo and Fulvestrant group (HR: 0.757; 95% CI [0.606–0.945]; p=0.0137). Additionally, this benefit was more pronounced in patients with visceral metastasis (HR: 0.675) and primary resistance to prior endocrine therapy (HR: 0.622). Furthermore, the time to require chemotherapy was also improved for patients treated with Abemaciclib compared to those in the placebo group (HR: 0.622; 95% CI [0.499–0.775]) [
25,
26].
Following the same line, MONARCH-3 is a phase III, randomised (2:1), double-blind clinical trial that included 493 patients with breast cancer, positive hormone receptors, and HER-2 negative, who presented with locoregional recurrence or distant metastasis. The patients were randomised such that one group received Abemaciclib (150 mg/12 hours) or placebo, in addition to a non-steroidal aromatase inhibitor (NSAI: Anastrozole 1 mg or Letrozole 2.5 mg). The majority of patients (79.1%) received Letrozole. The median follow-up time was 17.8 months. The median PFS was 14.7 months in the placebo-treated arm, and up to the cut-off date, the data for the Abemaciclib and NSAI treatment arm was not available (HR: 0.54; 95% CI [0.41-0.72]; p=0.00021). Although the data were still immature, the median survival was similar between both arms of the study, with 32 (9.8%) deaths in the Abemaciclib arm and 17 (10.3%) deaths in the placebo arm (HR: 0.97) [
27].
Additionally, the tMONARCH study was more innovative, as it introduced a third treatment arm. Thus, MONARCH is a phase II, multicentric, randomised (1:1:1), open-label clinical trial that included 234 women with metastatic breast cancer, positive hormone receptors, and HER-2 negative, who had progressed after receiving endocrine therapy and chemotherapy. The median follow-up was 27.2 months (cut-off date: 28 June 2019). In one treatment arm, patients received 150 mg of Abemaciclib and 20 mg of Tamoxifen; in another, they received only 150 mg of Abemaciclib, and in the third arm, they were given 200 mg of Abemaciclib along with prophylactic Loperamide (antidiarrhoeal, opioid μ receptor agonist). The median survival was 24.2 months for the group combining Abemaciclib and Tamoxifen; 20.8 months for patients who received 150 mg of Abemaciclib monotherapy (combination therapy vs 150 mg Abemaciclib: HR: 0.620; 95% CI [0.397–0.969]; p=0.034), and 17.0 months for those who received 200 mg of Abemaciclib with Loperamide (Abemaciclib 150 mg vs Abemaciclib 200 mg: HR: 0.956; 95% CI [0.635–1.438]; p=0.832) [
28].
Finally, MONARCH + was a phase III, randomised, double-blind clinical trial that included 463 postmenopausal women from China, Brazil, India, and South Africa, who had advanced breast cancer, positive hormone receptors, and HER-2 negative [
29].
The participants were divided into two cohorts [
29]:
• Cohort A (308 patients): had not received prior systemic treatment. 207 patients were treated with Abemaciclib (150 mg/12 hours) and 99 patients received placebo. Additionally, both arms received treatment with Anastrozole (1 mg/24 hours) or Letrozole (2.5 mg/24 hours).
• Cohort B (157 patients): had progressed after receiving endocrine therapy. 104 participants received treatment with Abemaciclib (same dose as Cohort A) and 53 patients were assigned to the placebo group. Both treatment arms also received Fulvestrant (500 mg).
In Cohort A, the median PFS was not reached in the Abemaciclib-treated group, while it was 14.7 months in the placebo arm. The 12-month PFS rate was 72.1% for patients in the Abemaciclib arm compared to 58.0% for those in the placebo group (p=0.0207). In Cohort B, the median PFS was 11.5 months in the Abemaciclib-treated group and 5.6 months in the placebo arm (HR: 0.376; 95% CI [0.240–0.588]; p<0.0001). Additionally, the 12-month PFS rates were 49.1% for the group that received Abemaciclib compared to 28.9% for the placebo group (p=0.0229) [
29].
4.1.4. Dalpiciclib
Dalpiciclib is another selective cyclin-dependent kinase 4 and 6 inhibitor, though it has not yet been approved for the treatment of breast cancer. Its use in this pathology was evaluated in the DAWNA-1 study.
DAWNA-1 was a phase III, randomised (1:2), double-blind, placebo-controlled clinical trial that included 361 women with advanced breast cancer, positive hormone receptors, and HER-2 negative, whose disease had progressed after receiving two lines of endocrine therapy prior to inclusion in the study. A total of 241 patients received treatment consisting of Dalpiciclib and Fulvestrant, and 120 patients were given placebo and Fulvestrant. The Dalpiciclib group was followed for a median of 10.7 months, while the placebo group had a median follow-up of 10.6 months (cut-off date: 15 November 2020). Regarding the patients' characteristics, the median age was 51 years, with most (55.7%) being postmenopausal women, and 60.1% had visceral metastasis. 27% of patients in the Dalpiciclib and Fulvestrant arm and 40.8% of those in the placebo group received at least one additional antitumour therapy after discontinuing the trial treatment. By the cut-off date, 86 (35.7%) and 76 (63.3%) deaths or disease progressions had occurred in the Dalpiciclib and placebo groups, respectively. Moreover, the median PFS was 15.7 months in the Dalpiciclib group, compared to 7.2 months in the placebo group (HR: 0.42; 95% CI [0.31–0.58]; p<0.0001). At 6 months, the PFS rate in the Dalpiciclib and Fulvestrant arm was 76.4% (95% CI [70.1–81.5]) compared to 53.2% in the placebo and Fulvestrant group (95% CI [43.5–62.0]). At 12 months, these rates were 51.8% (95% CI [43.2–59.8]) and 29.1% (95% CI [20.2–38.5]), respectively. The median survival was still immature at the time of the analysis [
30].
4.1.5. Inhibitors of Cyclin-Dpendent Kinase 4 and 6 (iCDK4/6)
PRAEGNANT is a study that included 1,803 women with breast cancer, positive hormone receptors, and HER-2 negative. The included patients received treatment with an iCDK4/6 in combination with endocrine therapy or endocrine therapy alone and were divided according to whether the treatment corresponded to first, second, or third-line therapy. Thus, in the first-line treatment, the median PFS was 24.7 months for those receiving combined iCDK4/6 and endocrine therapy (95% CI [11.9–not reached]) compared to 16.6 months for those treated with endocrine therapy alone (95% CI [10.9–22.6]). In the second-line treatment, the median PFS was 7.8 months (95% CI [5.8–15.4]) for patients receiving combined therapy versus 8.7 months for the monotherapy group (95% CI [6.0–11.5]). Finally, in the third-line treatment, the median PFS was 4.7 months (95% CI [3.4–8.3]) for the group treated with endocrine therapy alone compared to 4.2 months (95% CI [3.0–14.5]) for the combined treatment group. At the time of the analysis, the median survival was still immature [
31].
As a summary,
Table 4 presents the efficacy results of the various studies analysed in this article.
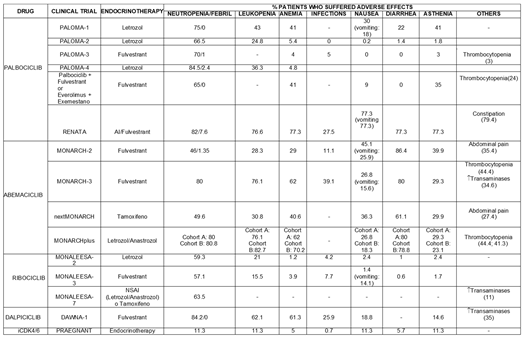
4.2. Saffety
4.2.1. Ribociclib
The most frequently reported adverse effect in the studies that added Ribociclib to endocrine therapy was neutropenia, which occurred with nearly the same frequency regardless of the selected hormone therapy.
Anaemia and infections were less common when Ribociclib was combined with Letrozole compared to other endocrine therapies. However, the Ribociclib-Letrozole combination was associated with a higher percentage of patients experiencing leukopenia, diarrhoea, asthenia, and nausea at some moment during the treatment.
4.2.2. Palbociclib
The most commonly reported adverse reaction when Palbociclib was combined with hormone therapy was again neutropenia. Palbociclib was the drug that led to the highest number of cases of febrile neutropenia, accounting for 7.6% of all neutropenia cases in the RENATA study.
The second most common adverse effect was leukopenia.
Furthermore, the combination with AI (Letrozole, Anastrozole) led to a higher incidence of anemia compared to the combination of Palbociclib with Fulvestrant.
4.2.3. Abemaciclib
As well as other cyclin-dependent kinase 4 and 6 inhibitors, the most common adverse effect was neutropenia, followed by leukopenia and anemia.
It is worth noting that when Abemaciclib was added to endocrine therapy, it was associated with a higher frequency of vomiting, diarrhoea, thrombocytopenia, and increased transaminases compared to other cyclin-dependent kinase 4 and 6 inhibitors.
4.2.4. Dalpiciclib
In the DAWNA-1 study, in which Dalpiciclib was combined with Fulvestrant, the most frequently reported adverse effect was neutropenia, as well as other cyclin-dependent kinase 4 and 6 inhibitors. This was followed by leukopenia and anemia.
Infections were also an important adverse reaction in these patients, in contrast to most studies involving other cyclin-dependent kinase 4 and 6 inhibitors, where infections were not commonly reported.
4.2.5. iCDK4/6
The PRAEGNANT study did not specify which cyclin-dependent kinase 4 and 6 inhibitor was used, but the data were similar to those of other studies, with neutropenia, leukopenia, diarrhoea, and asthenia being the most frequent adverse effects.
It should be noted that leukopenia was reported less frequently in studies combining iCDK4/6 with Tamoxifen compared to other endocrine therapies.
Finally,
Table 5 schematically presents the safety results for these compounds.
5. Discussion
The longest PFS was achieved when Ribociclib was the cyclin-dependent kinase 4 and 6 inhibitor combined with endocrine therapy. These figures were even greater when the treatment was provided as a first-line therapy. In all the studies analysed, the differences were statistically significant compared to placebo.
Regarding OS, with the available data (as some studies still had immature results), higher OS rates were also achieved when endocrine therapy was combined with Ribociclib.
It is worth noting that the adverse effects reported in trials studying the addition of Ribociclib to endocrine therapy were not observed with greater frequency compared to other CDK4/6 inhibitors. Therefore, Ribociclib demonstrated higher efficacy with a similar toxicity profile when compared to the other cyclin-dependent kinase 4 and 6 inhibitors. Additionally, it should be considered that adverse events (neutropenia, leukopenia, nausea, diarrhoea, and asthenia) were more frequent when Ribociclib was combined with Letrozole, except for anemia and infections, which were more common in patients receiving Ribociclib and Fulvestrant.
On the other hand, Abemaciclib showed broader differences in PFS compared to placebo when combined with hormonal therapy as a first-line treatment (MONARCH-3).
In addition, for Abemaciclib, it was observed in MONARCH-2 and MONARCH-3 that the most frequent adverse effect was diarrhoea (reported in more than 80% of patients). However, there is variability in the figures for other adverse effects reported in these studies. These differences may be due to the use of different treatment strategies, as the studies involved different lines of therapy (second line in MONARCH-2 and first line in MONARCH-3). Both used the same endocrine therapy (Fulvestrant); however, adverse events were generally more frequent in MONARCH-3.
As for Palbociclib, better PFS rates were achieved when it was combined with endocrine therapy as a first-line treatment. It is not possible to determine whether its effectiveness was greater with one type of endocrine therapy over another, as in all the studies analysed, Palbociclib was always combined with Letrozole when used as a first-line treatment. The differences in PFS between the groups treated with Palbociclib and those who received placebo appear to be more pronounced when Palbociclib is used in postmenopausal women. However, it is challenging to draw firm conclusions, as although studies were conducted exclusively on postmenopausal women (e.g., RENATA, PALOMA-1, and PALOMA-2), in those trials involving premenopausal women, postmenopausal women also participated.
Furthermore, when Palbociclib is combined with endocrine therapy (Fulvestrant) as a third-line treatment, it has been shown to achieve a lower median PFS compared to patients treated with a combination of Everolimus and Exemestane.
Regarding the OS figures achieved in the studies analysing Palbociclib use in this patient profile, it is noteworthy that, although better results were observed in the groups combining Palbociclib with endocrine therapy compared to placebo or endocrine therapy alone, none of the studies with OS data (PALOMA-1 and PALOMA-3) detected statistically significant differences.
As for the safety of Palbociclib, its figures do not differ significantly from those of other CDK4/6 inhibitors. However, it is worth noting that the combination of Palbociclib with Fulvestrant more frequently caused thrombocytopenia compared to its combination with other types of hormonal therapies.
Finally, Dalpiciclib nearly doubled the PFS compared to the placebo group in the DAWNA-1 study, a difference that was statistically significant. However, nearly 26% of patients treated with Dalpiciclib experienced some type of infection during the treatment period, representing one of the highest infection rates reported among all the studies analysed. It is worth highlighting that this adverse effect is more frequent when any of the CDK4/6 inhibitors is combined with Fulvestrant.
6. Conclusions
The addition of CDK4/6 inhibitors to endocrine therapy in the treatment of advanced or metastatic breast cancer with positive hormone receptors and HER-2 negative has significantly improved PFS, median survival, and chemotherapy-free intervals compared to the use of hormonal treatments alone or in combination with placebo.
Ribociclib demonstrated greater efficacy with a safety profile similar to that of other cyclin-dependent kinase 4 and 6 inhibitors.
Based on the analysed data, it is suggested that Palbociclib might be more effective when used in postmenopausal women. However, clinical trials involving only premenopausal women treated with Palbociclib are needed to confirm this with certainty.
Currently, cyclin-dependent kinase 4 and 6 inhibitors are becoming established as a new standard treatment for this pathology, potentially offering lower toxicity than chemotherapy, as they are selective inhibitors of cyclin-dependent kinases 4 and 6. In all cases, the most frequent adverse effects were haematological, primarily neutropenia and leukopenia.
However, given that in these patients the disease ultimately progresses, requiring chemotherapy, it is necessary to deeply investigate the mechanisms of treatment resistance and, in doing so, develop effective therapies to overcome them.
Author Contributions
Conceptualization, S.B.S. and A.S.A.; methodology, A.L.M.; investigation, A.L.M.; S.B.S. and A.S.A.; writing—original draft preparation, A.L.M.; S.B.S. and A.S.A; writing—review and editing, S.B.S; M.C.C.; L.B.MJ and A.B.M visualization, S.B.S.; M.C.C; L.B.MJ and A.B.M.; supervision, S.B.S; A.S.A and L.B.MJ;. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| iCDK4/6 |
Cyclin-dependent kinase 4 and 6 inhibitors |
| HER-2 |
Human epidermal growth factor receptor-2 |
| ER |
Estrogen receptors |
| PR |
Progesterone receptors |
| Ki67 |
Proliferation index |
| RB |
Retinoblastoma protein |
| E2F |
Transcription factor for DNA replication |
| CDK |
Cyclin-dependent kinases |
| HR |
Hazard Ratio |
| NSAI |
Non-steroidal Aromatase Inhibitors |
| AI |
Aromatase Inhibitors |
| CI |
Confidence Interval |
| OS |
Overall Survival |
| PFS |
Progression-Free Survival |
References
- Cáncer de mama [Internet]. [acceded on 3 may 2022]. https://www.who.int/es/news-room/fact-sheets/detail/breast-cancer.
- Cifras del cáncer en España 2021 [Internet]. [acceded on 3 may 2022]. https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf.
- Defunciones por causas (lista reducida) por sexo y grupos de edad (7947) [Internet]. INE. [acceded on 3 may 2022]. https://www.ine.es/jaxiT3/Datos.htm?t=7947.
- El cáncer de mama en España [Internet]. GEICAM - Investigación en Cáncer de Mama. [acceded on 3 may 2022]. https://www.geicam.org/sala-de-prensa/el-cancer-de-mama-en-espana.
- Cancer de mama - SEOM: Sociedad Española de Oncología Médica © 2019 [Internet]. [acceded on 3 may 2022]. https://seom.org/info- sobre-el-cancer/cancer-de-mama?start=8.
- Zapardiel Gutiérrez I. Actualización en tumores ginecológicos. Tres Cantos, Madrid: You & Us; 2016.
- Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17.
- Xu ZH, Zhang H, Wei DH, Xie LL, Xu CS. Cyclin-dependent kinase 4/6 inhibitor in combination with endocrine therapy versus endocrine therapy only for advanced breast cancer: a systematic review and meta-analysis. Transl Cancer Res. 2020;9(2):657-68.
- Cancer de mama - SEOM: Sociedad Española de Oncología Médica © 2019 [Internet]. [acceded on 3 may 2022]. https://seom.org/info- sobre-el-cancer/cancer-de-mama?start=11.
- verzenios-epar-product-information_es.pdf [Internet]. [acceded on 3 may 2022]. https://www.ema.europa.eu/en/documents/product- information/verzenios-epar-product-information_es.pdf.
- Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, et al. Potent Cell-Cycle Inhibition and Upregulation of Immune Response with Abemaciclib and Anastrozole in neoMONARCH, Phase II Neoadjuvant Study in HR+/HER2- Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26(3):566-80.
- Chigutsa E, Kambhampati SRP, Karen Sykes A, Posada MM, van der Walt JS, Turner PK. Development and Application of a Mechanistic Population Modeling Approach to Describe Abemaciclib Pharmacokinetics. CPT Pharmacomet Syst Pharmacol. 2020;9(9):523-33. [CrossRef]
- 93MO Overall survival (OS) results by age subgroup from the phase III MONALEESA-7 (ML-7) trial of premenopausal patients (pts) with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB) | Cochrane Library [Internet]. [acceded on 20 april 2022]. https://www.cochranelibrary.com/es/central/doi/10.1002/central/CN- 02303925/full.
- Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019;381(4):307-16. [CrossRef]
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. LBA17 Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32:S1290-1.
- Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res BCR. 2018;20(1):123. [CrossRef]
- Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival (OS) results of the phase III MONALEESA-3 trial of postmenopausal patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor 2-negative (HER2−) advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). Ann Oncol. 2019;30:v856-7. [CrossRef]
- Finn RS, Boer K, Bondarenko I, Patel R, Pinter T, Schmidt M, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat. 2020;183(2):419-28.
- Rugo HS, Finn RS, Diéras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow- up. Breast Cancer Res Treat. 2019;174(3):719-29. [CrossRef]
- Rugo HS, Finn RS, Gelmon K, Joy AA, Harbeck N, Castrellon A, et al. Progression-free Survival Outcome Is Independent of Objective Response in Patients With Estrogen Receptor-positive, Human Epidermal Growth Factor Receptor 2-negative Advanced Breast Cancer Treated With Palbociclib Plus Letrozole Compared With Letrozole: Analysis From PALOMA-2. Clin Breast Cancer. 2020;20(2):e173-80. [CrossRef]
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379(20):1926-36. [CrossRef]
- Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. 228MO PALOMA-4: Primary results from a phase III trial of palbociclib (PAL) + letrozole (LET) vs placebo (PBO) + LET in Asian postmenopausal women with estrogen receptor– positive/human epidermal growth factor receptor 2–negative (ER+/HER2–) advanced breast cancer (ABC). Ann Oncol. 2021;32:S457.
- Petracci F, Abuin GG, Pini A, Chacón M. RENATA study—Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer—real-world use [Internet]. 2020 [acceded on 22 april 2022]. http://ecancer.org/en/journal/article/1058-renata-study-latin-american- prospective-experience-clinical-outcome-of-patients-treated-with-palbociclib-in- hormone-receptor-positive-metastatic-breast-cancer-real-world-use.
- Orlandi A, Iattoni E, Pizzuti L, Fabbri A, Botticelli A, Di Dio C, et al. Palbociclib Plus Fulvestrant or Everolimus Plus Exemestane for Pretreated Advanced Breast Cancer with Lobular Histotype in ER+/HER2− Patients: A Propensity Score-Matched Analysis of a Multicenter Retrospective Patient Series. J Pers Med. 2020;10(4):291.
- Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(25):2875-84.
- MONARCH 2: overall survival of abemaciclib plus fulvestrant in patients with HR1, HER2-advanced breast cancer | Cochrane Library [Internet]. [acceded on 20 april 2022]. https://www.cochranelibrary.com/es/cetral/doi/10.1002/central/CN- 02075504/full.
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35(32):3638-46. [CrossRef]
- Hamilton EP, Cortés J, Ozyilkan O, Chen SC, Petrakova K, Manikhas A, et al. 273O nextMONARCH: Final overall survival analysis of abemaciclib monotherapy or in combination with tamoxifen in patients with HR+, HER2- metastatic breast cancer. Ann Oncol. 2020;31:S348. [CrossRef]
- Zhang QY, Sun T, Yin YM, Li HP, Yan M, Tong ZS, et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2– advanced breast cancer: the multinational randomized phase III study. Ther Adv Med Oncol. 2020;12:175883592096392.
- Xu B, Zhang Q, Zhang P, Hu X, Li W, Tong Z, et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021;27(11):1904-9. [CrossRef]
- Schneeweiss A, Ettl J, Lüftner D, Beckmann MW, Belleville E, Fasching PA, et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer - Data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast Edinb Scotl. 2020;54:88-95.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).