1. Introduction
Retinopathy of prematurity (ROP) is a sight-threatening condition that arises from disrupted retinal vascular development in premature infants. If left untreated, ROP can progress to severe visual impairment and even blindness, making it one of the leading preventable causes of childhood blindness worldwide (1,2). Advances in neonatal care have significantly improved the survival rates of extremely preterm infants; however, these improvements have been paralleled by a rising incidence of ROP (3,4). Managing this complex condition, particularly the complications associated with its treatment, remains a critical challenge in neonatal intensive care units (5).
For decades, laser photocoagulation has been the cornerstone of ROP treatment, effectively reducing disease progression through ablation of the avascular retina. However, this technique is not without drawbacks, including permanent retinal damage, loss of peripheral visual field, and high rates of myopia (6). The emergence of vascular endothelial growth factor (VEGF) inhibitors, such as intravitreal bevacizumab, has revolutionized the management of ROP, particularly for aggressive posterior forms of the disease (7). While VEGF inhibitors offer promising outcomes, their use has raised concerns about ocular and systemic complications, with limited long-term data to guide clinical practice (8).
Among the complications associated with ROP treatment, hemorrhagic events—including preretinal, vitreous, and intravitreal hemorrhages—are particularly concerning. These complications not only pose immediate risks but may also influence long-term visual outcomes (9). Identifying the factors that predispose neonates to such events is critical for optimizing treatment strategies. Factors such as neonatal intensive care unit (NICU) stay duration, low gestational age, and maternal characteristics are hypothesized to influence the risk of hemorrhagic complications, yet comprehensive studies addressing these associations are scarce (10).
This study aims to evaluate the frequency of hemorrhagic complications in premature infants treated with intravitreal bevacizumab and to investigate the potential risk factors contributing to these events. By addressing these knowledge gaps, the findings of this research are expected to enhance clinical decision-making and contribute to safer, more effective treatment protocols for ROP. Additionally, the study seeks to provide a foundation for further exploration into optimizing the balance between treatment efficacy and complication risk in this vulnerable population.
2. Materials and Methods
2.1. Study Design and Participants
This retrospective study evaluated the frequency of hemorrhagic complications and associated risk factors in premature infants treated with intravitreal bevacizumab for retinopathy of prematurity (ROP). The study included 132 patients treated at our institution. Ethical approval was obtained, and the study adhered to the principles of the Declaration of Helsinki. Inclusion criteria were infants diagnosed with ROP who received bevacizumab injections and had complete clinical and follow-up data. Patients with missing data or follow-up shorter than six months were excluded.
2.2. Data Collection
This study excluded patients with pre-existing hemorrhages to ensure the accuracy of the findings. Hemorrhages occurring after intravitreal injections were classified based on their location and extent: post-injection hemorrhages confined to the ROP ridge and not exceeding two optic disc diameters were classified as preretinal hemorrhages, while hemorrhages extending beyond two optic disc diameters into the vitreous cavity were classified as intravitreal hemorrhages. These distinctions were made using detailed ophthalmoscopic examinations to ensure precise categorization and to isolate treatment-related complications.
Demographic and clinical variables were collected from medical records, including gestational age, birth weight, maternal age, NICU length of stay, and the week of anti-VEGF administration. Hemorrhagic complications were classified as intravitreal or preretinal based on clinical assessment. Additional data included the presence of systemic adverse events or infections, though these were rare.
2.3. Ethical Approval
The study was designed and conducted in accordance with internationally recognized principles of Good Clinical Practice and the Declaration of Helsinki. Ethical approval for the study was obtained from the Antalya Training and Research Hospital Clinical Researc Ethics Committee. with decision number 9/10, granted on 13 June 2024.
2.4. Statistical Analysis
Descriptive statistics were expressed as frequencies, percentages, means with standard deviations (SD), and medians with interquartile ranges (IQR). The normality of continuous variables was assessed using the Shapiro-Wilk test. Continuous variables were compared between groups using the Independent Samples t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Categorical variables were compared using Pearson’s Chi-Square test or Fisher’s Exact test, as appropriate.
To identify independent risk factors for hemorrhagic complications, a binary logistic regression analysis was conducted. Variables with a p-value < 0.25 in univariate analysis were included in the regression model. Statistical significance was set at p < 0.05. All analyses were performed using SPSS 23.0 software.
3. Results
3.1. Patient Characteristics
A total of 132 patients were included in the analysis, of whom 23 (17.4%) experienced hemorrhagic complications. Among these, 21 cases were preretinal hemorrhages and 2 were intravitreal hemorrhages. Two of the 23 patients with retinal hemorrhagic complications had intravitreal hemorrhage. Both of these patients with vitreous hemorrhage had Type 1 ROP and were injected with a diagnosis of Zone 2, Stage 3 ROP. The remaining 109 patients (82.6%) had no hemorrhagic events. The mean gestational age of the cohort was 26.93 ± 2.57 weeks, and the mean birth weight was 979.52 ± 357.37 grams. Maternal age averaged 31.52 ± 6.88 years, and the average NICU length of stay was 48.05 ± 17.17 days. (
Table 1).
Figures 1 and 2.
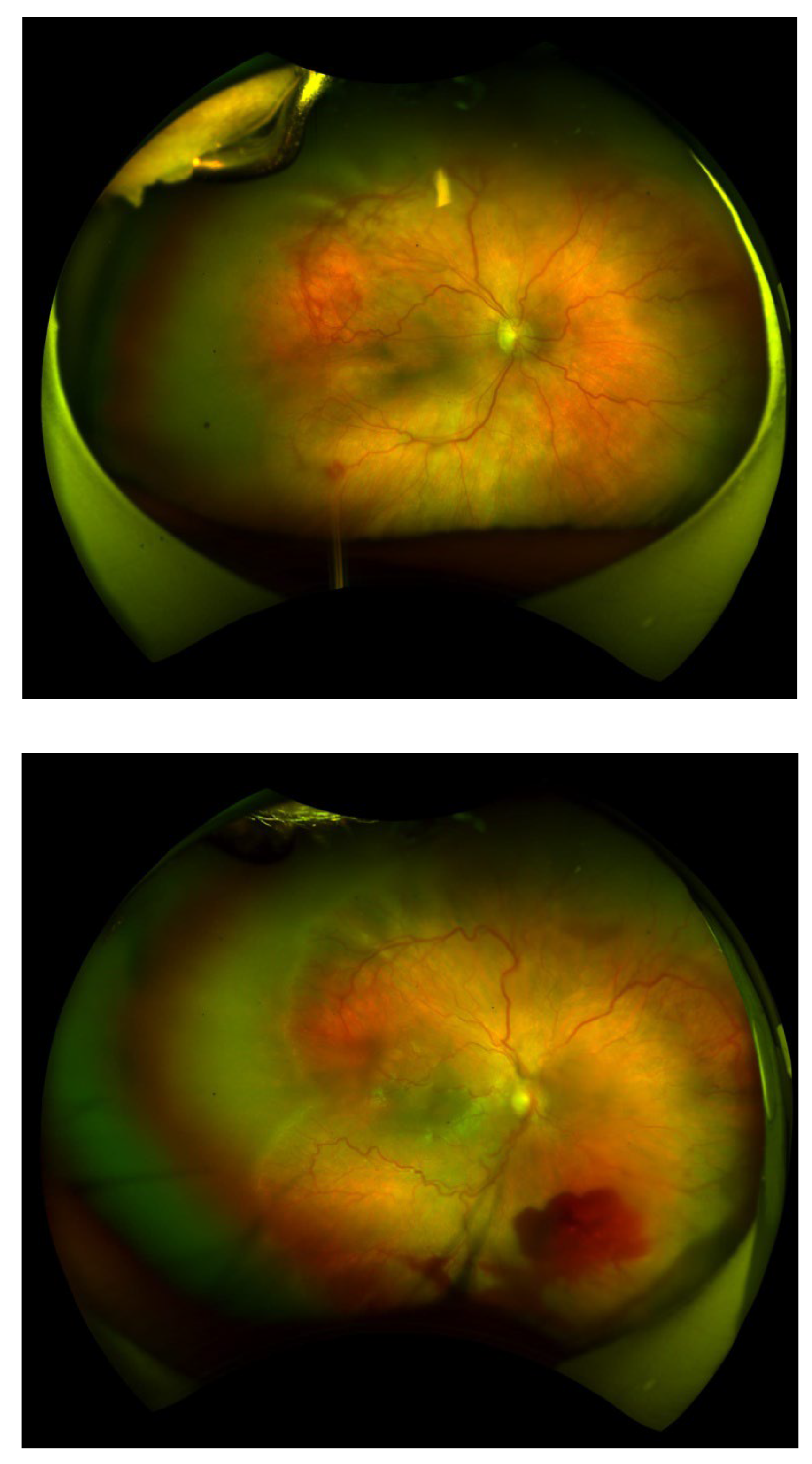
Figure 1 and Figure 2 depict representative fundus images highlighting hemorrhagic complications following treatment. Figure 1 demonstrates a normal fundus appearance, while Figure 2 illustrates preretinal hemorrhage. These images are provided to visually support the clinical findings discussed in this study.
Figures 1 and 2.
Figure 1 and Figure 2 depict representative fundus images highlighting hemorrhagic complications following treatment. Figure 1 demonstrates a normal fundus appearance, while Figure 2 illustrates preretinal hemorrhage. These images are provided to visually support the clinical findings discussed in this study.
3.2. Comparison Between Groups
Patients with hemorrhagic complications had a slightly lower mean gestational age (25.96 ± 2.08 weeks) compared to those without complications (27.15 ± 2.63 weeks); however, this difference was not statistically significant (p = 0.105). Similarly, no significant difference was observed in birth weight (897.13 ± 332.76 grams in the hemorrhage group vs. 996.91 ± 361.40 grams in the non-hemorrhage group; p = 0.177) or maternal age (p = 0.776). The week of anti-VEGF administration also showed no significant difference between the groups (p = 0.406).
In contrast, NICU length of stay was significantly longer in patients who experienced hemorrhagic complications (62.23 ± 12.87 days) compared to those without hemorrhages (45.78 ± 16.74 days; p < 0.0001). This finding indicates a potential link between prolonged hospitalization and increased vascular fragility in premature infants. (
Table 2).
3.3. Risk Factor Analysis
Logistic regression analysis identified NICU length of stay as the only independent risk factor for hemorrhagic complications. Each additional day in the NICU increased the odds of hemorrhage by 5.1% (p = 0.008, OR = 1.051, 95% CI: 1.013–1.091). Neither gestational age nor birth weight showed a significant association with hemorrhagic risk (p > 0.05 for both). The model’s Nagelkerke R² was 0.171, and the classification rate was 84%. (
Table 3).
4. Discussion
The objectives of this research are to assess the prevalence of and causes for the bleeding complications, in respect of neonates who have received intravitreal bevacizumab for retinopathy of prematurity. Efficacy data on the use of VEGF inhibitors in infants with ROP is of great value to the treatment of ROP.
According to the findings of our investigation the percentage of patients who had hemorrhagic complications was 17.4%. This rate is higher than such figure as it is available in the existing literature and this difference may be explained by the wide definition of our study which considered both intravitreal and preretinal haemorrhages. In the literature, only intravitreal haemorrhages are usually reported. For instance, Mintz-Hittner et al. (2011) have reported that intravitreal hemorrhage was rare (<1%) during the evaluation of the efficacy of this agent intravitreal ranibizumab (1). In the same manner, VanderVeen et al. (2016) noted a low incidence of serious adverse events in patients who received intravitreal injections but did not evaluate the effect of intravitreal injections of anti-VEGF therapy on preretinal hemorrhage (2). This has been noted also in other studies that make evaluations on the safety of anti-VEGF therapy (3,4).
Our research addresses this gap by considering pre-retinal haemorhages in the assessment. Although pre-retinal haemorhages are generally less severe than intraocular bleeds, they are nonetheless significant problems in the newborn population owing to the inflammatory features, scar formation, and other possible risks they pose to the body (5,6). In our study, 91.3% of the bleeding events were pre retinal and only 8.7% were intra Vitreo. These observations indicate that pre retinal haemorhage is a prevalent group of complication tendances and is evident to many yet is neglected to their severity and impact on other conditions.
Out of the 23 who had retinal bleeding complications, two patients had an intraocular bleed. Both of these patients were Type 1 ROP and were diagnosed at Zone 2, Stage 3 ROP, which consequently led to the injection. In patients of Stage III ROP, the presence of fibrous proliferation tends to evoke the chances of a vitreous contraction at the time of the injection or even post injection period which causes bleeding of the vessels. In Zone 2 and 3 ROP, due to the location of the injection and the fibrous force exerted by the ROP ridge on its site increases the chance of bleed (7,8).
Duration of a stay in a NICU is independent divisive factor for risk of complications caused by laser. Prolonged periods spent within a NICU can endanger patients through increasing their chances for both intravitreal and preretinal abnormality due to high vascular stress. This has also been the case in other studies, particularly MORIN et al (2016) and Lofqvist et al. (2009) who also observe negative vascular health association with extended NICU stays. Several limitations exist with these studies for instance the omission of detailed analysis of deficits as a result of hemorrhage which underscores the importance of our findings. Whenever the occurrence of hemorrhagic events took place in the course of this study, each of them had availed themselves of one additional day in a NICU which increases the chances of hemorrhaging by 5.1 percent further illustrating the need for management of duration, management of a NICU (9,10).
Lack of clear conclusions on systemic interactions and complications, the growth of heated discussions around the use of VEGF inhibitors in treatment of ROP. Neonatal babies who were stated to have been administered Treatment through VEGF inhibitors had their architecture changed suggesting some systemic effects. In these cases it is essential to develop a uniform classification of complications in the hemorrhagic process in a standardized form. Besides, it is necessary to develop research strategies that will allow reporting such findings as intravitreal and preretinal hemorrhagic episodes in the course of the research and once the study is achieved; suggest treatment that will increase the chances of avoidance of those complications (11-14)
Although the retrospective design of this study and the collection of data from a single centre are important limitations, the large patient population and detailed data analysis increase the reliability of the findings. The importance of multicentre, prospective studies in validating these findings has been emphasized in recent literature (15,16). These studies could also explore the long-term neurodevelopmental outcomes of neonates treated with VEGF inhibitors (17,18).
In conclusion, our study shows that haemorrhagic complications are not limited to rare intravitreal haemorrhages, and preretinal haemorrhages should also be considered. These findings, which highlight important risk factors such as NICU length of stay, aim to contribute to optimizing ROP treatment protocols and minimizing complications in this vulnerable patient group.
4.1. Limitations:
This study has some limitations. Firstly, the adoption of a retrospective design makes it difficult to establish a cause-effect relationship. The retrospective nature of the data may potentially introduce bias, especially as some patient information may be missing. Furthermore, our study was conducted in a single centre and the generalisability of the results to other centres and different patient populations may be limited. Extending the study with data obtained from different centres will increase the accuracy and generalisability of the findings.
Another limitation is that the methods used for grading bleeding complications may be subjective and lack a standardised protocol. Although the separate evaluation of preretinal and intravitreal haemorrhages contributes to the literature, more data on the long-term effects of these complications are needed. Finally, the lack of data on systemic complications makes it difficult to fully evaluate the safety profile of intravitreal bevacizumab.
4.2. Future Perspective:
Several important recommendations can be made for future studies. Firstly, a prospective and multicentre design should be adopted and the frequency and risk factors of bleeding complications in a larger patient population should be examined in more detail. In particular, the long-term visual and systemic effects of preretinal haemorrhages should be more focused.
The development of standardised protocols for the classification of bleeding complications will allow better comparison of the results of different studies. Furthermore, vascular biomarkers and parameters assessing microvessel health could be used to better understand the link between NICU length of stay and vessel fragility.
Future research should aim to optimise the conditions of administration of intravitreal VEGF inhibitors and reduce the risk of complications. In addition, studies evaluating the long-term safety profiles of these treatments will contribute to the development of safer treatment approaches in the neonatal population.
Finally, there is a need to focus more on systemic effects and to develop strategies for their management. This will provide guidance to healthcare professionals working in both neonatal and neonatal intensive care. In the light of these findings, it is concluded that a multidisciplinary approach should be adopted for safer and more effective administration of intravitreal injections.
Author Contributions
E.K.O. conceptualized and designed the study and drafted the manuscript and was responsible for data acquisition; S.S. and S.Y. drafted the manuscript; M.F.K. provided supervision and guidance throughout the study; M.K.E. and M.F.K. performed the editing of the manuscript; E.K.O. and S.S. reviewed the manuscript and curated the methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any specific grant from public, commercial, or non-profit funding agencies.
Informed Consent Statement
Verbal and written patient consent was taken in this study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zin A, Gole GA. "Retinopathy of prematurity—incidence today." Clin Perinatol. 2013;40(2):185-200. [CrossRef]
- Lepore D, Quinn GE, Molle F, et al. "Aflibercept, bevacizumab or ranibizumab for ROP: A comparison of efficacy and safety." Am J Ophthalmol. 2021;222:208-216. [CrossRef]
- Stahl A, Krohne TU, Eter N, et al. "Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of ROP: A randomized clinical trial." JAMA Ophthalmol. 2021;139(2):258-267. [CrossRef]
- Taher, N. O., Ghaddaf, A. A., Al-Ghamdi, S. A., Homsi, J. J., Al-Harbi, B. J., Alomari, L. K., & Almarzouki, H. S. (2022). Intravitreal anti-vascular endothelial growth factor injection for retinopathy of prematurity: A systematic review and meta-analysis. Frontiers in Medicine, 9, Article 884608. [CrossRef]
- Chen, J., Stahl, A., Hellstrom, A., & Smith, L. E. (2011). Current update on retinopathy of prematurity: Screening and treatment. Current Opinion in Pediatrics, 23(2), 173–178. [CrossRef]
- Sjöbom, U., Hellqvist, T., Humayun, J., Nilsson, A. K., Gyllensten, H., Hellström, A., & Löfqvist, C. (2024). Circulating VEGF-A levels in relation to retinopathy of prematurity and treatment effects: A systematic review and meta-analysis. Ophthalmology Science, 4(1), Article 100548. [CrossRef]
- Patel CK, Fung TH, Muqit MM, et al. "Advances in laser therapy for retinopathy of prematurity." Eye (Lond). 2013;27(6):725-735. [CrossRef]
- Hartnett ME, Lane RH. "Effects of oxygen on the development and severity of retinopathy of prematurity." J AAPOS. 2013;17(3):229-234. [CrossRef]
- Sanghi, G., Gangwe, A., & Das, P. (2024). Evidence-based management of retinopathy of prematurity: More than meets the eye. Clinical Epidemiology and Global Health, 18, Article 101530. [CrossRef]
- Fielder, A. R., Blencowe, H., O'Connor, A., et al. (2017). Retinopathy of prematurity: Guidelines for screening and treatment. Early Human Development, 114, 68–71. [CrossRef]
- Mintz-Hittner HA, Kennedy KA, Chuang AZ. "Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity." N Engl J Med. 2011;364(7):603-615. [CrossRef]
- VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB. "Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity." Ophthalmology. 2016;123(9):1985-1991. [CrossRef]
- Sun Y, Lin Z, Ke X, et al. "Efficacy and safety of anti-VEGF therapy in the treatment of retinopathy of prematurity: A meta-analysis." Br J Ophthalmol. 2020;104(5):577-583.
- Castellanos MA, Schwartz S, Hernandez-Da Mota SE. "Update on retinopathy of prematurity in Latin America." Am J Ophthalmol. 2019;204:40-47.
- Hartnett ME, Lane RH. "Effects of oxygen on the development and severity of retinopathy of prematurity." J AAPOS. 2013;17(3):229-234. [CrossRef]
- Chen SN, Lian I, Hsu CW, et al. "Changes in systemic vascular endothelial growth factor levels in infants treated for retinopathy of prematurity." Eye (Lond). 2014;28(6):734-741.
- Morin J, Luu TM, Superstein R, et al. "Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity." Pediatrics. 2016;137(4):e20153218.
- Löfqvist C, Andersson E, Johansson U, et al. "Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity." Arch Ophthalmol. 2009;127(5):622-627. [CrossRef]
- Slidsborg C, Olesen HB, Jensen PK, et al. "Treatment for retinopathy of prematurity: Volumes and outcome over 16 years at a Danish tertiary centre." Acta Ophthalmol. 2020;98(2):150-157.
- Lepore D, Quinn GE, Molle F, et al. "Aflibercept, bevacizumab or ranibizumab for ROP: A comparison of efficacy and safety." Am J Ophthalmol. 2021;222:208-216.
- Shah PK, Narendran V, Kalpana N, et al. "Safety and efficacy of intravitreal bevacizumab for zone 1 retinopathy of prematurity." Indian J Ophthalmol. 2011;59(7):9-15.
- Xu Y, Dai H, Zhang X. "Correlation of serum VEGF levels and ROP severity in premature infants." J Pediatr Ophthalmol Strabismus. 2018;55(3):188-194.
- Patel CK, Fung TH, Muqit MM, et al. "Advances in laser therapy for retinopathy of prematurity." Eye (Lond). 2013;27(6):725-735.
- Geloneck MM, Chuang AZ, Clark WL, et al. "Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment." JAMA Ophthalmol. 2014;132(11):1327-1333. [CrossRef]
- Chiang MF, Quinn GE, Fielder AR, et al. "ROP screening guidelines: Global and regional challenges." Am J Ophthalmol. 2017;180:64-74.
- Baskin DE. "Ocular complications of prematurity." Pediatr Clin North Am. 2013;60(6):1537-1552.
- Vinekar A, Dogra MR, Sangtam T, et al. "Retinopathy of prematurity in Asian Indian babies weighing greater than 1250 grams at birth." JAMA Ophthalmol. 2007;125(2):173-178.
- Shah PK, Narendran V, Kalpana N, et al. "Long-term neurodevelopmental outcomes after intravitreal bevacizumab for retinopathy of prematurity." Indian J Ophthalmol. 2014;62(1):110-114.
Table 1.
Baseline Characteristics and Clinical Variables of the Study Population.
Table 1.
Baseline Characteristics and Clinical Variables of the Study Population.
| Variable |
Value |
| Bleeding Status |
|
| - Absent |
82.6% (109) |
| - Present |
17.4% (23) |
| Gender |
|
| - Female |
47.9% (58) |
| - Male |
52.1% (63) |
| Gestational Age (weeks) |
26.93 ± 2.57 |
| Birth Weight (grams) |
979.52 ± 357.37 |
| Mother's Age (years) |
31.52 ± 6.88 |
| NICU Length of Stay (days) |
48.05 ± 17.17 |
Table 2.
Comparison Between Patients Without Bleeding and Those With Bleeding.
Table 2.
Comparison Between Patients Without Bleeding and Those With Bleeding.
| Variable |
No Bleeding (n=109) |
Bleeding (n=23) |
p-value |
| Gestational Age (weeks) |
27.15 ± 2.63 (22-35)Median: 27 (25-28) |
25.96 ± 2.08 (22-29)Median: 27 (24-28) |
0.105 (2) |
| Birth Weight (grams) |
996.91 ± 361.4 (550-2630)Median: 900 (720-1130) |
897.13 ± 332.76 (500-1695)Median: 820 (590-1080) |
0.177 (2) |
| Mother's Age (years) |
31.62 ± 6.97 (18-55)Median: 31 (27-36) |
31.17 ± 6.67 (20-50)Median: 30 (26-36) |
0.776 (2) |
| Week of Anti-VEGF Treatment |
8.94 ± 2.39 (3-15)Median: 9 (8-11) |
8.48 ± 2.21 (4-13)Median: 8 (7-9) |
0.406 (1) |
| NICU Length of Stay (days) |
45.78 ± 16.74 (21-109)Median: 42 (35-54) |
62.23 ± 12.87 (40-80)Median: 62 (50-70) |
<0.0001 (2) |
Table 3.
Comparison Between Patients Without Bleeding and Those With Bleeding.
Table 3.
Comparison Between Patients Without Bleeding and Those With Bleeding.
| Variable |
B |
S.E. |
Wald |
p-value |
Exp(B) [95% CI] |
| Gestational Age |
0.019 |
0.215 |
0.008 |
0.931 |
1.019 [0.668-1.553] |
| Birth Weight |
0.001 |
0.002 |
0.020 |
0.888 |
1.000 [0.996-1.003] |
| NICU Length of Stay |
0.050 |
0.019 |
7.082 |
0.008 |
1.051 [1.013-1.091] |
| Constant |
-4.765 |
5.002 |
0.907 |
0.341 |
0.009 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).