In 1833, French physician Frantz Glenard initially proposed the term "visceroptosis" in his doctoral thesis, defining it as a prolapse or sagging of the abdominal viscera (internal organs) below their normal position resulting from ligament laxity, and suggesting it as a potential cause of chronic abdominal pain [
5]. When the stomach is affected more specifically, it is defined as gastroptosis. Concurrently, Rokitansky (1842) proposed a different entity, also responsible for chronic abdominal pain, which he described as a phenomenon of obstruction and dilatation of the proximal intestine caused by compression of the third portion of the duodenum by the superior mesenteric artery (SMA) or, more specifically, by the SMA and the aorta [
6]. The condition, initially referred to as either "internal hernia" or "internal incarceration," received its first comprehensive account from David Wilkie in 1921. This detailed description led to the establishment of Wilkie syndrome, also known as superior mesenteric artery syndrome (SMAS) [
7,
8].
Although isolated cases have been documented in the literature, no one has hitherto reported these rare conditions simultaneously in the same patient, as in our case [
9,
10]. Previously, gastroparesis was estimated to affect 2-3% of the general population [
11]. However, a recent US study found the prevalence to be 0.16% [
12]. Moreover, in diabetic patients, 1-5% develop gastroparesis, with the risk being four times higher in type 1 diabetes than in type 2 [
13]. In SMAS, the data currently available show that women are mainly affected in the 20-50 age group, with a higher prevalence in women than in men (ratio 3:2) [
14]. A number of risk factors appear to be involved in this phenomenon. Among those identified are severe underweight due to certain catabolic conditions (such as cancer, neurologic injury, surgery, burns, malabsorption syndromes, trauma or psychiatric problems), postural defects (such as congenital scoliosis) and excessive laxity of the abdominal wall and mesenteric attachments, which become too thin and relaxed under the weight of the organ [
15]. The clinical manifestations are frequently numerous but not particularly specific, including a lengthy history of intermittent postprandial abdominal discomfort, bloating, or epigastric distress, recurrent episodes of nausea and vomiting, early satiety, and weight loss, all of which are exacerbated by standing or post-prandial activity [
16,
17].
The aetiology of abdominal pain is a complex phenomenon, with no single causal model proving wholly adequate. Indeed, gastroptosis and SMAS may be considerable overlap with more common gastrointestinal disorders, including gastritis, peptic ulcer disease, pancreatitis and pancreatic tumours (above a certain size), gastro-oesophageal reflux disease (GERD), inflammatory bowel diseases such as Crohn's disease affecting the upper gastrointestinal tract, celiac disease and irritable bowel syndrome [
18]. Additionally, less frequent yet equally pertinent conditions that may elucidate the symptomatology were discussed within the context of anatomical conditions, including congenital stenosis of the duodenum and intestines, intestinal malrotation, food allergies, and psychosomatic disorders [
18]. It is evident that all of the aforementioned causes have been excluded in our patient, as a result of the additional examinations conducted. The biological assessment did not yield any noteworthy findings. A gastroscopy was performed and revealed only a subtle erythematous gastritis, which did not fully explain our patient's underlying clinical condition.
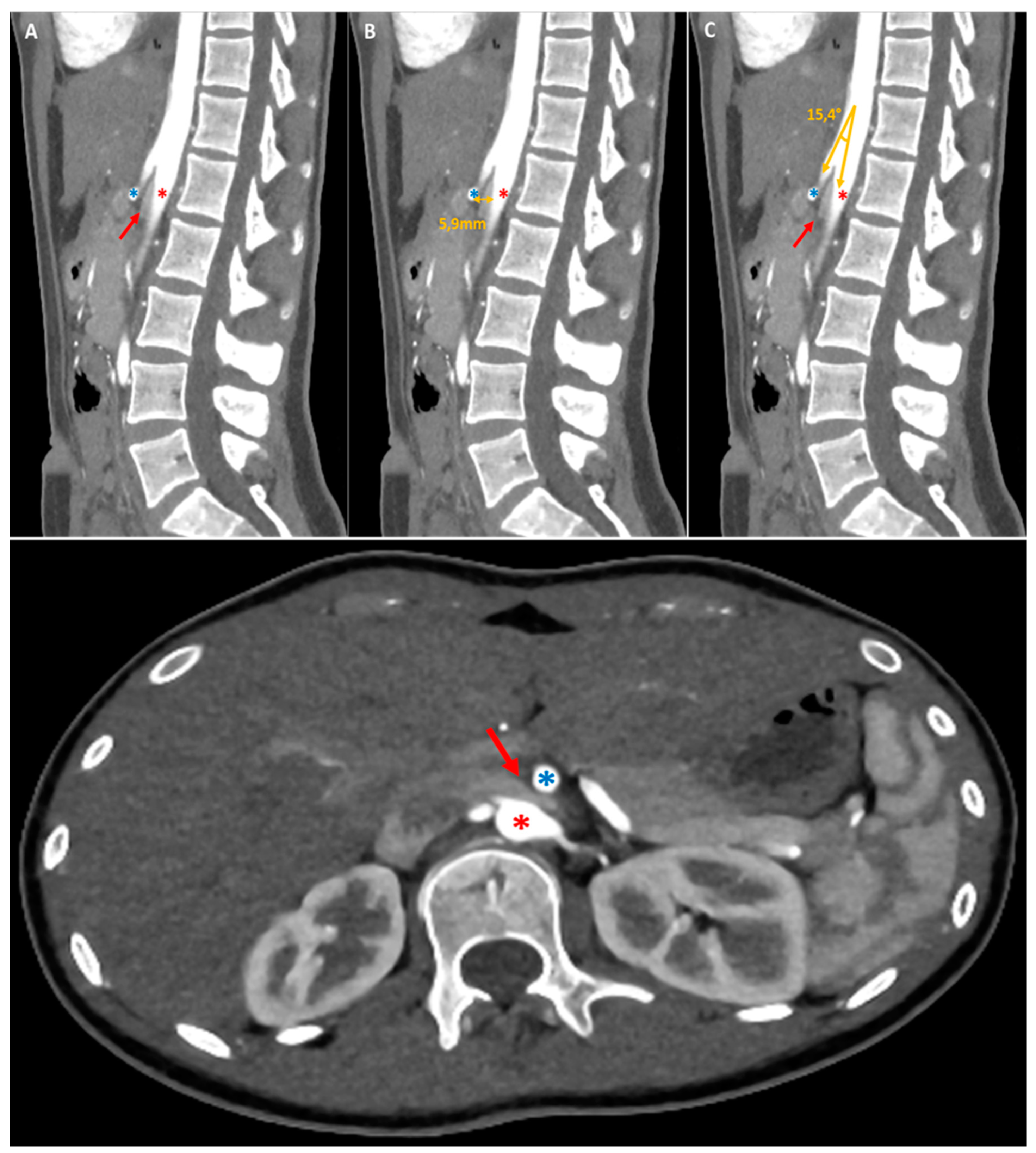
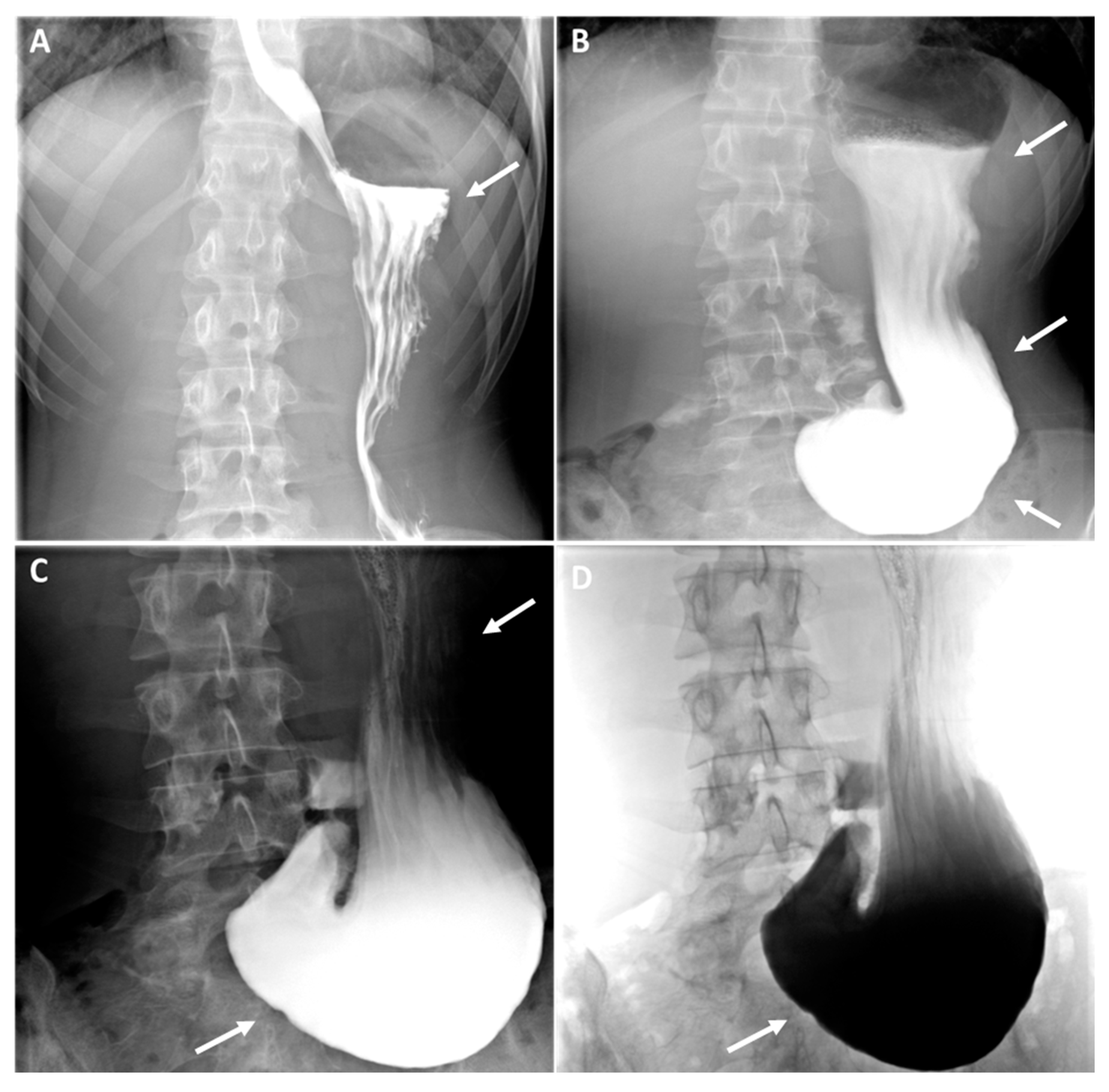
As evidenced by the existing literature, imaging has a crucial role in the diagnostic process of gastroptosis and SMAS [
19]. Fluoroscopy with oral barium or iodine contrast remains the primary assessment tool of the upper gastrointestinal tract, offering real-time dynamic visualisation that facilitates the correlation of clinical history with imaging [
4,
19,
20]. In both case, fluoroscopic findings generally include gastrointestinal distention (
Figure 2, white arrows where the stomach is displaced downwards, with the greatest curvature close to the iliac crest, while the antrum remains in place), delayed gastric emptying (
Figure 2, dynamic A-D) and narrowing of the third part of the duodenum (less obvious in our case). Further information can be obtained by computed tomography (CT), which, in combination with intravascular contrast, allows detailed assessment of the aorto-mesenteric vascularisation and calibre of the gastroduodenal structures, proving invaluable in the diagnosis. SMAS syndrome is characterised by gastroduodenal dilatation with marked narrowing at the junction of the AMA and the aorta (
Figure 1, red arrow), shortening of the aorto-mesenteric distance (normal: 10-34mm; 1B), and tightening of the aorto-mesenteric angle (normal: 28-65°;
Figure 1C) [
19,
20,
21]. Gastroptosis may also be visible, depending on whether or not the patient has eaten prior to the examination; however, it may be less visible if the examination is performed on an empty stomach.
In the current clinical practice, invasive treatment is reserved for a limited number of cases complicated by intestinal obstruction [
22]. The treatment of gastroptosis and SMAs is primarily focused on the management of the symptoms and correction of the underlying anatomical abnormalities. For gastroptosis, conservative management encompasses dietary modifications, postural adjustments, and physical therapy to strengthen the abdominal and spinal musculature. Surgical interventions, such as gastroplication or gastropexy, are employed in severe cases [
9,
22,
23]. For SMAS, it is primarily based on nutritional support, including the administration of high-calorie diets or enteral feeding to facilitate weight gain and relieve vascular compression. Additionally, postural modifications and, in certain cases, prokinetic drugs may be employed. In instances where conservative measures prove ineffective, surgical options, such as duodenojejunostomy or SMA release surgery, may be necessary to address the underlying anatomical issues [
24]. The management of both conditions requires a multidisciplinary approach, involving the expertise of gastroenterologists, nutritionists, and surgeons, tailored to the specific needs of each patient.
In conclusion, Gastroptosis and Wilkie syndrome are two rare entities that are underdiagnosed and rarely reported in the literature, representing a diagnostic and therapeutic challenge in clinical practice. Our case highlights the necessity for a multidisciplinary approach, involving general practitioner, gastroenterologists, nutritionist, radiologists and surgeons, to accurately diagnose and treat these overlapping conditions. A comprehensive history, thorough physical examination and appropriate imaging studies are crucial to circumvent such diagnostic pitfalls.
Author Contributions
Conceptualization, A.J.; methodology, N.Y, A.J. and S.M.; software, A.J. and NY.; validation, A.J., N.Y, M.E, S.M. and R.A.; formal analysis, M.E. and CK.; investigation, N.Y, A.J, S.M, A.G; resources, A.J., M.E and N.Y.; writing—original draft preparation, A.J.; writing—review and editing, A.J., N.Y, S.M. and R.A.; supervision, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not receive ethical review and approval due to the nature of the research (case report).
Informed Consent Statement
Written informed consent was obtained from the patient. The consent form was obtained on 1 March 2024.
Data Availability Statement
The data used and analyzed in this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chambers, Christine T.a,b,c,*; Dol, Justinea; Tutelman, Perri R.a,b; Langley, Charlotte L.a; Parker, Jennifer A.a; Cormier, Brittany T.a; Macfarlane, Gary J.d; Jones, Gareth T.d; Chapman, Darlenee; Proudfoot, Nicolea; Grant, Amyf; Marianayagam, Justinag. The prevalence of chronic pain in children and adolescents: a systematic review update and meta-analysis. PAIN 165(10):p 2215-2234, October 2024. [CrossRef]
- Cristina Maria Sabo, Simona Grad, Dan L. Dumitrascu; Chronic Abdominal Pain in General Practice. Dig Dis 11 November 2021; 39 (6): 606–614. [CrossRef]
- Bharucha AE, Chakraborty S, Sletten CD. Common functional gastroenterological disorders associated with abdominal pain. Mayo Clin Proc. 2016;91(8):1118–32. [CrossRef]
- Sarangapani, A.; Rasane, S.; Kohli, V.D.; Chandy, G.M. Glenard’s disease. Arch. Med. Health Sci. 2016, 4, 153–154.
- Beilin DS. When Has Visceroptosis Clinical Significance? Radiology. 1930 Aug 1;15(2):223–6.
- Rokitansky C. Third volume. Wien: Braumüller & Seidel; 1842. Innere-Hernien; pp. 215–219. (Handbuch der pathologischen Anatomie).
- Wilkie D.P.D. Chronic duodenal ileus. Br. Med. J. 1921;2(3176):793–795.
- Yale SH, Tekiner H, Yale ES. Historical terminology and superior mesenteric artery syndrome. Int J Surg Case Rep. 2020;67:282-283.
- Staszewska, A.; Jarzumbek, A.; Saran, A.; Gierak-Firszt, S.; Kwiecien, J. Postprandial Abdominal Pain Caused by Gastroptosis—A Case Report. Children 2023, 10, 116. [CrossRef]
- Abenavoli L, Imoletti F, Quero G, Bottino V, Facciolo V, Scarlata GGM, Luzza F, Laganà D. The Diagnosis of Wilkie's Syndrome Associated with Nutcracker Syndrome: A Case Report and Literature Review. Diagnostics (Basel). 2024 Aug 23;14(17):1844.
- Horowitz M, Su YC, Rayner CK, Jones Gastroparesis: prevalence, clinical significance and treatment. Can J Gastroen- terol. 2001 Dec;15(12):805-13.
- Syed AR, Wolfe MM, Calles-Escandon Epidemiology and Diagnosis of Gastroparesis in the United States: A Popula- tion-based Study. J Clin Gastroenterol. 2019 May 22.
- Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, et Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012 Jan;107(1):82-8.
- Oka A, Awoniyi M, Hasegawa N, Yoshida Y, Tobita H, Ishimura N, Ishihara S. Superior mesenteric artery syndrome: Diagnosis and management. World J Clin Cases. 2023 May 26;11(15):3369-3384.
- Superior Mesenteric Artery (SMA) Syndrome: Practice Essentials, Pathophysiology, Etiology. eMedicine [Internet]. 2023 Nov 15; Available from: https://emedicine.medscape.com/article/932220-overview?form=fpf.
- Mathenge N, Osiro S, Rodriguez II, Salib C, Tubbs RS, Loukas M. Superior mesenteric artery syndrome and its associated gastrointestinal implications. Clinical Anatomy. 2013 Aug 20;27(8):1244–52.
- Kusano, M.; Moki, F.; Hosaka, H.; Shimoyama, Y.; Kawamura, O.; Nagoshi, A.; Maeda, M.; Kuribayashi, S.; Zai, H.; Mizuide, M.; et al. Gastroptosis is associated with less dyspepsia, rather than a cause of dyspepsia, in Japanese persons. Intern. Med. 2011, 50, 667–671.
- American Gastroenterological Association. American Gastroenterological Association Medical Position Statement: Nausea and Vomiting. Gastroenterology 2001, 120, 261–286.
- Van Welie, A.J.M.; Klein, W.M.; Draaisma, J.M.T. The clinical or radiographic diagnosis of gastroptosis: Still relevant? Gastro Open J. 2017, 2, 14–19.
- Beilin, D.S. When has visceroptosis clinical significance? Radiology 1930, 15, 223–226.
- So C-Y, Chan K-Y, Au H-Y, et al. Superior mesenteric artery (SMA) syndrome: an unusual cause of intestinal obstruction in palliative care. Ann Palliat Med 2017;6:91–3.
- Christianakis E, Bouchra K, Koliatou A, Paschalidis N, Filippou D. Gastroparesis associated with gastroptosis presenting as a lower abdominal bulking mass in a child: a case report. Cases J. 2009 Nov 4;2:184. [PubMed]
- Hall-Edwards, J.F. The diagnosis and treatment of gastroptosis. Br. Med. J. 1921, 1, 698–700.
- Van Horne N, Jackson JP. Superior Mesenteric Artery Syndrome. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2023. PMID: 29489172.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).