Submitted:
28 November 2024
Posted:
29 November 2024
You are already at the latest version
Abstract
Keywords:
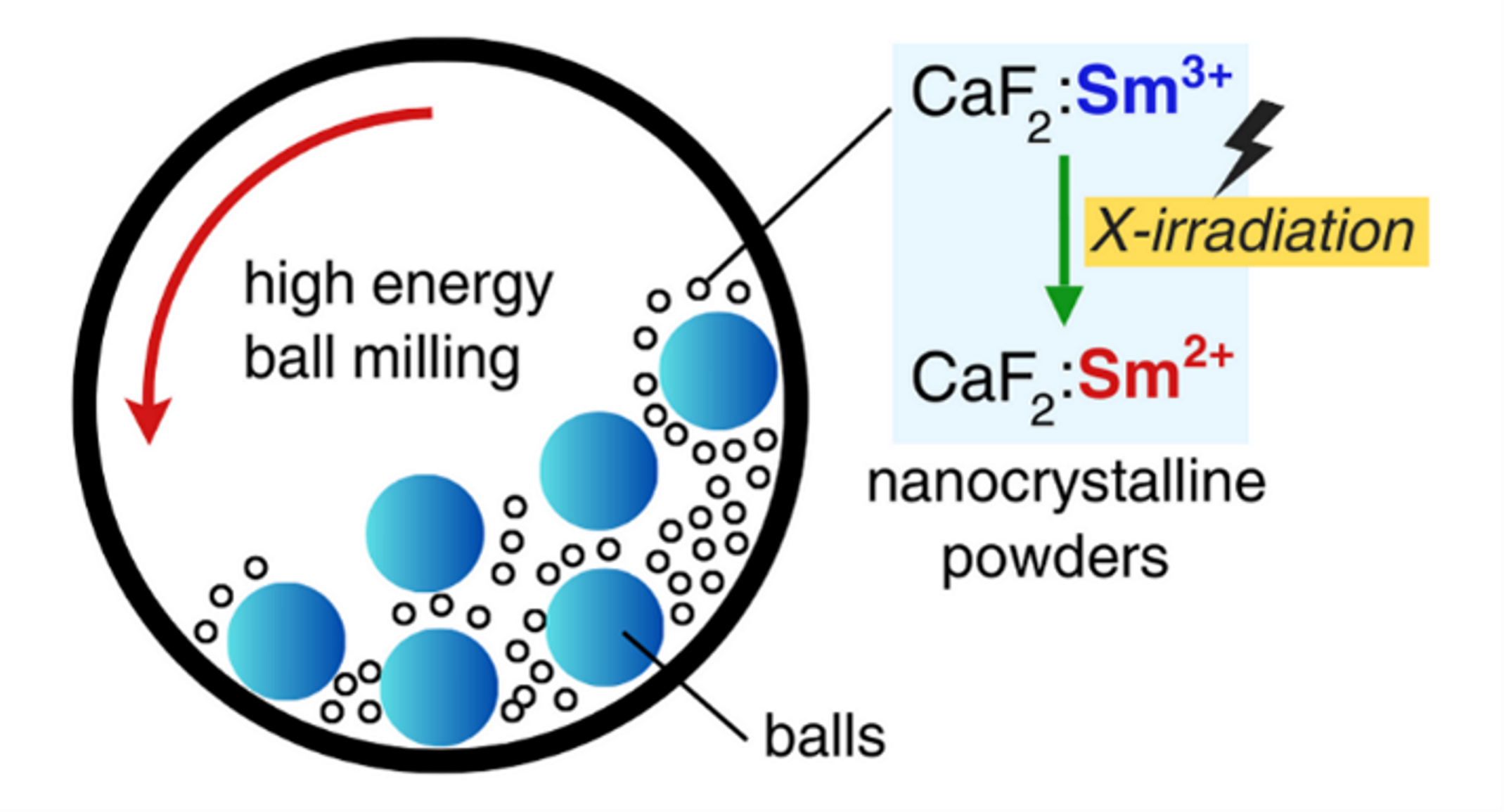
1. Introduction
2. Experimental Methods
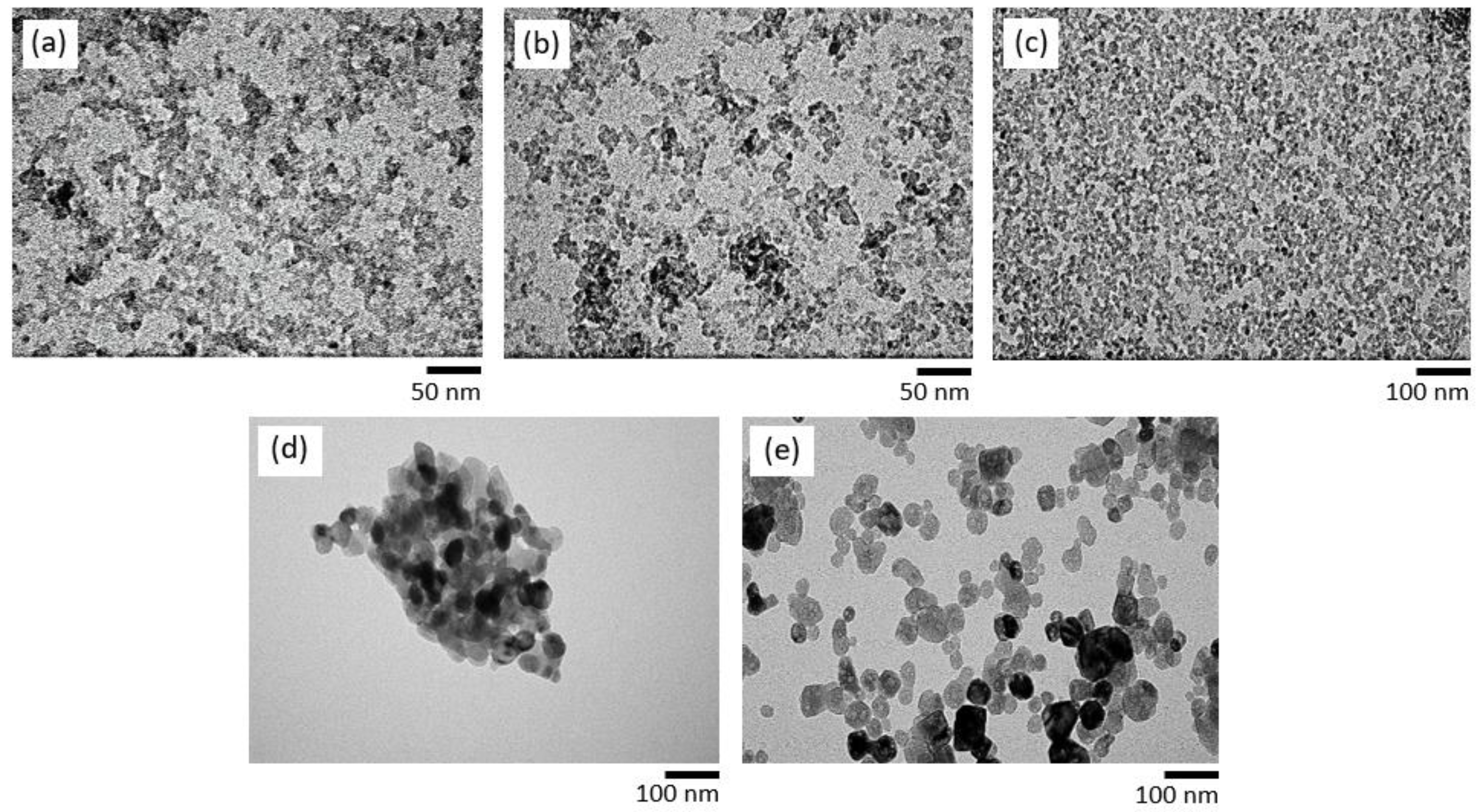
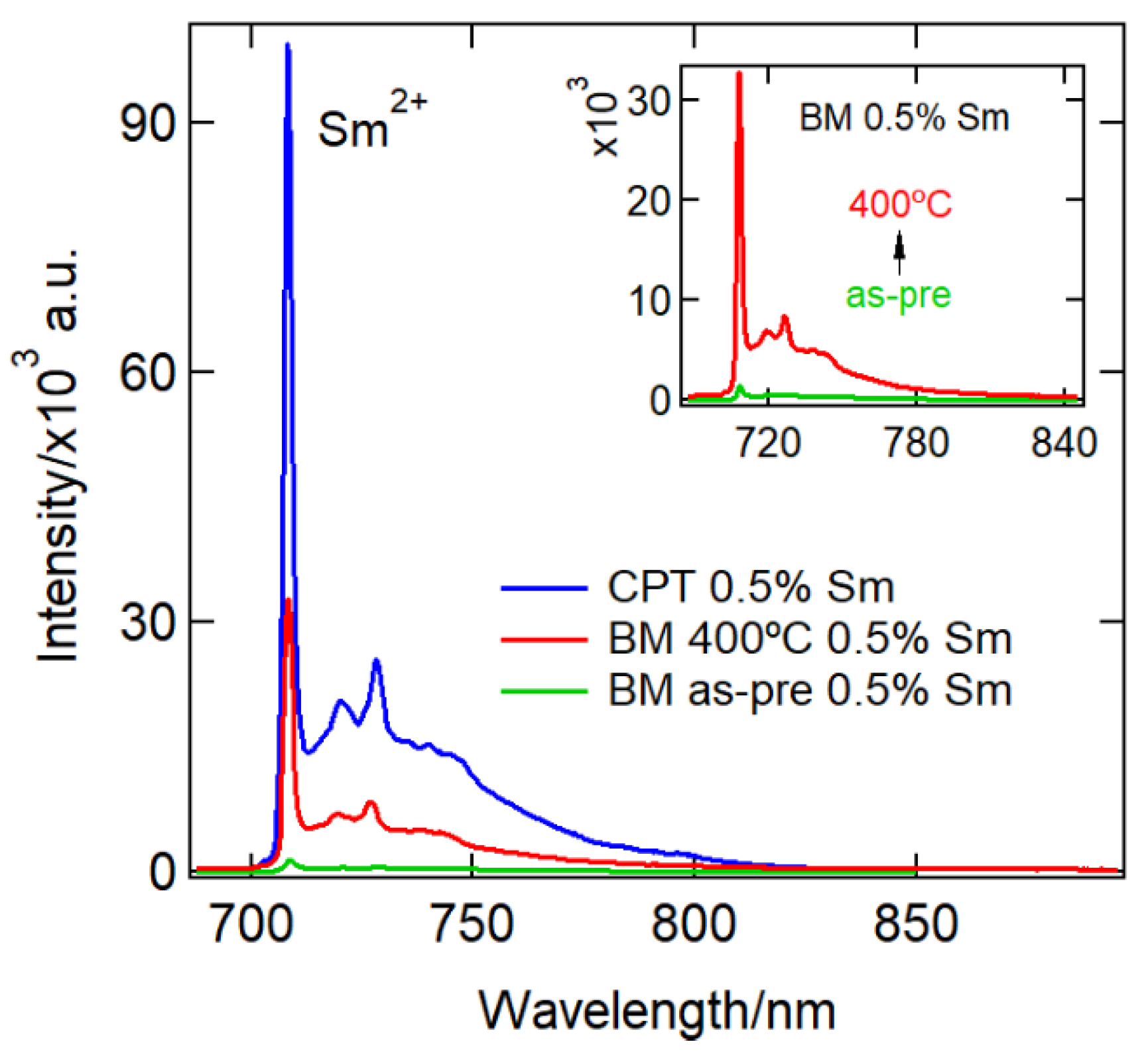
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerward, L.; Olsen, J. S.; Steenstrup, S.; Malinowski, M.; Åsbrink, S.; Waskowska, A. X-ray Diffraction Investigations of CaF2 at High Pressure. J. Appl. Crystallogr. 1992, 25, 578-581. [CrossRef]
- Hazen, R. M.; Finger, L. W. Calcium fluoride as an internal pressure standard in high-pressure cyrstallography. J. Appl. Crystallogr. 1981, 14, 234-236. [CrossRef]
- Song, K. S.; Williams, R. T. Alkaline Earth Fluorides. In Self-Trapped Excitons; Song, K. S., Williams, R. T. Ed.; Springer Berlin Heidelberg. 1993; pp 96-122.
- Cheetham, A.; Fender, B.; Cooper, M. Defect structure of calcium fluoride containing excess anions I. Bragg scattering. J. Phys. C: Solid State Phys. 2001, 4, 3107. [CrossRef]
- Ye, W.; Liu, X.; Qiying, H.; Zhou, Z.; Hu, G. Co-precipitation synthesis and self-reduction of CaF2:Eu2+ nanoparticles using different surfactants. Mater. Res. Bull. 2016, 83. [CrossRef]
- Cantelar, E.; Sanz-García, J. A.; Sanz-Martín, A.; Muñoz Santiuste, J. E.; Cussó, F. Structural, photoluminescent properties and Judd-Ofelt analysis of Eu3+-activated CaF2 nanocubes. J. Alloys Compd. 2020, 813, 152194. [CrossRef]
- Pandurangappa, C.; Lakshminarasappa, B. N. Optical studies of samarium-doped fluoride nanoparticles. Philos. Mag. 2011, 91(35), 4486-4494. [CrossRef]
- Ritter, B.; Krahl, T.; Rurack, K.; Kemnitz, E. Nanoscale CaF2 doped with Eu3+ and Tb3+ through fluorolytic sol–gel synthesis. J. Mater. Chem. C. 2014, 2(40), 8607-8613.
- Yuan, G.; Murai, S.; Tamura, S.; Tomita, K.; Tanaka, K. Enhancement of up- and downconversion photoluminescence from Yb3+, Er3+ co-doped CaF2 nanoparticles deposited on two-dimensional plasmonic arrays. In Proc. SPIE 11194, Plasmonics IV, Hangzhou, China, 18 November 2019, Eds.; 2019; Vol. 11194, pp 19.
- Nakhaei, O.; Shahtahmassebi, N.; Mahmood, R. Synthesis and Characterization of CaF2 NPs with Co-precipitation and Hydrothermal Method. J. Nanomed. Nanotechnol. 2011, 2(5), 116.
- Quan, Z.; Yang, D.; Yang, P.; Zhang, X.; Lian, H.; Liu, X.; Lin, J. Uniform Colloidal Alkaline Earth Metal Fluoride Nanocrystals: Nonhydrolytic Synthesis and Luminescence Properties. Inorg. Chem. 2008, 47(20), 9509-9517. [CrossRef]
- James, S.; Adams, C.; Bolm, C.; Braga, D.; Collier, P.; Friscic, T.; Grepioni, F.; Harris, K.; Hyett, G.; Jones, W., et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413-447. [CrossRef]
- Heise, M.; Scholz, G.; Düvel, A.; Heitjans, P.; Kemnitz, E. Mechanochemical synthesis, structure, and properties of solid solutions of alkaline earth metal fluorides: Ma1−xMbxF2 (M: Ca, Sr, Ba). Solid State Sci. 2016, 60, 65-74.
- Molaiyan, P.; Witter, R. Mechanochemical synthesis of solid-state electrolyte Sm1−xCaxF3−x for batteries and other electrochemical devices. Mater. Lett. 2019, 244, 22-26. [CrossRef]
- Chowdhury, N.; Riesen, N.; Riesen, H. Yb3+ and Er3+ Codoped BaLiF3 Nanocrystals for X-ray Dosimetry and Imaging by Upconversion Luminescence. ACS Appl. Nano Mater. 2021, 4(7), 6659-6667.
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3(1), 13-19. [CrossRef]
- Sadykov, V. A.; Mezentseva, N. V.; Bobrova, L. N.; Smorygo, O. L.; Eremeev, N. F.; Fedorova, Y. E.; Bespalko, Y. N.; Skriabin, P. I.; Krasnov, A. V.; Lukashevich, A. I., et al. Chapter 12 - Advanced Materials for Solid Oxide Fuel Cells and Membrane Catalytic Reactors. In Advanced Nanomaterials for Catalysis and Energy; Sadykov, V. A. Ed.; Elsevier. 2019; pp 435-514.
- Düvel, A.; Wilkening, M.; Uecker, R.; Wegner, S.; Sepelak, V.; Heitjans, P. Mechanosynthesized nanocrystalline BaLiF3: The impact of grain boundaries and structural disorder on ionic transport. Phys. Chem. Chem. Phys. 2010, 12, 11251-11262. [CrossRef]
- Heise, M.; Scholz, G.; Krahl, T.; Kemnitz, E. Luminescent properties of Eu3+ doped CaF2, SrF2, BaF2 and PbF2 powders prepared by high-energy ball milling. Solid State Sci. 2019, 91, 113-118. [CrossRef]
- Rozaila, Z. S.; Riesen, N.; Riesen, H. Luminescence and photoionization of X-ray generated Sm2+ in coprecipitated CaF2 nanocrystals. Dalton Trans. 2021, 50(44), 16205-16213.
- Crystallography Open Database. http://www.crystallography.net/cod/index.php. (accessed 2019-12-01).
- MAUD. http://maud.radiographema.eu/. (accessed 2019-12-02).
- Zhang, J.; Riesen, H. Photostimulated and persistent luminescence of samarium ions in BaFCl. J. Lumin. 2019, 207, 188-194. [CrossRef]
- Liu, Z.; Stevens-Kalceff, M. A.; Wang, X.; Riesen, H. Mechanochemical synthesis of nanocrystalline BaFCl:Sm3+ storage phosphor by ball milling. Chem. Phys. Lett. 2013, 588, 193-197.
- Bensalah, A.; Mortier, M.; Patriarche, G.; Gredin, P.; Vivien, D. Synthesis and optical characterizations of undoped and rare-earth-doped CaF2 nanoparticles. J. Solid State Chem. 2006, 179(8), 2636-2644. [CrossRef]
- Zhi, G.; Song, J.; Mei, B.; Weibing, Z. Synthesis and Characterization of Er3+ Doped CaF2 Nanoparticles. J. Alloys Compd. 2011, 509, 9133–9137. [CrossRef]
- Shannon, R. D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. A. 1976, 32(5), 751-767. [CrossRef]
- Wells, J.-P. R. Laser Spectroscopy of Alkaline Earth Fluoride Crystals Doped With Trivalent Samarium and Europium Ions. Ph.D. Thesis, University of Canterbury, Christchurch, New Zeland, 1996.
- Mikhail, P.; Ramseyer, K.; Frei, G.; Budde, F.; Hulliger, J. Bleaching of Sm2+ during photoluminescence and cathodoluminescence. Opt. Commun. 2001, 188, 111-117. [CrossRef]
- Rabbiner, N. Fluorescence of Sm3+ in CaF2. Phys. Rev. X. 1963, 130, 502-506.
- Bungala, C. J.; Kumar, M.; Gopal, K. Fluorescence properties and energy transfer mechanism of Sm3+ ion in lead telluroborate glasses. Opt. Mater. 2011, 33, 1643-1647. [CrossRef]
- Jamalaiah, B. C.; Rasool, S. N. Fluorescence properties of Sm3+ ions in yttrium aluminum borate phosphors for optical applications. J. Mol. Struct. 2015, 1097, 161-165. [CrossRef]
- Lakshminarayana, G.; Yang, R.; Mao, M.; Qiu, J.; Kityk, I. V. Photoluminescence of Sm3+, Dy3+, and Tm3+-doped transparent glass ceramics containing CaF2 nanocrystals. J. Non-Cryst. Solids. 2009, 355(52), 2668-2673. [CrossRef]
- Qiao, Y.-P.; Chen, P. Luminescence, energy transfer, and color adjustment of CaO-CaF2-Al2O3-B2O3-SiO2 glass co-doped with CeO2 and Sm2O3. J. Non-Cryst. Solids. 2021, 552, 120461.
- Wood, D. L.; Kaiser, W. Absorption and Fluorescence of Sm2+ in CaF2, SrF2, and BaF2. Phys. Rev. 1962, 126(6), 2079-2088.
- Kelly-Gorham, M. R. K.; DeVetter, B. M.; Brauer, C. S.; Cannon, B. D.; Burton, S. D.; Bliss, M.; Johnson, T. J.; Myers, T. L. Complex refractive index measurements for BaF2 and CaF2 via single-angle infrared reflectance spectroscopy. Opt. Mater. 2017, 72, 743-748. [CrossRef]
- Kaiser, W.; Spitzer, W. G.; Kaiser, R. H.; Howarth, L. E. Infrared properties of CaF2, SrF2, and BaF2. Phys. Rev. 1962, 127(6), 1950-1954.
- Qiao, Y.-P. Influence of Sm2O3 and CaF2 Concentration on the Enhancement of Luminescence and Red Colour in Borosilicate Glass. Trans. Indian Ceram. Soc. 2021, 80(3), 208-215.
- Liu, Z. Q.; Stevens-Kalceff, M. A.; Riesen, H. Effects of Postannealing on the Photoluminescence Properties of Coprecipitated Nanocrystalline BaFCl:Sm3+. J. Phys. Chem. A. 2013, 117(9), 1930-1934.
- Stevens-Kalceff, M. A.; Liu, Z.; Riesen, H. Cathodoluminescence Microanalysis of Irradiated Microcrystalline and Nanocrystalline Samarium Doped BaFCl. Microsc. Microanal. 2012, 18(6), 1229-1238. [CrossRef]
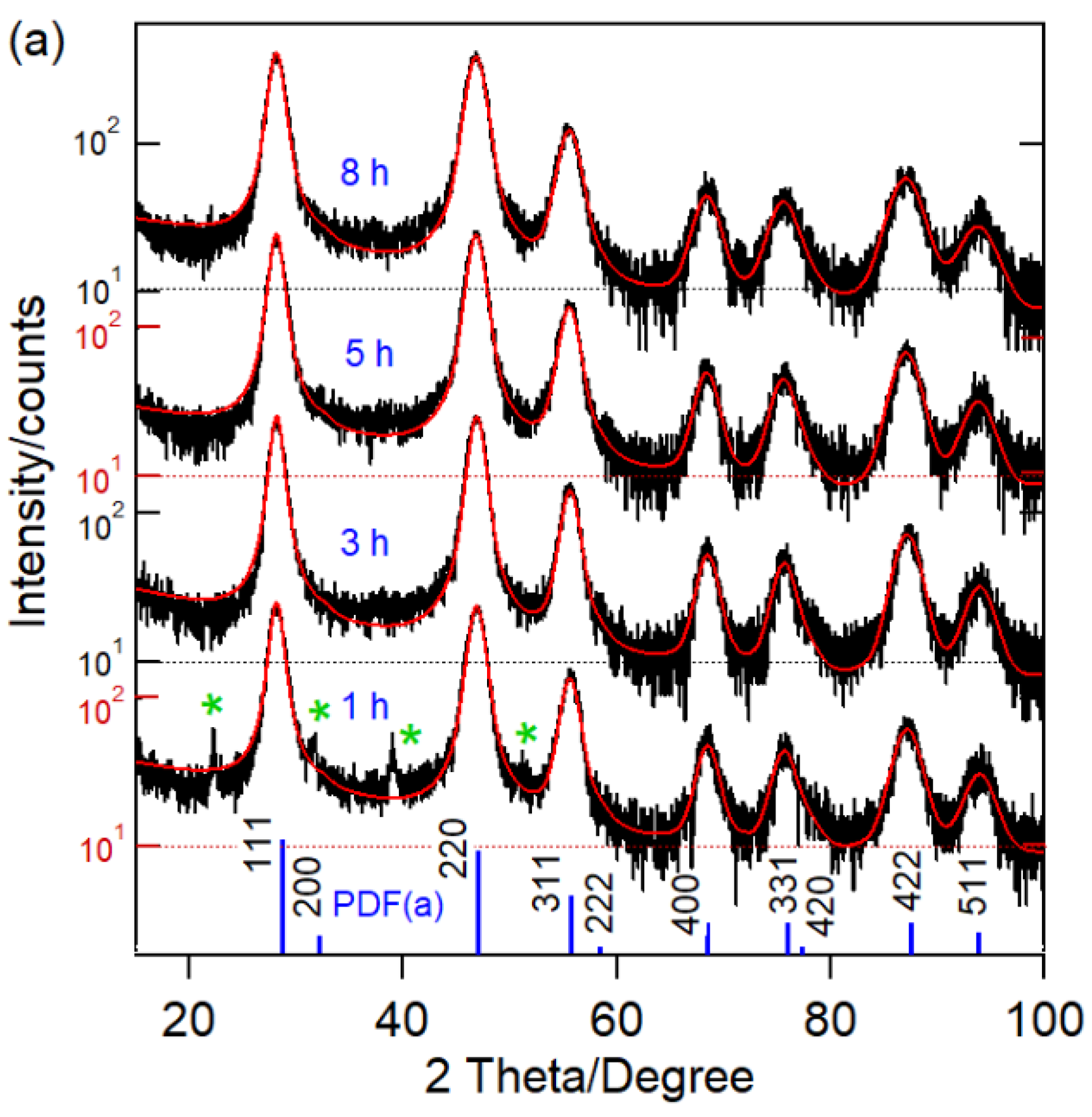
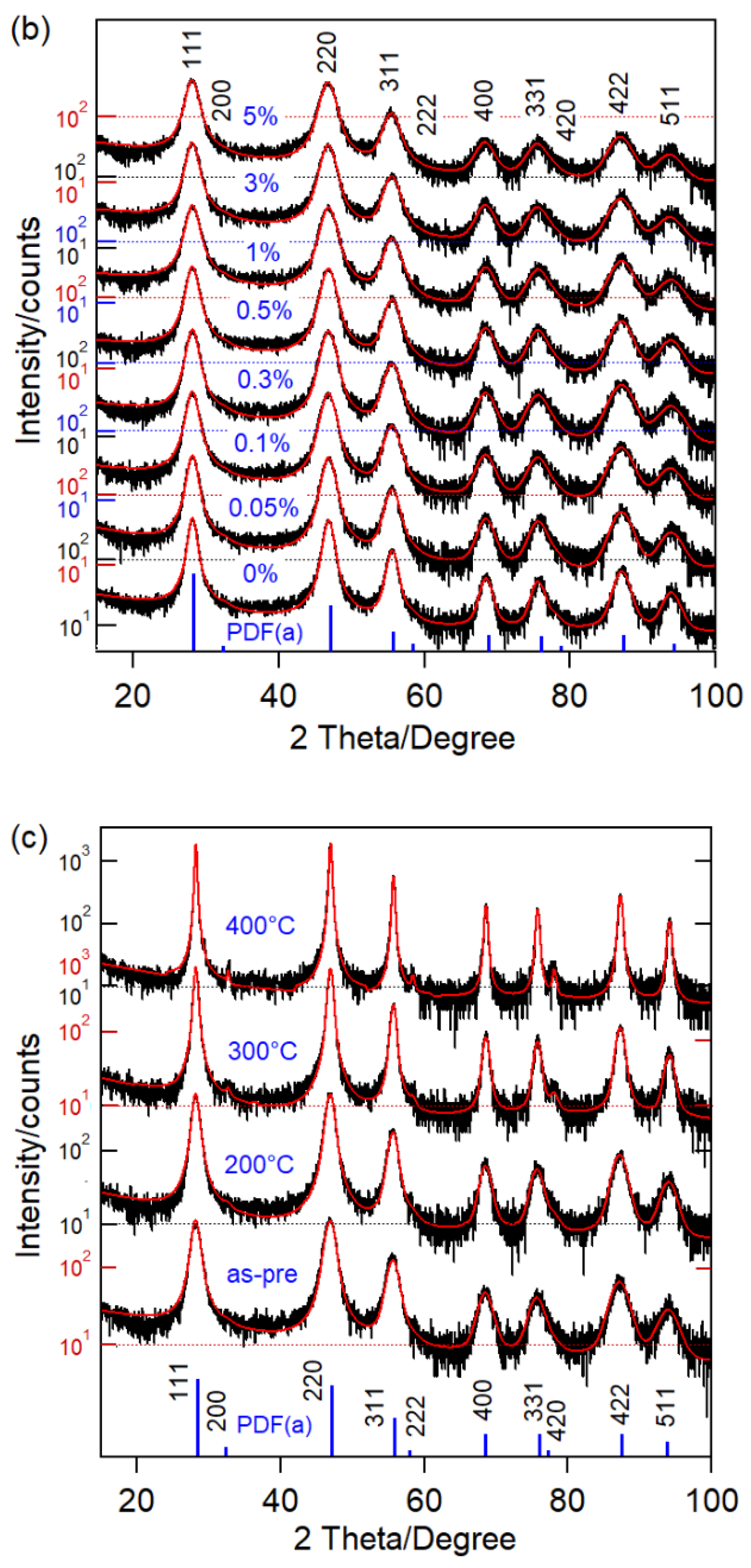
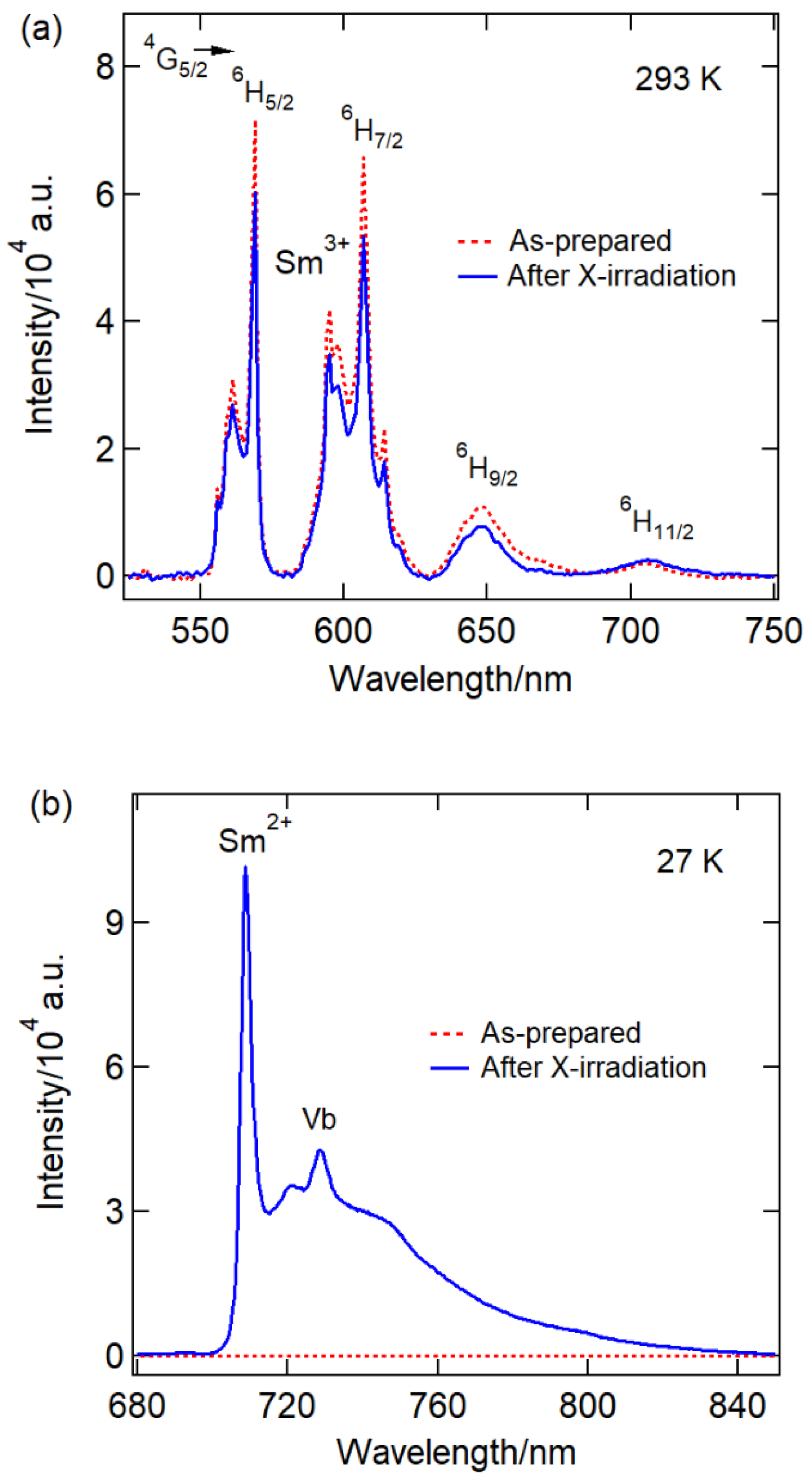
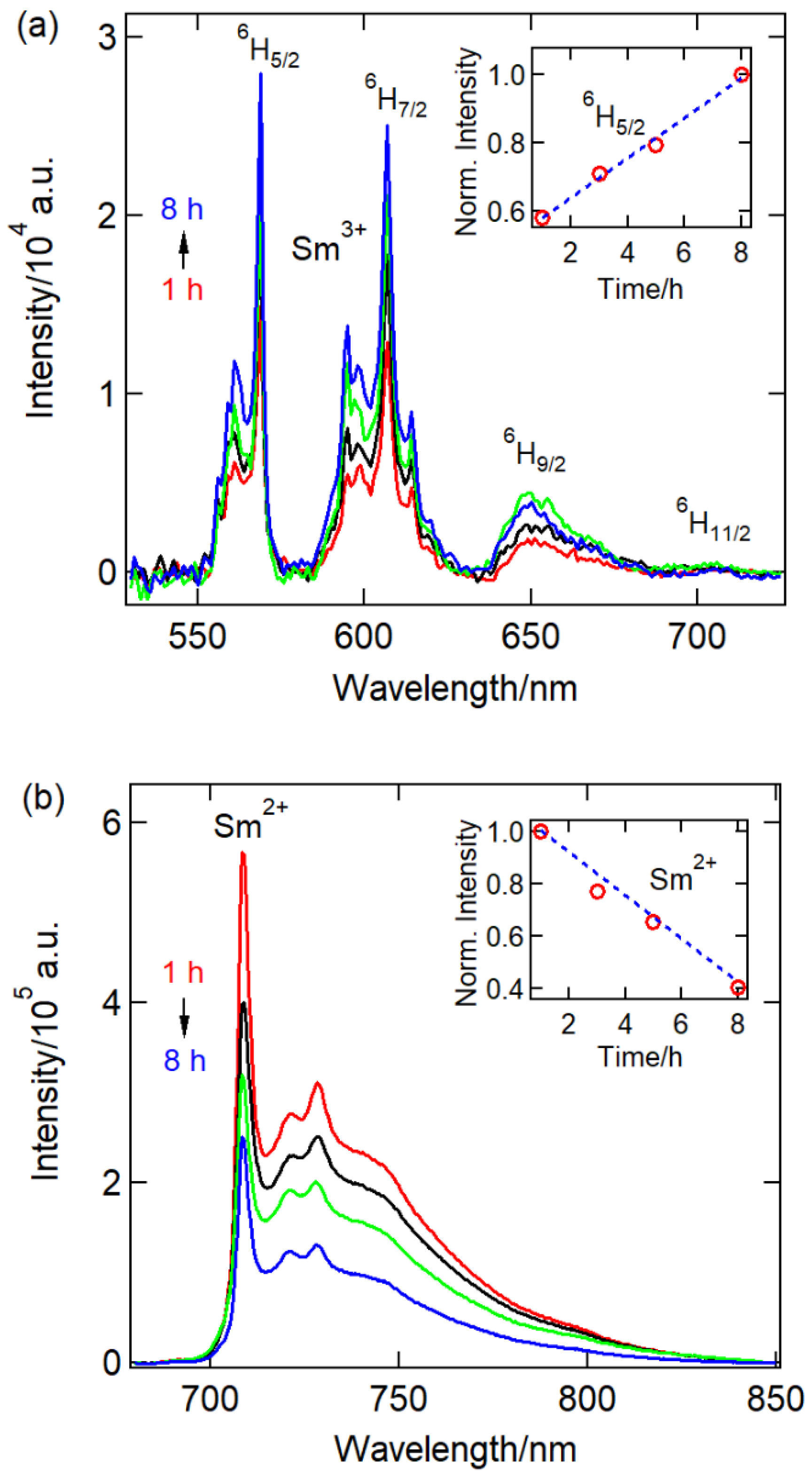
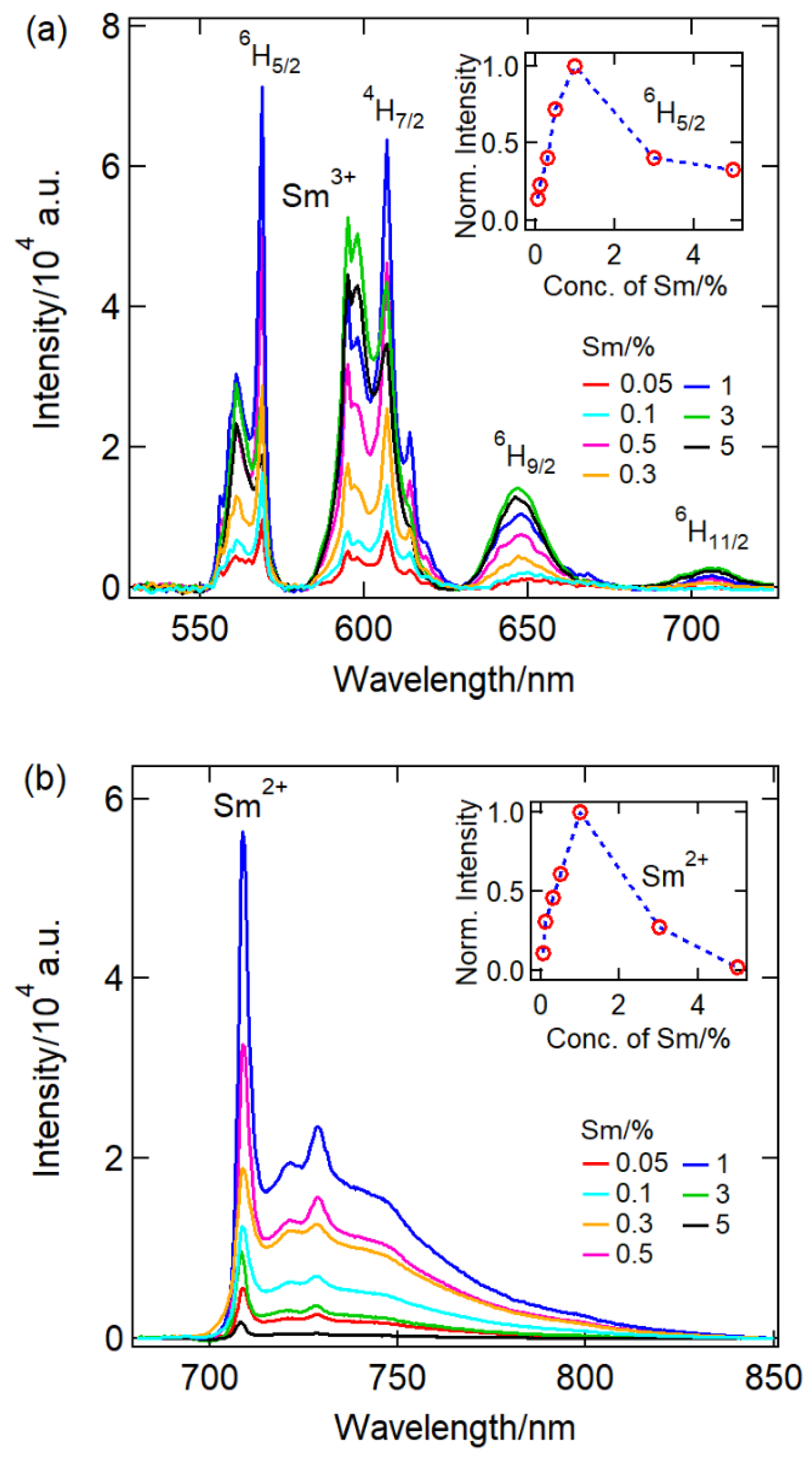
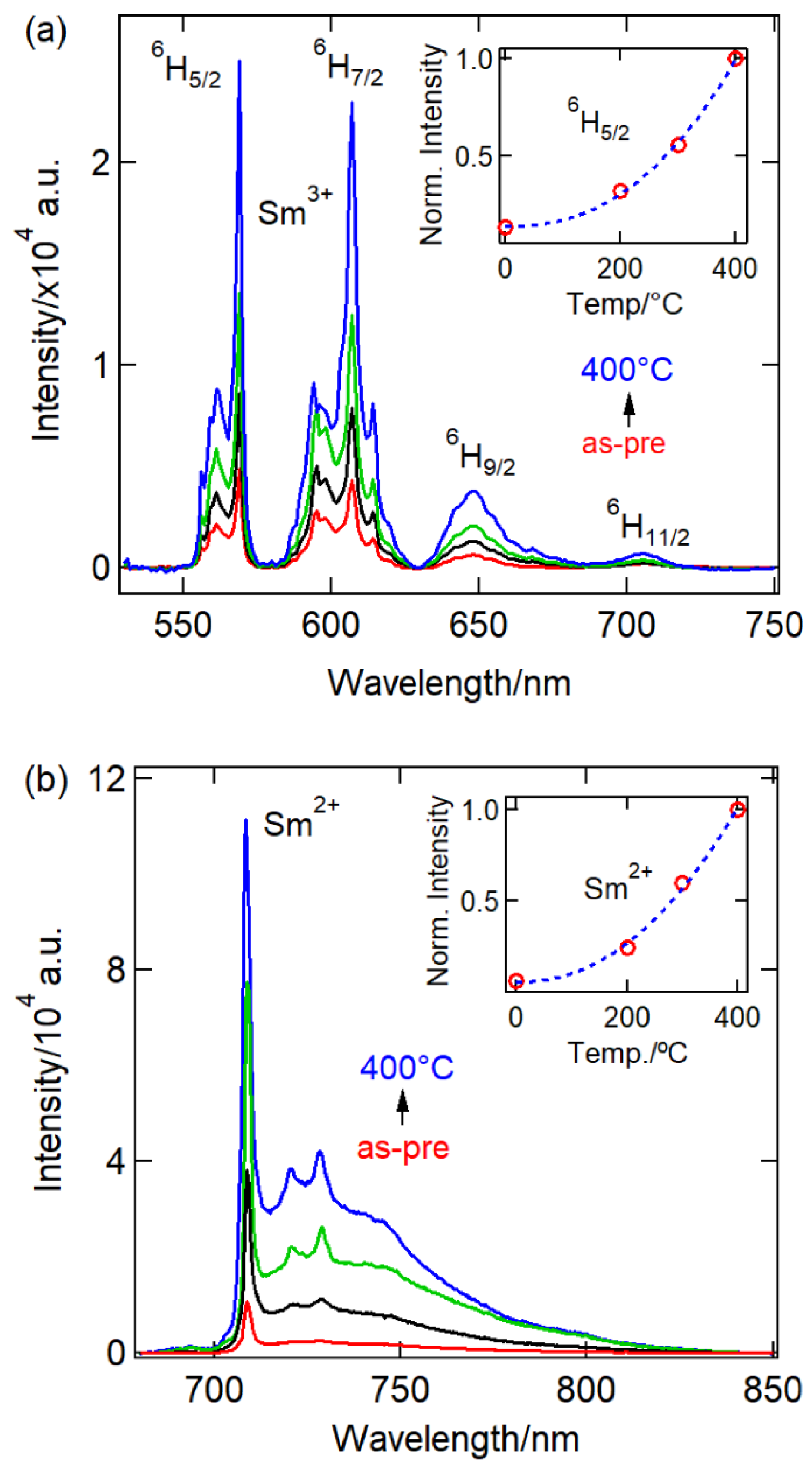
| Ball-milling time CaF2:0.1% Sm3+ | |||||||||||||
| time (h) | average crystallite size ± 1 (nm) |
lattice parameter, a (Å) | Rietveld refinement | ||||||||||
|
% |
% |
||||||||||||
| 1 | 12 | 5.4754 ± 0.0012 | 18.9 | 14.5 | 1.30 | ||||||||
| 3 | 11 | 5.4763 ± 0.0010 | 19.0 | 15.1 | 1.26 | ||||||||
| 5 | 9 | 5.4823 ± 0.0012 | 19.4 | 14.9 | 1.30 | ||||||||
| 8 | 8 | 5.4832 ± 0.0013 | 18.5 | 14.9 | 1.24 | ||||||||
| Concentration of Sm3+ CaF2:ySm3+, 8 h ball-milling time | |||||||||||||
| y% | average crystallite size ± 1 (nm) |
lattice parameter, a (Å) | Rietveld refinement | ||||||||||
|
% |
% |
||||||||||||
| 0 | 12 | 5.4824 ± 0.0011 | 15.9 | 13.9 | 1.14 | ||||||||
| 0.05 | 11 | 5.4826 ± 0.0012 | 16.8 | 13.8 | 1.22 | ||||||||
| 0.1 | 9 | 5.4832 ± 0.0013 | 18.5 | 14.9 | 1.24 | ||||||||
| 0.3 | 9 | 5.4838 ± 0.0012 | 17.4 | 14.4 | 1.21 | ||||||||
| 0.5 | 8 | 5.4844 ± 0.0011 | 17.8 | 15.2 | 1.17 | ||||||||
| 1 | 8 | 5.4864 ± 0.0010 | 17.3 | 14.6 | 1.18 | ||||||||
| 3 | 7 | 5.4880 ± 0.0014 | 17.1 | 14.5 | 1.18 | ||||||||
| 5 | 6 | 5.4915 ± 0.0017 | 17.2 | 14.6 | 1.18 | ||||||||
| Annealing temperature CaF2:0.1% Sm3+, 8 h ball-milling time | |||||||||||||
| temp. (°C) |
average crystallite size ± 1 (nm) |
lattice parameter, a (Å) | Rietveld refinement | ||||||||||
|
% |
% |
||||||||||||
| as-pre | 9 | 5.4774 ± 0.0011 | 20.9 | 15.0 | 1.39 | ||||||||
| 200 | 12 | 5.4753 ± 0.0007 | 19.3 | 15.4 | 1.25 | ||||||||
| 300 | 22 | 5.4701 ± 0.0004 | 18.7 | 15.3 | 1.22 | ||||||||
| 400 | 45 | 5.4687 ± 0.0002 | 18.7 | 15.2 | 1.23 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





