1. Introduction
Pharmaceutical molecules such as antibiotics are efficient treatments for several human and animal diseases. Environmental emissions in water of their non-consumed traces may cause unwanted effects. They are in particular suspected to participate to the development of antibio-resistant AR micro-organisms. They can be generated as traces on the outlets of hospital or large town and also around their fabrication sites. Antibiotics emission are carefully controlled by world organizations [
1] such as WHO, World Health Organization, and data concerning them in Europe are published in Journals such as
Environment International [
2] and in aquatic media in Current Opinion in Environmental Science & Health [
3].
The present study is devoted to the photocatalytic decomposition of one antibiotic, amoxyciline AMX in water by an Advanced Oxidation Process AOP as already studied in references 4 to 6 with several catalysts. We know that the initial method of R. Massard can be used to prepare iron oxides colloids [
4]. We have recently used maghemite ones for water depollution of large organic molecules [
5] and faced difficulties because of their important solubility. The studied catalytic reaction was in fact heterogeneous as well as homogeneous because of an important concentration of dissolved Fe (II) ions associated with the Fenton reaction, the liberation of Fe (III) species and H
2O
2 discovered by Fenton in 1895 and studied in details as a function of the pH in reference [
6]:
We have used here solid catalysts rich in nanoparticles less soluble in water than maghemite as zinc ferrite, ZFO, and hematite. Particles of ZFO are commun in Industry, mainly because they are formed by roasting the pollution dust inside EAFD, Electrical Arc furnace dust [
7]. By reduction, they are transformed into iron oxides (hematite) and zinc vapors. Zinc ferrite nanoparticles when, their size and morphology change, become very active in research Laboratories working in telecommunications, as captors. Their peculiar interest is correlated with their magnetic, thermal, optical and also electric properties which make them particularly important for several applications fields, catalysis and also as ferrofluids - cooling-down devices [
8,
9,
10,
11,
12]. Hematite nanoparticles also have very interesting magnetic and photocatalytic properties and their heterogeneous reactivity can be studied because of their stability in water, as described in references [
13,
14].
We will show that both ZnFe
2O
4 of spinel-type and mixtures of ZFO and α-Fe
2O
3 hematite nanoparticles are active for AOP, without addition of H
2O
2 and can be obtained starting from given well-selected silica SBA-15 grains. Calcination in air to generate the oxides nanoparticles is fixed at 700°C, at 2 °C by min and quenching, i.e. cooling down rapidly in air. This temperature is important to have a percentage of Fe (III) cations inside both octahedral and tetrahedral crystallographic sites and quenching is important to fix the chemical composition the % of inversion during the cooling down period. ZnFe
2O
4 and mixtures of ZnFe
2O
4 with hematite are obtained by changing the anions associated with the metallic Zn and Fe cations. Solids prepared by comparison between nitrates and chlorides salts of both Zn and Fe species are compared. Characterizations are performed by XRD, N
2 sorption and also using TEM ultrathin sections of the silica grains to evidence crystallized oxides nanoparticles formed inside the pores of the SBA-15 grains, as made by some of us recently for Co and Rh catalysts, used in methane reforming [
15] and by others for Co Fe and Ni Fe oxides nanoparticles SBA-15 tested for methanol oxidation reactions [
16]. The filling percentage of the pores will be evaluated by N
2 sorption. To obtain specific information about the % of inversion, the percentage of Fe (III) in tetrahedral sites, we will need information by Mössbauer spectroscopy and by Neutrons diffraction and they will be described in a next publication.
We have used UV visible spectra and liquid chromatography HPLC to follow the AMX molecules decomposition. Chromatographic results are more reliable because the most intense UV visible peaks are located in the UV spectral range and are broad. We have worked in water inside a double walls beaker of temperature maintained at 25°C by a fresh water Julabo set-up opened to air, the disappearance of a main peak due to AMX is detected at a wavelength of 230 nm for a retention time between 3.5 and 3.7 min in the used experimental conditions (with a Lachrom Elite, VWR - Hitachi apparatus), a C18 column, a fixed volume of injection of 20 μL, fixed eluant solution 90/9/1 methanol/water/ formic acid v. to v. and an eluant flow of 0.8 ml. min-1). The AMX molecule is stable at 25°C and at a pH 5.
2. Materials and Methods
2.1. Reagents
Inorganic salts, ferric nitrate nanohydrate, Fe (NO3)3. 9H2O and zinc nitrate hexahydrate Zn (NO3)2. 6 H2O are respectively from Sigma–Aldrich and PROLABO. The first salt is commercialized at an ACS grade with a degree of purety of 98%, the second is in a purum crystallized form, with a purety larger than 99.0 %.
To prepare the blank silica SBA-15 support necessary to disperse the Zinc and Iron salts, a Pluronic co-polymer, with PEO standing for Poly Ethylene Oxide and PPO for Poly Propylene Oxide, of general formulae PEOx-PPOy-PEOx, PEO20-PPO70-PEO20 (P123) was used. An HCl aqueous solution of concentration 2 mol. L-1 was obtained by mixing 201.5 g of HCl at 37% with 815.6 g of distilled water. A double walls beaker was used and maintained stirred at 35.00 ± 0.15 °C with a Julabo Circulating Water Bath. 85 ml of tetraethoxy-orthosilicate was added drop by drop and stirring was stopped. The mixture was then let at 35°C for 24h. After this maturation time, the powder was recovered by filtration on paper. 770 g of the moist powder and 700 mL of its liquor were transferred into an autoclave of 1L. Hydrothermal treatment was made at 130°C, 24h. Inside plastic flasks a similar treatment at 90°C, 24h was made. Powders were recovered by filtration on paper Whatman. A calcination at 500°C in air (at 5°C by min then 6h in air inside a muffle oven, cooling down inside the same furnace) was used to eliminate the surfactant and condense silica.
2.2. Synthesis by the Two Solvents Methods
Freshly calcined silica was suspended in dry cyclohexane (1g for 35 mL). An aqueous solution containing a mixture of Fe (NO3)3 .9H2O and Zn (NO3)2 .6H2O (0.295 mL, both with concentrations 2 mol. L-1) was then added drop by drop. Calcination in air was used to obtain oxides particles, ZFO nanoparticles replicated inside the SBA-15 mesopores were formed by calcination in air at 700°C at 2°C by min then quenching to room temperature. The quenching was necessary to avoid modifications of the iron proportion in Td sites during cooling down. The mixture was manually stirred until a homogeneous coloration was obtained. Cyclohexane was removed rapidly, with less than 12 min of solid/solution contact. Filtration was made with a paper. The obtained sample after calcination was called 2S (1) nitrate. Impregnation can be reproduced a second and a third times to obtain 2S (2) nitrate and 2S (3) nitrate calcined samples. The same method was used for chlorides, FeCl3. 6 H2O and ZnCl2 using similar conditions, a first, a second and a third step of impregnation and a calcination to obtain 2S (1) chloride, 2S (2) chloride and 2S (3) samples.
2.3. Characterization Methods
2.3.1. N2 Sorption Results
Specific surface areas, pore volumes, and diameters of the main pores were measured by N2 sorption using a BET apparatus (Micromeritics). Specific surface areas were calculated from the analysis of the relative pressure range (0.05-0.25) using the Brünauer-Emmett-Teller (BET) method. The overall pore volume was measured from a single point at a relative pressure of P/P0 = 0.98. The BJH method was also applied to the desorption branch of the isotherm. t-plots or alpha plots were performed to obtain microporous volumes.
2.3.2. SEM and TEM
We have used a scanning electrons microscope SEM and a transmission microscope, TEM, JEOL JEM2011, operating at 200 kV. TEM micrographs offered unique advantages. They provided the possibility to study a solid with a resolution that can be as small as 2Å and enabled us to obtain local compositions and electron diffraction results on specific kinds of nanoparticles (selected area electron diffractions, SAED). Calcined catalytic powders were suspended in ethanol and dried on carbon-coated copper grids.
2.3.3. X-Ray Diffraction
X-ray diffractions were measured with an ADVANCE D8 Bruker diffractometer with a Bragg-Brentano geometry working at 40 kV and 30 mA and an irradiation at 1.5418 Å which is a mixture of Cu Kα1 at 1.54056 Å and Cu K 2 at 1.54439 Å with relative proportions of 1:2. A precise Lynx Eye detector was used. Diffractions reported here were recorded within the 5 < 2θ < 90° range. Crystalline phases were identified, mostly by simulation with the program EVA. FullProf software was also used. Identifications were made with ICDD data (The International Center for Diffraction Data, ICDD 4+). With each diffraction peak, peak positions, full widths at half-maxima (H or FWHM) were measured. Four main crystalline phases were identified: Zn-ferrite of spinel structure (ICDD 04-006-1542), hematite of hexagonal structure (ICDD 033-0664) and ZnO, wurtzite, also of hexagonal structure (ICDD 01-089-1397).
FullProf software was also used. Identifications were made with ICDD data (The International Center for Diffraction Data, ICDD 4+). With each diffraction peak, peak positions, full widths at half-maxima (H or FWHM) were measured. Four main crystalline phases were identified: Zn-ferrite of spinel structure (ICDD 04-006-1542), hematite of hexagonal structure (ICDD 033-0664) and ZnO, wurtzite, also of hexagonal structure (ICDD 01-089-1397) and cubic structure ZnO was also detected.
A pseudo-void description of a XRD peak is associated with a linear combination of the two possible shapes, L for Lorentzian and G for Gaussian. The line-width, PV, can be written as a function of a coefficient ETA η:
The observed diffraction peaks are broad and we have used the Fullprof simulation program to discriminate the influence of the nanoparticles sizes and of crystallographic microcontraints. Also to know if the enlargement is isotropic or does it depends upon diffractions peaks hkl indexation with h, k and l Miller. We have simulated the coherent X-ray diffraction domains to obtain the nanoparticle shape in our 2S (3) chloride sample, using the GFourier program.
2.4. Photocatalytic Tests of AMX Decomposition
A LED lamp (UV visible no NIR, Diall 1055 Lumens, power 75 W) was placed 35cm above the used double-glass walled reactor. An aqueous solution of 10.00 ± 0.04 ppm of AMX in distilled water was prepared. 80 mL of this solution were introduced into the reactor and then 80 mg of solid catalyst was dispersed in it. The pH of reaction was adjusted at pH=5 and we have checked that AMX was stable at this pH during 220 min. The reactor was placed into a black box and thermalized by a (16 L) water bath equipped with a Julabo agitator. The temperature was set at 25°C to suppress the heating due to the lamp and the system was left to equilibrate during 30 min, and the lamp was turned on. The first sample time t = 0 min was taken when the catalyst was added to the AMX solution and a second after absorption. Samples were then taken after 35, 40, 45, 75, 105, 165 and 225 min with a 1 mL syringe fitted with a 15 cm needle and filtered with opening at 0.45 μm.
Control was also carried out by keeping the reaction medium in the dark for 225 min. AMX concentration in water was measured as a function of time with an HPLC (Elite from Hitachi) apparatus using a C18 column (150 mm × 4.6 mm, 4 μm) and a UV detector. This stationary column, very commun in HPLC Laboratories, is characterized by its strong hydrophobic character, due to chains of 18 carbons atoms and for the retention of the most hydrophobic compounds. The flow-rate of the elution solvent (methanol/water; (90/10% v /v) was set at 0.8 mL.min-1 and the injected volume was 20 μl. Detection at the column outlet was carried out using a multi-diode array detector at λ = 230 nm.
3. Results
It is important to know that our blank silica samples have been prepared with a calcination in air at 500°C at 5°C by min 6 h and cooling down inside the furnace of calcination. These conditions are allowing the elimination of the Pluronic molecules and opened the silica pores. We have checked the validity of this method by checking 13C NMR spectra (not shown). With Zn and Fe containing samples, calcination is also performed in air but with a rate of 2°C by min until 700°C and a quenching is used by extracting the quartz reactor from the furnace and letting it cooled down spontaneously in air at room temperature. This « quenching » in itself is important to fixe and reproduce the percentage of Zn and Fe in tetrahedral sites of the spinel structure. We have supposed that the Zn2+ ions are located in the tetrahedral sites, where their energy of stabilization is better because of their sizes, as given in Shannon publication (17) and of their 3+ charge.
1.1. Percentage of Open Pores by N2 Sorption
Except for silicas, all the described considerations concern samples calcined at 700°C. The samples labelled 2S (i) chloride and nitrates were calcined in these conditions. N2 sorption analysis were made using the book of F. Rouquerol et al (18).
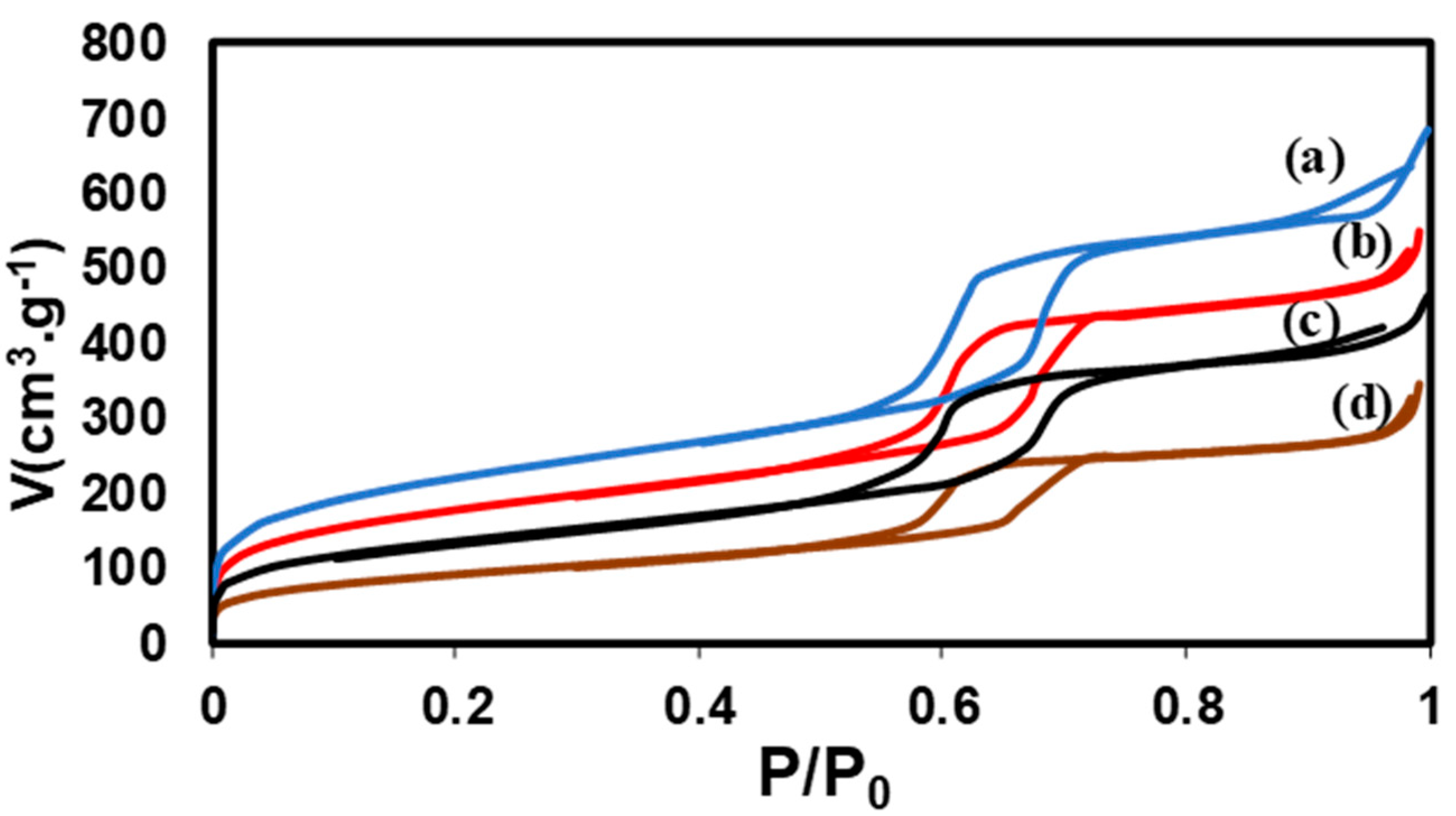
Figure 1.
N2 sorption isotherms before and after impregnation (a) SBA-15 silica alone (b) 2S (1) nitrate (c) 2S (2) nitrate (d) 2S (3) nitrate impregnated.
Figure 1.
N2 sorption isotherms before and after impregnation (a) SBA-15 silica alone (b) 2S (1) nitrate (c) 2S (2) nitrate (d) 2S (3) nitrate impregnated.
Figure 2.
N2 sorption isotherms before and after impregnation (a) SBA-15’ alone (b) 2S (1) chloride (c) 2S (2) chloride, two impregnations (d) 2S (3) chloride.
Figure 2.
N2 sorption isotherms before and after impregnation (a) SBA-15’ alone (b) 2S (1) chloride (c) 2S (2) chloride, two impregnations (d) 2S (3) chloride.
Blank silicas were calcined at 500°C in air, with a rate of 5°C by min and a treatment of 6h. These conditions have been selected to be sure that all the Pluronic template molecules were removed. This point has been independently checked by 13C NMR (not shown). The SBA-15 and SBA-15’ were prepared using different hydrothermal conditions and flasks inside the same furnace: 1) one in a plastic flask at 90°C 24h, 2) one in a special cell covered with Teflon® at 130°C for 24h which tightness under pressure, even at 130°C, is warranted. The fixed pressure gives a larger mesoporous volume circa P/P0 0.6, a smaller microporous volume bellow P/P0 0.3 and macropores due to textural porosity are absent between 0.9 and 1.0 P/P0. The fixed pressure is also associated with more vertical positions of the lines delaminating the hysteresis loop, between the absorption and the desorption branches of the isotherm indicating more parallel and ordered mesopores.
The samples containing Zn and Fe precursors were calcined using a different method. With nitrates precursors, the main obtained phase is zinc ferrite. For its thermal treatment, temperature was increased up to 700°C at 2°C by min to obtain a degree of inversion between 0.0 and 0.3 in 2S (3) nitrate and quenched to maintain the % of inversion constant and reproducible inside the biggest formed oxides nanoparticles crystals. Fixed percentages were necessary to allow clean Mössbauer spectra and neutron diffraction with a given % of inversion between Zn (II) and Fe (III) cations, even after a long-time storage in air. The Fe containing nanoparticles in 2S (3) chloride were external and rich in hematite crystals.
Table 1.
N2 sorption results.
Table 1.
N2 sorption results.
| Sample |
Calcination |
Specific surface area |
Porous volume |
Remaining porous volume after 2S impregnation and calcination at 700°C |
Silica treatment
HT 90°C in polypropylene flask, 24h
Autoclave
Teflon lining, 130°C, 24h |
500°C in air
2°C/min, 6h
|
770
|
0.96 |
|
| 477 |
0.89 |
|
| 2S (1) chloride |
700°C in air 2°C/min
and
quenching |
623 |
0.77 |
80 % |
| 2S (2) chloride |
476 |
0.64 |
67 % |
| 2S (3) chloride |
329 |
0.45 |
47 % |
| 2S (1) nitrate |
377 |
0.70 |
79 % |
| 2S (2) nitrate |
317 |
0.56 |
63 % |
| 2S (3) nitrate |
259 |
0.51 |
57 % |
Samples are all prepared after suspension of the calcined at 500°C blank silica in cyclohexane and knowing the accessible pores volume of each sample given by N
2 sorption to associate it with the volume of the added aqueous solution of inorganic salts (4M) employed. The relative volumes of Fe and Zn precursors are of 2 by 1 for the same concentration of 4 mol. L
-1. Details are indicated in
Table 2.
We have also used a microscope, the JEOL JEM2011, operating at 200 kV, to measure the size and shapes of the silica grains.
3.3. Identification of Fe-Containing Nanocrystals by XRD
In the samples prepared with nitrates Fe and Zn salts, a zinc ferrite cubic unit cell and similar to data in ICDD is observed. For given calcination conditions, its a unit cell parameter of samples rich in Zinc ferrite is decreasing with the number of impregnation (and calcination). A hexagonal unit-cell which parameters are staying constant is observed with samples rich in hematite prepared with chloride salts (
Table 3).
The sizes of the Zinc ferrite nanoparticles after one, two and three impregnations and calcinations are almost similar. There is a decrease of unit-cell corresponding well with the size of the Zinc ferrite nanoparticles. It can be associated with Fe (3+) cations location or to the presence of oxygen vacancies. For more demanding Mössbauer spectroscopy and neutrons diffraction methods, only the samples rich in Zn and Fe species after 3 impregnations have been selected.
3.4. SEM and TEM: Location and Nature of Fe-Containing Nanoparticles
SEM micrographs are grouped in the figures (
Figure 3a,b, captured at 1 keV). In
Figure 3a, we see different silica grains with elongated shapes and we do not see the supported metal oxide nanoparticles. In order to show that the NPs are mainly located inside the pores, it is necessary to analyze the grain section that is presented shown in
Figure 3-c. At a scale twice as large as that of
Figure 3a,b shows that some of the metal oxide nanoparticles prepared with the metal chloride precursors are clear, external, and positioned on the surface of the SBA-15 grains. Other NPs remain localized within the pores. This is a significant observation because, in the absence of zinc, the growth of iron oxide NPs under the same operational conditions only occurred outside the silica grains (as noted in Nabil Tabaja’s thesis work).
2. Photocatalytic Results
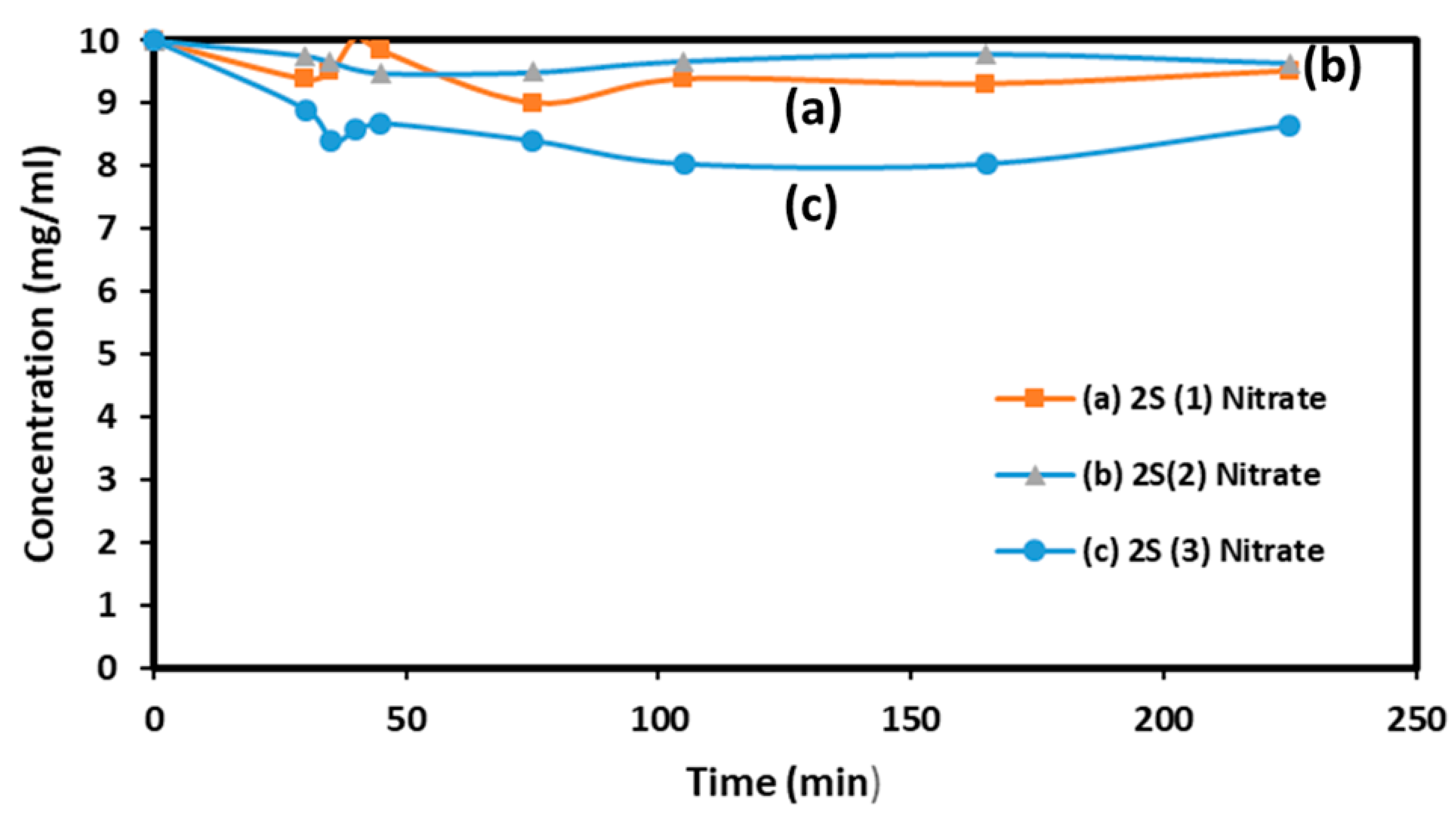
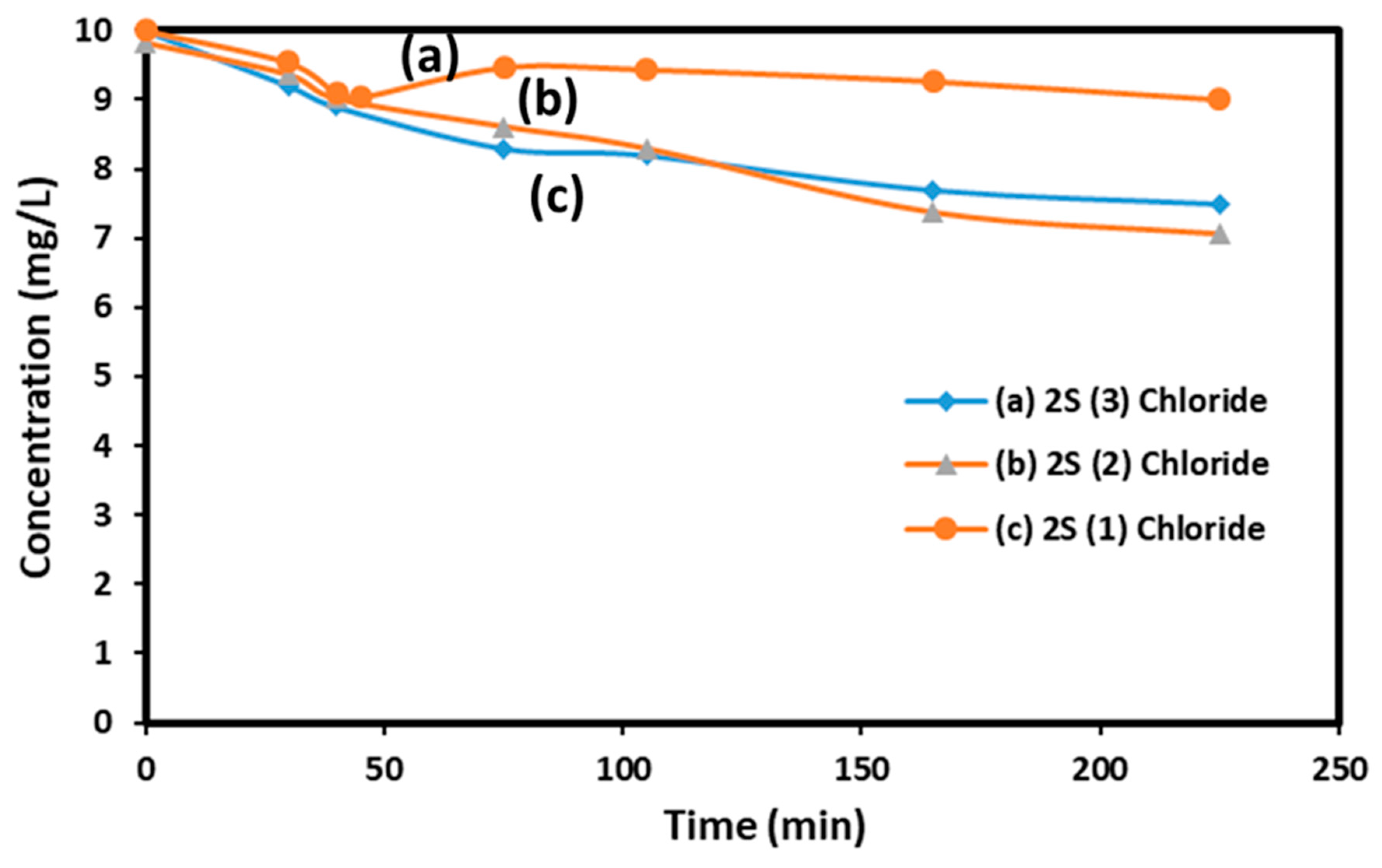
AMX decomposition in water has been studied in several previous publications and two are cited in references (19), about the photocatalytic decomposition of AMX in water and (20) about mixtures of AMX and ampicillin by Mass Spectroscopy. To evaluate the relative catalytic activity of our solids, experiments were conducted on 80 ml of solution prepared using 1 liter of distilled water and 10 mg of AMX at pH= 5 stored at 4 °C before to be used. 80 mL of this solution were introduced inside the reaction beaker and 80 mg of the solid catalyst was dispersed in it. The light was turned on after 30 min of equilibration in the dark. A first blank experiment with light illumination for 225 min showed no significant decrease in AMX concentration. The results obtained from the tests performed with the 2S (1), 2S (2) and 2S (3) samples prepared with nitrate and chloride precursors and calcined at 700°C are grouped in
Figure 4A and 4B.
If we consider the AMX concentration value measured after 250 minutes of test, the three samples obtained with successive impregnations using chloride salts (iron chloride and zinc chloride) are all slightly more active than the samples obtained with nitrate salts. This seems logical because, in the first samples, the nanoparticles are external, on the surface of the silica grains, and accessible across their entire surface. With the first samples, the activity increases as the metal species content rises, increasing from 10 to 30% when moving from one to three impregnations. More AMX decomposition is obtained on 2S (2) and 2S (3) samples than on 2S (1).
4. Conclusions
The samples prepared by impregnation 2S with cyclohexane, chlorides and nitrate salts and calcined at 700°C (2°C by min, quenching) have been characterized first using conventional Laboratory methods (TEM, XRD, N2 sorption). More demanding methods like Neutrons diffraction and Mossbauer spectroscopy are introduced here to confirm there is a degree of inversion in samples rich in Zinc ferrite nanoparticles and also to evidence some superparamagnetism in the samples rich in hematite.
All the solids are tested and found very active for AOP reactions, performed on an antibiotic (AMX). Solids rich in Zinc ferrite can be contaminated by hematite and ZnO wurtzite phases, observed and quantified by XRD. They are associated with an increase of catalytic activity. However, the spontaneous formation of heterojunctions detected by UV visible spectra on coprecipitated samples are not observed for samples prepared here using the 2S technique and calcined at 700°C in air at 2°C by min and quenched.
Author Contributions
Conceptualization, A.J., A.D., J.T. and T.H.; methodology, A.J. and A.D.; software, A.J. and A.D.; validation, A.J., A.D., G.W., J.T. and T.H.; formal analysis, A.J. and A.D.; investigation, A.J..; resources, A.J., A.D., and J.T.; data curation, A.J., A.D., G.W., J.-M. G., J.T. and T.H.; writing—original draft preparation, A.J. and A.D.; writing—review and editing, A.J., A.D., J.T. and T.H.; visualization, G.W., J.-M. G., and J.T.; supervision, A.D. and J.T.; project administration, A.D. and J.T.; funding acquisition, A.D. and J.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
There are no additional data. The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO guidance on waste and waste water management in pharmaceutical manufacturing with emphasis on antibiotic productions, 2023, for public consultation. WHO-MHP-HPS-EML- 2022.02-free. [Online].
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; Elpers, C. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environment international. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M. Antibiotics traces in the aquatic environment: persistence and adverse environmental impact. Current opinion in environmental science & health. 2020, 13, 68–74. [Google Scholar]
- Massard, R.; Cabuil, V. Synthèse en milieu alcalin de magnétite colloïdale : contrôle du rendement et de la taille des particules. J. Chim. Phys. 1987, 84, 967–973. [Google Scholar] [CrossRef]
- Ferroudj, N.; Nzimoto, J.; Davidson, A.; Talbot, D.; Briot, E.; Dupuis, V.; Bée, A.; Medjram, M.S.; Abramson, S. Maghemite nanoparticles and maghemite/silica nanocomposite microspheres as magnetic Fenton catalysts for the removal of water pollutants. Applied Catalysis B: Environmental. 2013, 136, 9–18. [Google Scholar] [CrossRef]
- Lin, Y.; Qiao, J.; Sun, Y.; Dong, H. The profound review of Fenton process: What's the next step? Journal of Environmental Sciences. 2025, 147, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Suetens, T.; Guo, M.; Van Acker, K.; Blanpain, B. Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. Journal of hazardous materials. 2015, 287, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kmita, A.; Pribulova, A.; Holtzer, M.; Futas, P.; Roczniak, A. Use of specific properties of zinc ferrite in innovative technologies 2016. Archives of Metallurgy and Materials. 2016, 61. [Google Scholar]
- Zhu, J.; Zhu, Y.; Chen, Z.; Wu, S.; Fang, X.; Yao, Y. Progress in the preparation and modification of zinc ferrites used for the photocatalytic degradation of organic pollutants. International Journal of Environmental Research and Public Health. 2022, 19, 10710. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Urbánek, P.; Machovsky, M.; Masař, M.; Holek, M. Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4 spinel ferrite nanoparticles synthesized via honey-mediated sol-gel combustion method. Journal of Physics and Chemistry of Solids. 2017, 110, 87–99. [Google Scholar] [CrossRef]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M. Optical, magnetic and structural properties of ZnFe2O4 nanoparticles synthesized by conventional and microwave assisted combustion method: a comparative investigation. Optik. 2017, 129, 57–68. [Google Scholar] [CrossRef]
- Kumar, G.Y.; Naik, H.B.; Roy, A.S.; Harish, K.N.; Viswanath, R. Synthesis, optical and electrical properties of ZnFe2O4 nanocomposites. Nanomaterials and Nanotechnology. 2012, 2, 19. [Google Scholar] [CrossRef]
- Asif, A.H.; Wang, S.; Sun, H. Hematite-based nanomaterials for photocatalytic degradation of pharmaceuticals and personal care products (PPCPs): A short review. Current Opinion in Green and Sustainable Chemistry. 2021, 28, 100447. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Walter, E.; Zong, M.; Wang, Y.; Zhang, X.; Qafoku, O.; Wang, Z.; Rosso, K.M. Facet-specific photocatalytic degradation of organics by heterogeneous fenton chemistry on hematite nanoparticles. Environmental Science & Technology. 2019, 53, 10197–10207. [Google Scholar]
- El Hassan, N.; Kaydouh, M.N.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H. Massiani Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Applied Catalysis A: General. 2016, 520, 114–121. [Google Scholar] [CrossRef]
- Tabaja, N.; Brouri, D.; Casale, S.; Zein, S.; Jaafar, M.; Selmane, M.; Toufaily, J.; Davidson, A.; Hamieh, T. Use of SBA-15 silica grains for engineering mixtures of oxides CoFe and NiFe for Advanced Oxidation Reactions under visible and NIR. Applied Catalysis B: Environmental. 2019, 253, 369–378. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Foundations of Crystallography. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K. Adsorption by powders and porous solids: principles, methodology and applications. Academic press. 2013.
- Trovo, A.G.; Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process–chemical and toxicological assessment. Water research. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Frański, R.; Czerniel, J.; Kowalska, M.; Frańska, M. Electrospray ionization collision-induced dissociation tandem mass spectrometry of amoxicillin and ampicillin and their degradation products. Rapid Communications in Mass Spectrometry. 2014, 28, 713–722. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).