1. Introduction
Extra-corporeal membrane oxygenation (ECMO) is a form of mechanical cardiopulmonary support used in critically ill patients [
1]. Components of the ECMO circuit include a venous cannula, a pump, an oxygenator, and an arterial cannula. Venous blood is pumped through an extracorporeal membrane simulating a lung membrane and is returned into the venous or arterial circulation.
Patients undergoing ECMO often present with or are at high risk for severe infections. Optimal dosing of antibiotics is crucial for successful outcomes in patients on ECMO. Though ECMO provides critical support, it is documented to impact pharmacokinetics (PK) of many types of medications, including antibiotics, sedatives, and analgesics. The ECMO circuit acts as an additional PK compartment with the potential to affect the therapeutic levels of the antibiotics as they travel through the circuit and back to the patient. The mechanisms by which ECMO changes drug pharmacokinetics include increasing volume of distribution, sequestering medications, or altering drug clearance. ECMO is also thought to impact drug PK through direct interaction between the circuit and drug, along with the physiological changes occurring in these patients due to their critical illness [
1,
2,
3,
4].
Beta-lactams are commonly utilized in patients on ECMO. They are hydrophilic and primarily undergo renal elimination. Though ECMO more commonly affects PK of lipophilic, highly protein bound drugs (i.e., ceftriaxone, voriconazole), there is data indicating alterations in PK of beta-lactams [
5]. Current data regarding the therapeutic dosing of beta-lactams in adult patients on ECMO is limited, however; ECMO is thought to have minimal impact the serum concentrations of cefepime, due to it’s relative hydrophilicity [
3,
5]. The findings between different studies are conflicting. One study found inadequate beta-lactam levels in 12% of patients on ECMO, suggesting the potential for increased dosing in early therapy [
6]. Another study did not find a difference in beta-lactam levels in ECMO compared to non-ECMO patients [
7]. Volume of distribution may be increased because of systemic inflammatory response (SIRS) often present in critically ill patients with severe infection [
8]. According to current literature, it is unclear if standard dosing of beta-lactams, and particularly cefepime, in patients requiring ECMO is sufficient to achieve therapeutic levels capable of treating susceptible organisms [
1,
2,
3,
4,
5]. Cefepime is a broad spectrum fourth generation cephalosporin. (
Figure 1).
Its spectrum of activity includes the Enterobacteriaceae family,
Pseudomonas aeruginosa, and has gram-positive activity like first and second generation cephalosporins. It has demonstrated a high resistance to pathogen-mediated enzymatic hydrolysis [
10]. Clinical uses include soft tissue and skin infections, lower respiratory tract infections, intra-abdominal and genitourinary infections. Cefepime is widely used in critically ill patients on ECMO. Its bactericidal activity depends on the amount of time the serum concentration is above the minimum inhibitory concentration of the drug, which in turn is based on dosing intervals. Cefepime can be administered as an intermittent infusion over 30 minutes for all gram-negative infections except for
Pseudomonas, for which an extended infusion over 3 to 4 hours or continuous infusion over 24 hours is preferred [
11,
12]. Cefepime serum protein binding values range from approximately 16%-19% [
13]. Its primary route of elimination is through the kidneys. At high doses, cefepime can cause neurotoxicity. Therefore, therapeutic drug monitoring is crucial and correct dosing strategies in ECMO are necessary to establish treatment efficacy and avoid toxicity [
13,
14].
The aim of this prospective study was to identify differences in pharmacokinetic (PK) parameters of beta-lactam antibiotics in adult patients receiving ECMO by comparing PK parameters and serum concentrations of cefepime prior to ECMO circuit and during the ECMO circuit at steady-state. Assessment of these serum concentrations and PK parameters along with subsequent correlation of the levels to the time spent above minimal inhibitory concentration (MIC) will provide guidance on the impact of standard beta-lactam dosing in patients receiving ECMO. It will raise the question of whether dosage adjustments are needed in these patients to prevent either therapeutic failure or drug toxicity.
2. Results
A total of 8 patients were identified. Six patients received cefepime; two patients received other beta-lactam antibiotics (cefazolin, piperacillin-tazobactam, respectively) and were excluded from the results.
A description of patient demographics and clinical characteristics while undergoing ECMO is presented (
Table 1). All patients were male. Average ±SD age was 45.8±14.7. Four patients received ECMO for severe SARS-CoV-2 infection, one for
Pneumocystis jirovecii pneumonia infection, and one for severe influenza A infection. Two patients received continuous renal replacement therapy (CRRT) during ECMO but after cefepime serum levels were obtained. Mean ±SD APACHE II and SOFA scores prior to ECMO were 24.6±7.1 and 11.0±3.9, respectively (
Table 1). Admission serum creatinine averaged 1.2 ± 0.5mg/dl and albumin averaged 2.2 ± 0.6G/dl. ECMO sweep gas flow rate averaged 4.3±0.9 and blood flow rate averaged 4.6±0.7. All but one patient received venovenous (VV) ECMO.
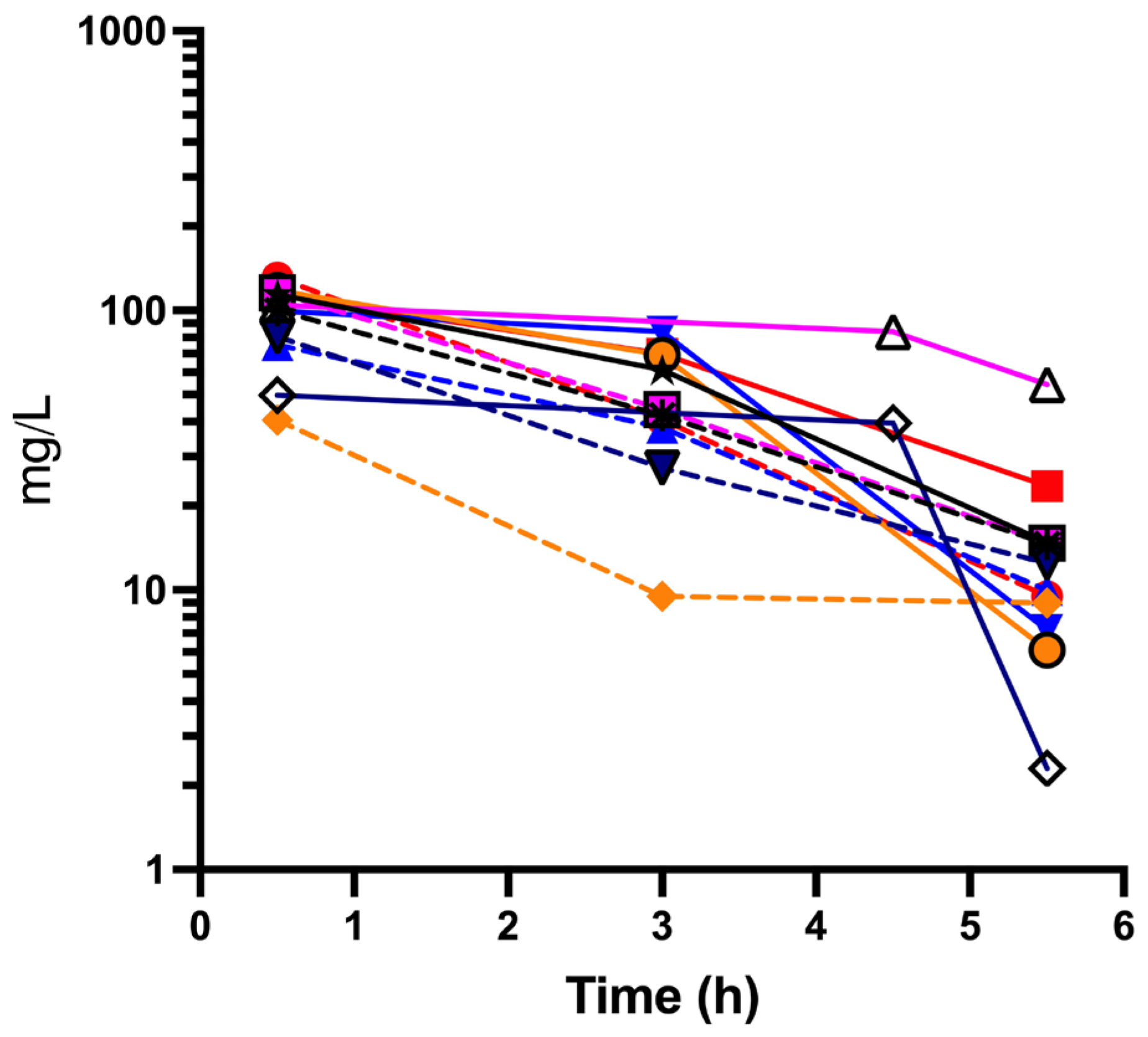
One patient was unable to capture peak cefepime concentration pre-ECMO due to severity of illness and urgency for ECMO circuit. In
Figure 2 an analysis of the serum concentration of cefepime versus time is presented.
Mean (± SD) peak concentration was 82.2 ± 45.7 mcg/mL (pre-ECMO) and 87.6 ± 33.6 mg/L (intra-ECMO) (
Figure 2). Average (± SD) trough cefepime concentration was 9.5 ± 0.5 mg/L pre-ECMO and 26.0 ± 27.3 mg/L intra-ECMO (
Table 2).
AUC0-6 averaged ± SE 211.9 ± 72.5 pre-ECMO and 329.6± 79.0 mg*h/L intra-ECMO, p=0.023. The elimination half-life was 3.85 ± 0.48 h pre-ECMO and 5.84 ± 4.61 h intra-ECMO p>0.05. Cefepime volume of distribution and total clearance averaged 5.1 ± 1.6 pre-ECMO and 4.2 ± 2.4 L intra-ECMO and 5.56 ± 3.09 pre-ECMO and 3.12 ± 0.94 L/hr intra-ECMO, respectively. One patient (intra-ECMO and CRRT) had a trough cefepime concentration > 50 mg/L. Patients received ECMO for 43.1±30 days. All patients expired during hospitalization. Neurotoxicity secondary to cefepime was unable to be assessed due to patients’ clinical condition at the time of investigation. No other cefepime side effects were noted. Only one patient had a pathogen isolated (Citrobacter koseri) from respiratory culture; the remaining patients’ cultures were negative.
3. Discussion
The initiation of ECMO introduces several variables which factor into achieving optimal dosing of medications. These include ECMO circuitry, increased volume of distribution, and further variables that affect the pharmacokinetic parameters of antimicrobials.
Previously, three primary hypotheses have been described in which ECMO affects pharmacokinetics: direct sequestration by the circuit, increased volume of distribution, and altered clearance [
7]. The ECMO circuitry can vary according to the type of ECMO membrane, tubing, and pump materials. The material used in tubing (polymers, silicone, etc) can have variable absorption of the medication being administered as well. Studies have demonstrated increased volume of distribution and decreased drug clearance in ECMO [
8]. This increased volume of distribution is attributed to system related factors leading to increased sequestration of the medication being used. Hollow-fiber membranes have demonstrated reduced sequestration. This is one example of how circuit material effects extraction [
15]. Drug lipophilicity and protein binding have been demonstrated to affect sequestration of the drug in ECMO circuitry. Altered clearance in ECMO is a variable to consider in antibiotic dosing. Factors such as coexisting or secondary kidney failure, variable organ perfusion and tissue oxygenation are also contributors to altered clearance in ECMO. Extracorporeal life support organization guidelines report an incidence of acute kidney injury while on ECMO anywhere from 30% to 85% [
14]. ECMO can also potentially affect clearance of hepatically-metabolized drugs, secondary to decreased flow to the liver [
7,
8,
14].
Pharmacokinetic variables are affected by additional patient variables such as total body weight and creatinine clearance. While we could not identify any side effects or toxicity from cefepime in our study patients, decreased drug clearance can lead to accumulation and can be a basis for a modified dosing regimen in patients with ECMO [
16]. The objective of antimicrobial therapy use is to obtain drug levels above minimal inhibitory concentration at the site of infection. In our study, levels above MIC were difficult to confirm as only one patient had an organism identified on culture, and all patients eventually expired.
A limitation of this study is the small sample size that was able to be enrolled into this study. The number of patients that would qualify for this study became difficult to enroll. While each patient served as their own control, more study on cefepime PK in ECMO patients may be required to make a solid conclusion.
The results obtained in this study allow for the analysis of the serum concentration time curve, which reflects the amount of antimicrobial, in this case cefepime, that has effectively reached the systemic circulation. Having this result allows for determination of area under curve (AUC), a parameter that can be used to evaluate the volume of distribution, total clearance, and bioavailability [
17]. The results demonstrate the variability in pharmacokinetics of cefepime in ECMO. The area under the curve (AUC) reflects the efficacy of standard dosing of cefepime in ECMO. In
Figure 2, an analysis of the serum concentration of cefepime vs time is presented. A significant increase in AUC intra-ECMO as compared to pre-ECMO was seen which could reflect accumulation with multiple doses or changes in cefepime PK from the ECMO circuit. Cefepime volume of distribution (Vd), the drug relative hydrophilicity and low protein binding resulted in less circuit sequestration leading to only a modest increase in the Vd. Cefepime clearance was not significantly changed intra-ECMO compared to pre-ECMO.
4. Materials and Methods
Study Population
This case study was done on patients hospitalized in a one major hospital of a U.S. Midwest healthcare system composed of 12 hospitals located in 2 states. Institutional IRB (Institutional Research Board for Human Studies) granted approval and determined the study as human subject research. After IRB approval, we identified the patients based on the inclusion and exclusion criteria. The first patient was recruited on September 30, 2021 and the last patient was recruited on August 28, 2023. A signed informed consent form was obtained from patients or their representative. The inclusion criteria for enrollment were age > 19 years receiving ECMO and receiving a beta-lactam antibiotic. Exclusion criteria included age < 19 years, pregnancy, identifying as a Jehovah’s Witness, hospice or comfort care, and receiving a beta-lactam antibiotic for more than 72 hours.
The Getinge Cardiohelp® ECMO (Maquet Getinge Cardiopulmonary AG, Rastatt, Germany) device was used for all patients according to the manufacturer recommendations. The ECMO tubing used was sterile heparin-bonded polyvinyl chloride (PVC). Cefepime was administered to patients as a 1-gram dose over 2 minutes every 6 hours as part of hospital policy.
Data Collection and Analysis
Data collected from the electronic medical record included age, gender, number of days on ECMO, serum creatinine on admission, admission SOFA score and APACHE II score, serum albumin, ECMO parameters and mortality. Blood (4 mL) was collected during two periods; before the placement of ECMO (pre-ECMO) and after placement of ECMO circuit (intra-ECMO). Samples were collected using red-top vacutainer (B-D, Franklin Lakes, NJ) tubes. Tubes were allowed to clot at room temperature for 10-15 minutes, centrifuged at 3000 x G and stored at –80°C until LC-MS/MS analysis. Cefepime serum concentrations (peak 0.5 h, midpoint 3-4 h, and trough 5.5 h after dosing) were obtained after patients received at least 24 hours of continuous cefepime dosing. Cefepime concentrations were determined at Creighton University using ABI QTRAP
® 5500 LC-MS/MS system coupled with a Exion liquid chromatography station, including LC controller, degasser, 2 liquid pumps, column oven, and LC autosampler, and peak gas generator running MultiQuant software. Cefepime assay methodology has been reported [
11]. The lower limit of detection for the assay was 0.01 µg/mL. Mean (± SD) or percentages are reported. Cefepime concentrations were fitted to a one-compartment model (
Table 2). Prism GraphPad was used for fitting using least-squares regression and weighting by 1/Y
2. AUC
0-6 was calculated in GraphPad and compared between pre-ECMO and intra-ECMO. Pharmacokinetic parameters including area under the serum-concentration time curve were derived from one-compartment model analysis. Intra-day and inter-day variability of cefepime assay was < 10%.
5. Conclusions
The current conventional dosing of cefepime at 1 gram every 6 hours is shown to achieve adequate serum levels in critically ill patients on ECMO, suggesting dose adjustments are not required. Variables affecting pharmacokinetics in ECMO should be considered on a case-by-case basis.
Author Contributions
Conceptualization, CJD and MV; methodology, CJD; validation, EJN; formal analysis, CD and EJN.; writing—original draft preparation, CD, DK, and RI; writing—review and editing, RP, DK, MV, CD ; LC-MS/MS determination MRS; supervision, EJN; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Creighton University (protocol #2002134 and approved on 8/1/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data availability is available to those who request it from the corresponding author.
Acknowledgments
The authors thank the ICU nurses that assisted in identifying potential patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sherwin, J.; Heath, T.; Watt, K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: A review of the current literature. Clin. Ther. 2016, 38, 1976–1994. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741–e9. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.A.; Sieg, A.C. Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacotherapy 2017, 37, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Tukacs, M. Pharmacokinetics and extracorporeal membrane oxygenation in adults: A literature review. AACN Adv. Crit. Care 2018, 29, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10 (Suppl. 5), S629–S641. [Google Scholar] [CrossRef] [PubMed]
- Bakdach, D.; Elajez, R.; Bakdach, A.R.; Awaisu, A.; De Pascale, G.; Ait Hssain, A. Pharmacokinetics, Pharmacodynamics, and Dosing Considerations of Novel β-Lactams and β-Lactam/β-Lactamase Inhibitors in Critically Ill Adult Patients: Focus on Obesity, Augmented Renal Clearance, Renal Replacement Therapies, and Extracorporeal Membrane Oxygenation. JCM 2022, 11, 6898. [Google Scholar] [PubMed]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; De Backer, D.; Vincent, J.L.; et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Alshaer, M.H.; Peloquin, C.; Venugopalan, V.; Alnuaimat, H.M.; Converse, M. Cefepime pharmacokinetics in adult extracorporeal membrane oxygenation patients. Pulm. Pharmacol. Ther. 2024, 84, 102271. [Google Scholar] [CrossRef] [PubMed]
- https://pubchem.ncbi.nlm.nih.gov/compound/Cefepime#section=2D-Structure.
- Okamoto, M.P.; Nakahiro, R.K.; Chin, A.; Bedikian, A. Cefepime clinical pharmacokinetics. Clin. Pharmacokinet. 1993, 25, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Corley, A.; Diehl, A.; Jakob, S.M.; Levkovich, B.J.; Pellegrino, V.; Que, Y.A.; et al. Population pharmacokinetics of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Int. J. Antimicrob. Agents 2021, 58, 106466. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: a systematic review. Crit. Care 2017, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.; Heath, T.; Watt, K. Pharmacokinetics and Dosing of Anti-infective Drugs in Patients on Extracorporeal Membrane Oxygenation: A Review of the Current Literature. Clin. Ther. 2016, 38, 1976–1994. [Google Scholar] [CrossRef] [PubMed]
- Bridges, B.C.; Dhar, A.; Ramanathan, K.; Steflik, H.J.; Schmidt, M.; Shekar, K. Extracorporeal Life Support Organization Guidelines for Fluid Overload, Acute Kidney Injury, and Electrolyte Management. ASAIO J. 2022, 68, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wildschut, E.D.; Ahsman, M.J.; Allegaert, K.; Mathot, R.A.; Tibboel, D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010, 36, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.F.; Bulitta, J.; Lipman, J.; Kirkpatrick, C.M.J. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J. Antimicrob. Chemother. 2006, 58, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.P.; Sarver, J.G. Pharmacokinetic Modeling. In Pharmacology; Elsevier, 2009; pp. 201–277. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).