Submitted:
04 September 2024
Posted:
05 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
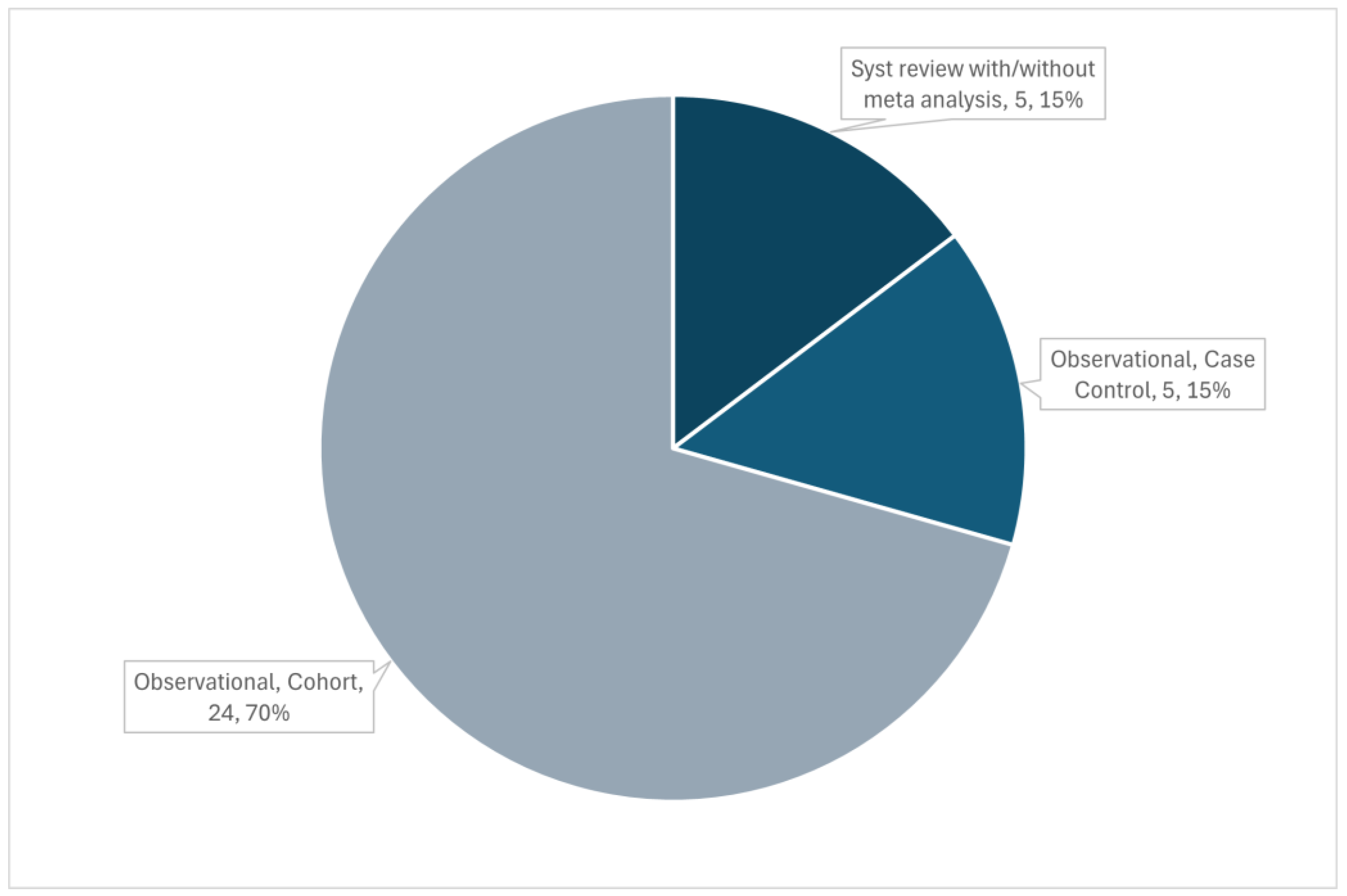
Search Strategy
Eligibility Criteria
Types of Participants
Concept
Context
Type of Sources
Source of Evidence Screening and Selection
Data Extraction Process
Results
Perfusion Abnormalities in Sepsis
Cerebral Blood Flow, Pulsatility Index, Resistance Index, Cerebrovascular Resistances and Other Intracranial Haemodynamics Indexes Alterations
| Study | Main findings | Metric used | Sample size (septic patients) |
|---|---|---|---|
| Straver 1996 [22] | Inverse relationship between systemic vascular resistance index and mean and diastolic MCAv. MCA/ICA index and MAP showed an inverse relationship (changes in MCAv more pronounced than changes in ICA). MCA and ICA flow velocities abnormalities are more pronounced in severe disease and in non-survivors. | MCAv; PI; MCA/ICA index | 20 |
| Thees 2007 [29] | CO2R seemed not to be impaired. They didn't observe abnormal findings explaining neurological abnormalities. CCP increased as expected during hyperventilation (25±11 to 39±15mmHg). | CO2R, CCP; CBF calculated with thermodiluition and indocyanine green dye; CMRO2 | 10 |
| Pfister 2008 [30] | 12/16 patients presented SAD. No differences in CBF between SAD and non-SAD groups. | MCAv, Mx | 16 |
| Szatmàri 2010 [17] | PI was higher in the group with sepsis. Vasomotor response was slower and lower in sepsis (less CRC and lower systolic MCAv). | PI, acetazolamide test, cerebrovascular reactivity, CRC | 14 |
| Fülesdi 2012 [18] | PI was higher in septic patients. CRC was similar in the two groups while cerebrovascular reactivity decreased slower in the septic group (more prolonged vasodilatory response). | Acetazolamide test, cerebrovascular reactivity, CRC | 16 |
| Pierrakos 2013 [16] | TCD has a feasibility of 91% vs. 85%, p = 0.89 (septic vs controls) due to acoustic bone window. PI and RI were higher in patients with sepsis than controls and higher in the first day. Cerebral vascular constriction is detectable by TCD in the early stage of sepsis. | MCAv, PI, RI, eCBF | 20 |
| Pierrakos 2014 [25] | PI on the first day was a good predictor of the presence of confusion (AUC = 0.908, 95%, CI 0.80-0.98, p < 0.01). For a cut-off value of 1.3, there was a 95% sensitivity and an 88% specificity. | PI | 40 |
| Toksvang 2014 [31] | The increase in MAP with noradrenaline generated a mean increase in MCAv of 14% (2-22%). There was poor agreement between TCD and NIRS for CBF estimation. | MCAv | 8 |
| Berg and Plovsing 2016 [21] | Hyperventilation was associated with a 36% increase in CVR, and a consequent 22% reduction in MCAv. CO2R is preserved in septic patients. | CVR, CO2R | 16 (only 7 underwent hyperventilation) |
| Pierrakos 2017 [26] | PI was higher in patients with CD (2.2 ± 0.7 vs. 1.4 ± 0.5, p = 0.02) and CBFi was lower (363±170 vs. 499±133, p = 0.03). In univariate analysis, delirium and PI on the first day of the study were related to CD but in the multivariate analysis PI was not found to be related to CD independently of the presence of delirium. | PI, CBFi | 28 |
| Le Dorze 2018 [19] | Baseline CO and HR were higher, and MAP lower in the sepsis group when compared to a brain injury and an anesthetised group of patients (controls). PSV, was higher in the sepsis group than in the control group but not with BI group. After a fluid challenge PSV and EDV increased significantly only in the sepsis group. No significant correlations between systemic and cerebral hemodynamic changes were observed in any group. | PSV, EDV | 38 |
| Feng 2021 [23] | The SAD group exhibited lower levels of EDV and a higher PI but all within normal range (0.98±0.19 vs. 0.84±0.20, p=0.019). | MCAv, CBFi, PI, THRR | 51 |
| Zheng 2023 [20] | Patients with SAE showed significantly elevated PSV (107 [69–138] cm/s vs 85 [69–101] cm/s, P=.002) and mean MCAv (57 [37–93] vs 54 [42–66], P=.045) even if only in the left MCA and with mean MCAv within the normal range. The PI and RI were significantly higher in the SAE group than in the non-SAE group (even if the values were within the normal range). Patients with agitation had higher MCAv and lower PI and RI than patients with decreased consciousness, suggesting lower CVR. | MCAv, PSV, EDV, PI, RI, FV, CBF volume | 198 |
| Mei 2024 [24] | The SAE group displayed significantly elevated levels of PI, RI, and CCT, while EDV was lower. CCT emerged as the most efficacious predictor for SAE, with an AUC of 0.846. S100β, PI, and CCT were identified as the independent predictors for SAE. | PI, RI, CCT | 67 |
Autoregulation Estimation and Other Forms of Vessels’ Reactivity
| Study | Main findings | Metric used | Sample size (septic patients) |
|---|---|---|---|
| Matta and Stow 1996 [35] | Mean IOR was 0.92 (intact autoregulation). | IOR | 10 |
| Pfister 2008 [30] | CAR was altered in the SAD patients, with no differences on perfusion in respect to the non-SAD group. | Mx | 16 |
| Steiner 2009 [37] | Correlation between Mx and another index of autoregulation from near infrared spectroscopy showed a strong positive association (R = 0.81; P < 0.0001). PaCO2-induced dilatation of flow-regulating vessels was associated with worse autoregulation. | Mx | 23 |
| Taccone 2010 [36] | CAR was impaired in 66% of patients, and impairment increased for higher PaCO2 values. | CAI | 21 |
| Schramm 2012 [38] | CAR was impaired in 88% of the patients, with a decreasing prevalence during the days (day1 - 60%, day2 - 59%, day3 - 41%, day4 - 46%). The status of CAR at day 1 was related to SAD development at day 4. SAD was associated with age. | Mx | 30 |
| Crippa 2018 [32] | 50% of patients presented impaired CAR. There was no difference in Mxa between survivors and non-survivors (at ICU discharge). Mxa was higher in patients with SAE. The best Mxa cut-off to predict SAE was 0.18 (sensitivity 79%, specificity 47%). | Mxa | 100 |
| Feng 2021 [23] | The SAD group had a significantly higher level of cerebrovascular dysfunction (THRR index < 1.09, 40 vs. 10%, p=0.01). THRR index<1.09 was a SAD predictor (OR=5.77, 95% CI: 1.222–27.255, p=0.027). | THRR | 51 |
| Crippa 2022 [33] | 53% patients had impaired CA. | THRT | 40 |
| Caldas 2022 [34] | Median ARI and Mxa values were 4.38 [2.83–6.04] and 0.32 [0.14–0.59], respectively. Impaired CAR according to the ARI threshold was observed in 42% of patients; impaired CAR according to Mxa threshold was observed in 53% patients. Mx and ARI had a weak correlation and a poor agreement to classify CAR. |
ARI, Mx | 95 |
Resuscitation
Non-Invasive Cerebral Perfusion Pressure and Estimation of Intracranial Pressure
Evaluation of the Neurologic Outcome
Discussion
| TCD/TCCS metrics | Acronym | Index explanation | Reference for calculation | Article |
|---|---|---|---|---|
| Acceleration | acc | Acceleration is defined as the maximal increase in FV per second during the systolic upstroke and was obtained by taking the maximum of the first order derivative of the ensemble average during the period lasting from systolic onset until first local maximum. | Schaafsma A. Improved parameterization of the transcranial Doppler signal. Ultrasound Med Biol 2012;38:1451–1459. |
De Goede 2017 |
| Autoregulation index | ARI | The signals were filtered, interpolated and resampled at 5Hz. Then the Welch method was used for smoothing spectral estimates derived from the fast Fourier transform (FFT) over segments of 102.4 seconds with 50% overlap. ARI values were obtained by fitting a second-order polynomial to minimize the error, using neighboring integer ARI values as a reference. ARI ranges from 0 (absent dynamic cerebral autoregulation, dCA) to 9 (most efficient dCA). | Caldas et al, Dynamic autoregulation is impaired in circulatory shock. Shock Augusta Ga. (2020) 54:183–9. Czosnyka et al, Monitoring of cerebral autoregulation. Neurocrit Care. (2014) 21(Suppl. 2):S95–102. Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res Off J Clin Auton Res Soc. (2009) 19:197–211. |
Caldas 2022 |
| Cerebral autoregulation index | CAI | Ratio of the relative changes in cerebrovascular resistances (CVR) and MAP Normal value: 0-2. |
Bouma GJ, Muizelaar JP. Cerebral blood flow, cerebral blood volume, and cerebrovascular reactivity after severe head injury. J Neurotrauma. 1992 Mar;9 Suppl 1:S333-48. | Taccone 2010 |
| Cerebral capillary closing pressure | CCP | Zero-flow velocity pressure as extrapolated by regression analysis of arterial pressure/MCAV plots, averaged over two respiratory cycles. | Thees et al, Anesthesiology. 2002 Mar;96(3):595-9 | Thees 2007 |
| Cerebral circulation time (assessed via contrast enhanced ultrasound) | CCT | Similarly to TCD, CCT measures the interval between the entry of arterial blood in the internal carotid artery (ICA) and its exit through the internal jugular vein (IJV). Utilizing a C5-1 convex array transducer, both the ICA and IJV were visualized in a transverse cross-sectional plane, specifically at a location 1.5 cm superior to the bifurcation of the common carotid artery. settings were switched to “contrast mode” with reduced mechanical and thermal indices. An FDA-approved microbubble contrast agent (SonoVue, Bracco, Milan, Italy) was prepared in 5mL of isotonic saline and rapidly administered via the median cubital vein, followed by a 5mL saline flush. Bolus administration and subsequent CCT assessments were performed on the side demonstrating higher blood flow velocity in earlier TCD measurements. Analysis of the imaging data was executed through uninterrupted video capture, with time-intensity curves being isolated post-recording by a seasoned ultrasonographer. The inbuilt software automatically processed these curves after targeting the ICA and IJV. | Liu X, et al. A new method of measurement of cerebral circulation time: contrast-enhanced ultrasonography in healthy adults and patients with intracranial shunts. Ultrasound Med Biol. (2014) 40:2372–8. | Mei 2024 |
| Cerebral metabolic rate of oxygen | CMRO2 |
With PvO2 as the pressure of oxygen in the jugular vein. |
- | Thees 2007 |
| Cerebrovascular reserve capacity | CRC | The maximal % increase of the blood flow velocity after acetazolamide administration. |
- | Szatmári 2010, Fülesdi 2012 |
| CO2 reactivity | CRCO2 | Difference between the MCAv at hypocapnia and hypercapnia expressed as a percentage of the baseline MCAv per kPa change in ETCO2. | - | Bowie 2003 |
| Absolute CO2R: change in MCAv per kPa change in PaCO2 Relative CO2R: percentage change in MCAv at PaCO2 5.3 kPa per kPa change in PaCO2 |
Matta and Stow 1996 | |||
| Percentage change in MCAv per kPa change in PaCO2 | Thees 2007 | |||
| CO2 reactivity, normalised | NCR | % change in CBF velocity per 1% increase in EtCO2 | Terborg 2001 | |
| Cerebrovascular resistances | CVR | CVR = MAP/MCAv | - | Taccone 2010, Berg 2016 |
| Cerebrovascular reactivity | CVR | CVR = (MCAacz - MCAv rest)/MCAv rest ; MCAvacz is the MCA mean blood flow velocity measured at 5, 10, 15 and 20 minutes after acetazolamide, and MCAvrest is the MCA mean blood flow velocity measured at rest. | - | Szatmári 2010, Fülesdi 2012 |
| Diastolic FV | Dias@560 | Dias@560 was obtained by calculating the mean blood FV during the interval 520–600 ms after stroke onset. Finally, the acc, sys1 and sys2 values were divided by the dias@560 value for normalization. | Schaafsma A. Improved parameterization of the transcranial Doppler signal. Ultrasound Med Biol 2012;38:1451–1459. |
De Goede 2017 |
| Estimated CBF (CBF index) | CBFi or CBF | - | Pierrakos 2013 | |
| Pierrakos 2013 | Pierrakos 2014 | |||
| Pierrakos 2013 | Pierrakos 2016 | |||
| - | Feng 2019 | |||
| A 25 mg dose of indocyanine green dye, dissolved in 40 ml of iced 5% glucose solution, was used as a double-indicator and injected into the right atrium through a central venous line. Dilution curves for both the dye and temperature were recorded simultaneously using thermistor-tipped fiber-optic catheters placed in the aorta (via a 30 cm catheter inserted into the femoral artery) and the jugular bulb. All measurements were taken from the sonographically controlled dominant (right) internal jugular vein. CBF was calculated based on the mean transit time of the first pass of the thermal and dye indicators using a specialized computer system. | Wietasch GJK, et al. Bedside assessment of cerebral blood flow by double-indicator dilution technique. Anesthesiology 2000, 92:367-375.13. Mielck F, et al. Reliability of cerebral blood flow measurements by transcerebral double-indicator dilution technique. Eur J Anaesth 2001, 18:653-661. |
Thees 2007 | ||
| Estimated CPP | eCPP | Czosnyka et al, Cerebral perfusion pressure in head-injured patients: A noninvasive assessment using transcranial Doppler ultrasonography. J. Neurosurg. 1998, 88, 802–808. | Crippa 2022, Crippa 2024 | |
| Schmidt et al, Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003 Jan;34(1):84-9. | Pfister 2008 | |||
| Intravascular flow volume | FV | For a defined vessel, FV was defined as the product of time-averaged flow velocity (TAV) and its cross-sectional area (A) according to the formula: D= diameter. The CBF volume was determined as the sum of the FVs of the internal carotid artery and vertebral artery of both sides. |
Scheel et al, Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 2000; 31:147–150 | Zheng 2024 |
| Index of autoregulation | IOR | Ratio of percentage change in estimated cerebral vascular resistance (CVRe) to percentage change in MAP, using the equations CVRe = MAP/MCAv and IOR =%∂CVRe/ %∂ MAP, where MAP at the time of MCAv measurement | Matta BF, Lam AM, Strebel S, Mayberg TS. Cerebral pressure autoregulation and CO2-reactivity during propofolinduced EEG suppression. British Journal of Anaesthesia 1995; 74: 159–163. | Matta and Stow 1996 |
| Mean flow index | Mx or Mxa | General definition: the Mx or Mxa index is calculated as a moving correlation coefficient between short-term fluctuations in two signals over a specific time window (e.g., 5-10 seconds). Mx usually refers to a calculated index between CPP and MCAv, conversely Mxa refers to ABP and MCAv. In septic patients, thus, Mxa is used, even if in the papers is commonly referred as Mx or Mxa alternatively. A positive correlation suggests that increases in blood pressure lead to increases in MCAv, indicating impaired autoregulation (Mxa>0.3). In contrast, a near-zero or negative correlation indicates effective autoregulation, where CBF remains stable despite changes in MAP. |
||
| In this article: values of MAP and FV averaged every 10”. Mx is calculated every 60“ as the moving linear correlation coefficient between the last 30 consecutive values of MAP and FV (5 minutes). | Piechnik SK, et al. The continuous assessment of cerebrovascular reactivity: a validation of the method in healthy volunteers. Anesth Analg 1999, 89:944-949. |
Pfister 2008 (2) | ||
| In this article: values of MAP and FV averaged every 6”. Mx is calculated every 60” as the moving linear correlation coefficient between the last 30 consecutive values of MAP and FV (3 minutes). | Czosnyka et al, Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–34. Piechnik SK et al, The continuous assessment of cerebrovascular reactivity: a validation of the method in healthy volunteers. Anesth Analg. 1999;89:944–9. | Schramm 2012 | ||
| In this article: values of MAP and FV averaged every 10”. Mx is calculated every 60“ as the moving linear correlation coefficient between the last 30 consecutive values of MAP and FV (5 minutes). | Czosnyka et al, Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–34. Piechnik SK et al, The continuous assessment of cerebrovascular reactivity: a validation of the method in healthy volunteers. Anesth Analg. 1999;89:944–9. | Steiner 2009, Caldas 2022, Crippa 2022 (2), Crippa 2024 | ||
| The Pearson’s correlation coefficient between the averaged ABP and flow velocity averaged on 10s-consecutive windows with 50% overlap. | Czosnyka et al, Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27(10):1829–34. | Crippa 2018 | ||
| Non-invasive ICP or estimated ICP | nICP or eICP (Crippa 2022, Crippa 2024) | Mathematical algorithm built up starting from various TCD waveform parameters and ABP, that aims to estimate with precision the nICP. | Schmidt et al, Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003 Jan;34(1):84-9. | Pfister 2008 |
| Czosnyka, M. et al. Cerebral perfusion pressure in head-injured patients: A noninvasive assessment using transcranial Doppler ultrasonography. J. Neurosurg. 1998, 88, 802–808. Rasulo FA, et al. The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: a multicenter prospective pilot study. Crit Care. 2017;21(1):44. |
Crippa 2022, Crippa 2024 | |||
| Resistance index | RI | RI = (PSV – EDV)/PSV | Berg 2015, Berg and Plovsing 2016, Caldas 2022, Zheng 2023, Mei 2024 | |
| Systolic component 1 and 2 | Sys1 and Sys2 | Sys1 and sys2 are the maximal flow velocities within the first and second systolic peaks and were obtained by taking the zero-line crossing of the first (if necessary second) order derivative of the ensemble average during the first 100ms and during the remaining part of systole, respectively. | Schaafsma A. Improved parameterization of the transcranial Doppler signal. Ultrasound Med Biol 2012;38:1451–1459. |
De Goede 2017 |
| Percentage of waveforms without the second systolic peak | %no_sys2 | Percentage of 10-s intervals in which no sys2 was detected. | Schaafsma A. Improved parameterization of the transcranial Doppler signal. Ultrasound Med Biol 2012;38:1451–1459. |
De Goede 2017 |
| Transient hyperemia response ratio or Transient hyperemia response test | THRR (Feng 2021) or THRT (Crippa 2022) | CBF is analysed before, during and after the ipsilateral compression of the carotid artery at the neck level. Flow must undergo a reduction of 30-50% from baseline to ensure a proper compression. Compression duration is between 3 and 9s. After the occlusion is released, blood flow rapidly increases (hyperemia) and velocity is usually higher than the baseline due to a vasodilation occurring during compression. Ratio between maximal post-release (5 heartbeats) and baseline PSV is measured. A THRR index above 1.09 (>10% increase) is regarded as indicating dynamic cerebral vascular autoregulation function; if the level falls below 1.09, this is regarded as indicating impairment of CAR. | Cavill et al Factors affecting assessment of cerebral autoregulation using the transient hyperaemic response test. Br J Anaesth. (1998) 81:317–21 |
Feng 2021 |
| Zeileret et al, Pressure Autoregulation Measurement Techniques in Adult Traumatic Brain Injury, Part I: A Scoping Review of Intermittent/Semi-Intermittent Methods. J. Neurotrauma 2017, 34, 3207–3223. | Crippa 2022 | |||
| Gain, phase, coherence | - | Gain phase and coherence are transfer function analysis metrics that compare two signals in their spectrum frequency (ABP and MCAv). They quantify the effectiveness of dynamic CAR as a filter that dampens MAP-induced changes in CBF. In particular, gain compares the amplitude of the signals hypothesising that high amplitude oscillations in ABP should be dampened in CBF. Phase refers to the displacement of the CBF signal relative to the MAP signal, which reflects the response time of dynamic CAR. Coherence quantifies the linearity between the spectral power of CBF and the spectral power of MAP, assuming that when signals are highly related changes in ABP are passively transmitted to CBF and CAR is impaired. | Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 1998;274:H233–41. Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol 1999;277:H1089–99. Meel-van den Abeelen AS, van Beek AH, Slump CH, Panerai RB, Claassen JA. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med Eng Phys 2014;36:563–75. |
Berg 2015, Berg and Plovsing 2016 |
| Optic nerve sheet diameter | ONSD | Wang, L.J.; et al. Non-invasive and quantitative intracranial pressure estimation using ultrasonographic measurement of optic nerve sheath diameter. Sci. Rep.2017, 7, 42063 | Czempik 2020 |
| Dynamic AR | Static AR | |
|---|---|---|
| Snapshot metrics, qualitative | THRT - Transient hyperemia response test | - |
| Prolonged monitoring required, quantitative | ARI – Autoregulation index Mxa – Mean flow index (assessed between ABP and MCAv) Transfer function analysis indexes (Phase, gain, coherence) |
CAI – Cerebral autoregulation index IOR – Index of autoregulation |
Research Gaps, Awaited Studies, and Future Directions
Conclusions
Funding sources/sponsors
Conflicts of interest
Abbreviations
References
- Sonneville R, Benghanem S, Jeantin L, de Montmollin E, Doman M, Gaudemer A, et al. The spectrum of sepsis-associated encephalopathy: a clinical perspective. Crit Care. 2023 Oct 5;27(1):386. [CrossRef]
- Czempik PF, Pluta MP, Krzych ŁJ. Sepsis-Associated Brain Dysfunction: A Review of Current Literature. Int J Environ Res Public Health. 2020 Aug;17(16):5852. [CrossRef]
- Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996 Feb 14;275(6):470–3. [PubMed]
- Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013 Jan;84(1):62–9. [CrossRef]
- Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990 Aug;18(8):801–6. [CrossRef]
- Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J Clin Med [Internet]. 2020 Mar;9(3). Available from: https://pubmed.ncbi.nlm.nih.gov/32150970/. [CrossRef]
- Calviello LA, Cardim D, Czosnyka M, Preller J, Smielewski P, Siyal A, et al. Feasibility of non-invasive neuromonitoring in general intensive care patients using a multi-parameter transcranial Doppler approach. J Clin Monit Comput. 2022 Dec;36(6):1805–15. [CrossRef]
- Klawitter F, Jager M, Klinkmann G, Saller T, Söhle M, von Möllendorff F, et al. Sepsis-assoziierte Enzephalopathie. Anaesthesist. 2021 Feb 1;70(2):112–20. [CrossRef]
- Battaglini D, Pelosi P, Robba C. The Importance of Neuromonitoring in Non Brain Injured Patients. Crit Care Lond Engl. 2022 Mar 22;26(1):78. [CrossRef]
- Robba C, Wong A, Poole D, Al Tayar A, Arntfield RT, Chew MS, et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med. 2021 Dec;47(12):1347–67. [CrossRef]
- Tavazzi G, Spiegel R, Rola P, Price S, Corradi F, Hockstein M. Multiorgan evaluation of perfusion and congestion using ultrasound in patients with shock. Eur Heart J Acute Cardiovasc Care. 2023 May 4;12(5):344–52. [CrossRef]
- Bertuetti R, Gritti P, Pelosi P, Robba C. How to use cerebral ultrasound in the ICU. Minerva Anestesiol [Internet]. 2020 Mar [cited 2024 Sep 3];86(3). Available from: https://pubmed.ncbi.nlm.nih.gov/31922373/. [CrossRef]
- Lau VI, Arntfield RT. Point-of-care transcranial Doppler by intensivists. Crit Ultrasound J. 2017 Oct 13;9(1):21. [CrossRef]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018 Oct 2;169(7):467–73. [CrossRef]
- Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, et al. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022 Apr 1;20(4):953–68. [CrossRef]
- Pierrakos C, Antoine A, Velissaris D, Michaux I, Bulpa P, Evrard P, et al. Transcranial doppler assessment of cerebral perfusion in critically ill septic patients: a pilot study. Ann Intensive Care. 2013;3:28. [CrossRef]
- Szatmári S, Végh T, Csomós A, Hallay J, Takács I, Molnár C, et al. Impaired cerebrovascular reactivity in sepsis-associated encephalopathy studied by acetazolamide test. Crit Care Lond Engl. 2010;14(2):R50. [CrossRef]
- Fülesdi B, Szatmári S, Antek C, Fülep Z, Sárkány P, Csiba L, et al. Cerebral vasoreactivity to acetazolamide is not impaired in patients with severe sepsis. J Crit Care. 2012 Aug;27(4):337–43. [CrossRef]
- Le Dorze M, Huché F, Coelembier C, Rabuel C, Payen D. Impact of fluid challenge increase in cardiac output on the relationship between systemic and cerebral hemodynamics in severe sepsis compared to brain injury and controls. Ann Intensive Care. 2018 Jun 28;8(1):74. [CrossRef]
- Zheng Y, Shen M, Xuan L, Pan S, Chen S, Zhong M, et al. Cerebral Blood Flow Alterations in Sepsis-Associated Encephalopathy: A Prospective Observational Study. J Ultrasound Med Off J Am Inst Ultrasound Med. 2023 Aug;42(8):1829–39. [CrossRef]
- Berg RMG, Plovsing RR. Effects of short-term mechanical hyperventilation on cerebral blood flow and dynamic cerebral autoregulation in critically ill patients with sepsis. Scand J Clin Lab Invest. 2016;76(3):226–33. [CrossRef]
- Straver JS, Keunen RW, Stam CJ, Tavy DL, De Ruiter GR, Smith SJ, et al. Transcranial Doppler and systemic hemodynamic studies in septic shock. Neurol Res. 1996 Aug;18(4):313–8. [CrossRef]
- Feng Q, Ai M, Huang L, Peng Q, Ai Y, Zhang L. Relationship Between Cerebral Hemodynamics, Tissue Oxygen Saturation, and Delirium in Patients With Septic Shock: A Pilot Observational Cohort Study. Front Med. 2021;8:641104. [CrossRef]
- Mei J, Zhang X, Sun X, Hu L, Song Y. Optimizing the prediction of sepsis-associated encephalopathy with cerebral circulation time utilizing a nomogram: a pilot study in the intensive care unit. Front Neurol. 2024 Jan 11;14:1303075. [CrossRef]
- Pierrakos C, Attou R, Decorte L, Kolyviras A, Malinverni S, Gottignies P, et al. Transcranial Doppler to assess sepsis-associated encephalopathy in critically ill patients. BMC Anesthesiol. 2014;14:45. [CrossRef]
- Pierrakos C, Attou R, Decorte L, Velissaris D, Cudia A, Gottignies P, et al. Cerebral perfusion alterations and cognitive decline in critically ill sepsis survivors. Acta Clin Belg. 2017 Feb;72(1):39–44. [CrossRef]
- de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012 Aug;17(1):58–66. [CrossRef]
- de Azevedo DS, Salinet ASM, de Lima Oliveira M, Teixeira MJ, Bor-Seng-Shu E, de Carvalho Nogueira R. Cerebral hemodynamics in sepsis assessed by transcranial Doppler: a systematic review and meta-analysis. J Clin Monit Comput. 2017 Dec;31(6):1123–32. [CrossRef]
- Thees C, Kaiser M, Scholz M, Semmler A, Heneka MT, Baumgarten G, et al. Cerebral haemodynamics and carbon dioxide reactivity during sepsis syndrome. Crit Care Lond Engl. 2007;11(6):R123. [CrossRef]
- Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care Lond Engl. 2008;12(3):R63. [CrossRef]
- Toksvang LN, Plovsing RR, Petersen MW, Møller K, Berg RMG. Poor agreement between transcranial Doppler and near-infrared spectroscopy-based estimates of cerebral blood flow changes in sepsis. Clin Physiol Funct Imaging. 2014 Sep;34(5):405–9. [CrossRef]
- Crippa IA, Subirà C, Vincent JL, Fernandez RF, Hernandez SC, Cavicchi FZ, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care Lond Engl. 2018 Dec 4;22(1):327. [CrossRef]
- Crippa IA, Alvaro Quispe Cornejo A, Taccone FS. Changes in Arterial Carbon Dioxide Partial Pressure Do Not Affect Cerebral Autoregulation in Septic Patients. Neurocrit Care. 2022 Oct;37(2):572–4. [CrossRef]
- Caldas J, Quispe-Cornejo AA, Crippa IA, Subira C, Creteur J, Panerai R, et al. Cerebral Autoregulation Indices Are Not Interchangeable in Patients With Sepsis. Front Neurol. 2022;13:760293. [CrossRef]
- Matta BF, Stow PJ. Sepsis-induced vasoparalysis does not involve the cerebral vasculature: indirect evidence from autoregulation and carbon dioxide reactivity studies. Br J Anaesth. 1996;76(6):790–4. [CrossRef]
- Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre’ J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010 Feb;12(1):35–42. [CrossRef]
- Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–8. [CrossRef]
- Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care Lond Engl. 2012 Oct 4;16(5):R181. [CrossRef]
- Terborg C, Schummer W, Albrecht M, Reinhart K, Weiller C, Röther J. Dysfunction of vasomotor reactivity in severe sepsis and septic shock. Intensive Care Med. 2001 Jul;27(7):1231–4. [CrossRef]
- Bowie RA, O’Connor PJ, Mahajan RP. Cerebrovascular reactivity to carbon dioxide in sepsis syndrome. Anaesthesia. 2003 Mar;58(3):261–5. [CrossRef]
- de Goede AA, Loef BG, Reidinga AC, Schaafsma A. Fluid Resuscitation in Septic Patients Improves Systolic but not Diastolic Middle Cerebral Artery Flow Velocity. Ultrasound Med Biol. 2017 Nov;43(11):2591–600. [CrossRef]
- Pfister D, Schmidt B, Smielewski P, Siegemund M, Strebel SP, Rüegg S, et al. Intracranial pressure in patients with sepsis. Acta Neurochir Suppl. 2008;102:71–5. [CrossRef]
- Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998 May;88(5):802–8. [CrossRef]
- Crippa IA, Vincent JL, Zama Cavicchi F, Pozzebon S, Gaspard N, Maenhout C, et al. Estimated Cerebral Perfusion Pressure and Intracranial Pressure in Septic Patients. Neurocrit Care. 2023 Jul 7; [CrossRef]
- Czempik PF, Gąsiorek J, Bąk A, Krzych ŁJ. Ultrasonic Assessment of Optic Nerve Sheath Diameter in Patients at Risk of Sepsis-Associated Brain Dysfunction: A Preliminary Report. Int J Environ Res Public Health. 2020 May 22;17(10):3656. [CrossRef]
- Wood MD, Boyd JG, Wood N, Frank J, Girard TD, Ross-White A, et al. The Use of Near-Infrared Spectroscopy and/or Transcranial Doppler as Non-Invasive Markers of Cerebral Perfusion in Adult Sepsis Patients With Delirium: A Systematic Review. J Intensive Care Med. 2022 Mar;37(3):408–22. [CrossRef]
- Longhitano Y, Iannuzzi F, Bonatti G, Zanza C, Messina A, Godoy D, et al. Cerebral Autoregulation in Non-Brain Injured Patients: A Systematic Review. Front Neurol. 2021 Nov 16;12:732176. [CrossRef]
- Fan TH, Premraj L, Roberts J, Lydston M, Robba C, Hager D, et al. In-Hospital Neurologic Complications, Neuromonitoring, and Long-Term Neurologic Outcomes in Patients With Sepsis: A Systematic Review and Meta-Analysis. Crit Care Med. 2024 Mar;52(3):452–63. [CrossRef]
- Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989 May;17(5):389–93. [PubMed]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992 Jun;101(6):1644–55. [CrossRef]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003 Apr;29(4):530–8. [CrossRef]
- Calandra T, Cohen J, International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005 Jul;33(7):1538–48. [CrossRef]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801–10. [CrossRef]
- Kostoglou K, Bello-Robles F, Brassard P, Chacon M, Claassen JA, Czosnyka M, et al. Time-domain methods for quantifying dynamic cerebral blood flow autoregulation: Review and recommendations. A white paper from the Cerebrovascular Research Network (CARNet). J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2024 Apr 30;271678X241249276. [CrossRef]
- Liu X, Czosnyka M, Donnelly J, Cardim D, Cabeleira M, Lalou DA, et al. Assessment of cerebral autoregulation indices – a modelling perspective. Sci Rep. 2020 Jun 15;10(1):9600. [CrossRef]
- Caldas J, Rynkowski CB, Robba C. POCUS, how can we include the brain? An overview. J Anesth Analg Crit Care. 2022 Dec 27;2(1):55. [CrossRef]
- Alonso JV, Turpie J, Farhad I, Ruffino G. Protocols for Point-of-Care-Ultrasound (POCUS) in a Patient with Sepsis; An Algorithmic Approach. Bull Emerg Trauma. 2019 Jan;7(1):67–71.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg Med Clin North Am. 2010 Feb;28(1):29–56, vii. [CrossRef]
- Sweeney DA, Wiley BM. Integrated Multiorgan Bedside Ultrasound for the Diagnosis and Management of Sepsis and Septic Shock. Semin Respir Crit Care Med. 2021 Oct;42(5):641–9. [CrossRef]
- Corradi F, Via G, Tavazzi G. What’s new in ultrasound-based assessment of organ perfusion in the critically ill: expanding the bedside clinical monitoring window for hypoperfusion in shock. Intensive Care Med. 2020 Apr;46(4):775–9. [CrossRef]
- Noitz M, Szasz J, Dünser MW. Regional perfusion monitoring in shock. Curr Opin Crit Care. 2020 Jun;26(3):281–8. [CrossRef]
- Corradi F, Brusasco C, Via G, Tavazzi G, Forfori F. Renal Doppler-Based Assessment of Regional Organ Perfusion in the Critically Ill Patient. Shock Augusta Ga. 2021 Jun 1;55(6):842–3. [CrossRef]
- Tas J, Beqiri E, van Kaam CR, Ercole A, Bellen G, Bruyninckx D, et al. An Update on the COGiTATE Phase II Study: Feasibility and Safety of Targeting an Optimal Cerebral Perfusion Pressure as a Patient-Tailored Therapy in Severe Traumatic Brain Injury. Acta Neurochir Suppl. 2021;131:143–7. [CrossRef]
- Tamagnone FM, Cheong I, Luna E, Previgliano I, Otero Castro V. Ultrasound-guided cerebral resuscitation in patients with severe traumatic brain Injury. J Clin Monit Comput. 2023 Apr;37(2):359–63. [CrossRef]
- Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012 Aug;40(8):2456–63. [CrossRef]
- Bögli SY, Cucciolini G, Cherchi MS, Motroni V, Olakorede I, O’Leary R, et al. Feasibility and Safety of Integrating Extended TCD Assessments in a Full Multimodal Neuromonitoring Protocol After Traumatic Brain Injury. Ultrasound Med Biol. 2024 Aug;S0301562924002710. [CrossRef]
- Rasulo FA, Togni T, Romagnoli S. Essential Noninvasive Multimodality Neuromonitoring for the Critically Ill Patient. Crit Care Lond Engl. 2020 Mar 24;24(1):100. [CrossRef]

| Study | Main findings | Metric used | Sample size (septic patients) |
|---|---|---|---|
| Matta and Stow 1996 [35] | CO2R was within normal limits for all patients. | CO2R | 10 |
| Terborg 2001 [39] | During septic shock NCR was significantly reduced. | NCR | 8 |
| Bowie 2003 [40] | CO2R was abnormal in 10/12 patients. This was not related to mortality or other clinical parameters. | CO2R | 12 |
| Thees 2007 [29] | CO2R was not impaired. However, the survivors showed a pathologic neurological examination. | CO2R | 10 |
| Berg and Plovsing 2016 [21] | CO2R is preserved in sepsis. Short term hyperventilation does not necessarily enhance CAR. | Phase, gain, coherence | 16 (only 7 underwent hyperventilation) |
| Szatmàri 2010 [17] | Vasomotor response was slower and lower in sepsis (less CRC and lower systolic MCAv). | Acetazolamide tes, CVR, CRC | 14 |
| Fülesdi 2012 [18] | CRC was similar in the two groups while CVR decreased slower in the septic group (more prolonged vasodilatory response). | Acetazolamide tes, CVR, CRC | 16 |
| Study | Main findings | Metric used | Sample size (septic patients) |
|---|---|---|---|
| Pfister 2008 [42] | 47% of patients showed nICP>15 mmHg in at least one day. nICP increases were moderate and never exceeded 20 mmHg. nICP was strongly correlated with MAP but did not differ between survivors and non-survivors. 73% of patients had eCPP<60 mmHg (20% falling <50 mmHg). Low eCPP was associated with high S-100β levels. There was no link between nICP and fluid administration. | nICP, eCPP | 16 |
| Crippa 2022 [33] | 53% of patients had impaired CAR, 55% had low eCPP, and 38% had high nICP. Low eCPP and high nICP was seen in 35% of patients. Pupillary dilation velocity was significantly lower in those with impaired CAR. Patients with low eCPP or high nICP had lower Neurological Pupil index (NPi) values. | THRT, nICP, eCPP | 40 |
| Crippa 2023 [44] | The median eCPP was 63 mmHg, with 33% having low eCPP. The median nICP was 8 mmHg, with 4% having high nICP. Most patients (65%) had normal eCPP and nICP. 31% had low eCPP with normal nICP. 2% had low eCPP and high nICP. 2% had normal eCPP and high nICP. There were no significant differences in SAE occurrence or in-hospital mortality between patients with altered eCPP or nICP compared to those with normal values. |
eCPP, nICP, Mxa. | 132 |
| Study | Main findings | Metric used | Sample size (septic patients) |
|---|---|---|---|
|
Pfister 2008 [42] |
No significant correlations between nICP, daily change in nICP or relative change in nICP and overall or daily fluid administration or balance. | MCAv, nICP, eCPP | 16 |
|
Pfister 2008 [30] |
Mx was altered in SAD patients. No differences in CBF between the SAD and non-SAD group. | MCAv, Mx | 16 |
|
Schramm 2012 [38] |
25 patients (88%) showed impaired CAR during the four days with a decreasing prevalence during days (day1 - 60%, day2 - 59%, day3 - 41%, day4 - 46%). Delirium developed in 76% of patients. The status of CAR at day 1 was related to development of delirium at day 4. | Mx | 30 |
|
Pierrakos 2014 [25] |
Twenty-one patients (55%) presented delirium (positive CAM-ICU test). ROC curve analysis showing only PI on the first day and not the third day was a good predictor of the presence of confusion (AUC = 0.908, 95%, CI 0.80-0.98, p < 0.01). PI was related to confusion independently from age or APACHE II score. | MCAv, PI, CBFi | 40 |
|
Pierrakos 2017 [26] |
Fourteen patients (50%) presented CD at the time of discharge. Only on the first day of the study PI was higher in patients with CD (2.2 ± 0.7 vs. 1.4 ± 0.5, p = 0.02) and CBFi was lower (363 ± 170 vs. 499 ± 133, p = 0.03). In univariate analysis, delirium and PI on the first day were related to CD (OR: 36.1, 95%CI 4.3–299.1, p = 0.01, OR:4.1, 95%CI 1.1–15.2, p = 0.03), but in the multivariate analysis PI was not found to be related to CD independently of the presence of delirium. | MCAv. PI, CBFi | 28 |
|
Crippa 2018 [32] |
There was no difference in Mxa between survivors and non-survivors at ICU discharge. SAE was more common in patients with altered CAR than in those with intact CAR (34 of 50 [68%] vs 23 of 50 [46%]; p = 0.04), and Mxa was higher in patients with SAE (0.47 [0.21–0.64] vs 0.23 [- 0.12–0.52]; p <0.01). In multivariable analysis, higher Mxa, vascular disease and mechanical ventilation were independent predictors of SAE. The best Mxa cut-off to predict SAE was 0.18 (sensitivity 79%, specificity 47%). | Mxa | 100 |
|
Czempik 2020 [45] |
49/80 ONSD measurements exceeded 5.7 mm. No correlations between ONSDs and CRP concentrations, highest daily lactate, or SOFA. ONSD measurement should be applied for screening of SAE cautiously. | ONSD | 10 |
| Feng 2021 [23] | The logistic regression analysis demonstrated that several independent risks were SAD predictors: rSO2 <55% [OR=3.864, 95% CI: 1.026-14.550, p=0.046] and the THRR index<1.09 [OR=5.77, 95% CI: 1.222–27.255, p=0.027]. Patients with SAD have a close correlation with poor outcomes. | MCAv, CBFi, PI, THRR | 51 |
|
Crippa 2023 [44] |
SAE occurrence and mortality did not differ between patients with low and normal eCPP or between patients with high and normal nICP. | eCPP, nICP, Mxa | 132 |
| Mei 2024 [24] | The SAE group displayed significantly elevated levels of NSE, S100β, PI, RI, and CCT, while EDV was lower (all P-values < 0.05). CCT emerged as the most efficacious predictor for SAE, with an AUC of 0.846. S100β, PI, and CCT were identified as independent predictors for SAE. | MCAv, PSV, EDV, PI, RI, CCT | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





