Submitted:
01 August 2024
Posted:
02 August 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
SAFER-Study
Breakthrough COVID-19
COVID-19 diagnosis
Outcomes
Inclusion and exclusion criteria
Study variables
Data management and monitoring
Ethical considerations
Statistical analysis
Results
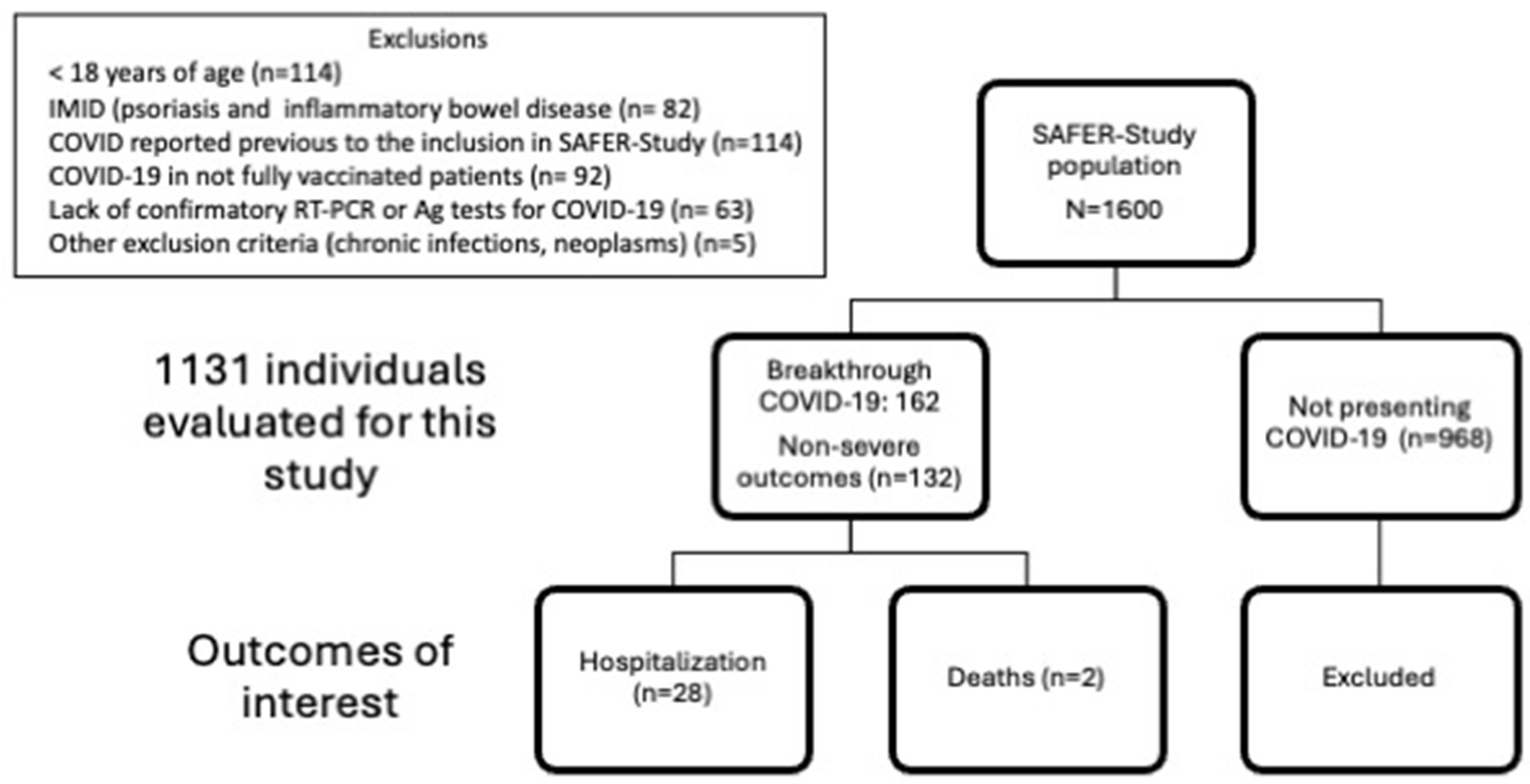
Study population
Vaccination
Breakthrough COVID-19
Discussion
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoff, L. S., Ravichandran, N., Shinjo, S. K., Day, J., Sen, P., Junior, J. G., Lilleker, J. B., Joshi, M., Agarwal, V., Kardes, S., Kim, M., Milchert, M., Makol, A., Gheita, T., Salim, B., Velikova, T., Gracia-Ramos, A. E., Parodis, I., O'Callaghan, A. S., Nikiphorou, E., … COVAD Study Group (2023). COVID-19 severity and vaccine breakthrough infections in idiopathic inflammatory myopathies, other systemic autoimmune and inflammatory diseases, and healthy controls: a multicenter cross-sectional study from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) survey. Rheumatology international, 43(1), 47–58. [CrossRef]
- Isnardi, C. A., Alpizar-Rodriguez, D., Calderaro, D. C., Marques, C. D. L., Pons-Estel, G. J., Xavier, R. M., Saurit, V., Pisoni, C. N., Tissera, Y. S., D'Angelo Exeni, M. E., Alba, P., Pereira, D., Gobbi, C. A., Gamba, M. J., Alfaro, M. A., Virasoro, B. M., Colunga-Pedraza, I. J., Irazoque-Palazuelos, F., Reyes-Cordero, G., Rodriguez-Reyna, T. S., … Martínez-Martínez, M. U. (2024). Factors Associated With Mortality in Patients With Immune-Mediated Rheumatic Diseases and COVID-19 From Latin America: Data From Argentina, Mexico, and Brazil. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases, 30(1), e9–e17. [CrossRef]
- Hasseli, R., Richter, J. G., Hoyer, B. F., Lorenz, H. M., Pfeil, A., Regierer, A. C., Schmeiser, T., Strangfeld, A., Voll, R. E., Krause, A., Reckert, S., Gräßler, A., Saar, P., Kapelle, A., Backhaus, M., Blank, N., Henes, J., Osiek, S., Knothe, A., Hoese, G., … COVID19-Rheuma.de collaborators (2023). Characteristics and outcomes of SARS-CoV-2 breakthrough infections among double-vaccinated and triple-vaccinated patients with inflammatory rheumatic diseases. RMD open, 9(2), e002998. [CrossRef]
- Liew, J., Gianfrancesco, M., Harrison, C., Izadi, Z., Rush, S., Lawson-Tovey, S., Jacobsohn, L., Ja, C., Hyrich, K. L., Gossec, L., Strangfeld, A., Carmona, L., Schäfer, M., Frãzao-Mateus, E., Bulina, I., Stafford, F., Tufan, A., Graver, C., Yardımcı, G. K., Zepa, J., … Yazdany, J. (2022). SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry. RMD open, 8(1), e002187. [CrossRef]
- Cook, C., Patel, N. J., D'Silva, K. M., Hsu, T. Y., DiIorio, M., Prisco, L., Martin, L. W., Vanni, K., Zaccardelli, A., Todd, D., Sparks, J. A., & Wallace, Z. S. (2022). Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Annals of the rheumatic diseases, 81(2), 289–291. [CrossRef]
- Alshukairi, A. N., Al-Omari, A., Albeity, A., Alandijany, T. A., Hassan, A. M., El-Kafrawy, S. A., Dada, A., Al Hroub, M. K., El-Saed, A., Bissar, L. S., Daghmush, R. M., Al-Ghamdi, S. M. G., Perlman, S., Azhar, E. I., & Halabi, H. (2022). COVID-19 breakthrough infections in rheumatic diseases patients after vaccination. Journal of infection and public health, 15(6), 685–688. [CrossRef]
- Galmiche, S., Luong Nguyen, L. B., Tartour, E., de Lamballerie, X., Wittkop, L., Loubet, P., & Launay, O. (2022). Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases, 28(2), 163–177. [CrossRef]
- CDC COVID-19 study shows mRNA vaccines reduce risk of infection by 91 percent for fully vaccinated people, 2021. Available: https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html [Accessed 28 Oct 2023].
- Strangfeld, A., Schäfer, M., Gianfrancesco, M. A., Lawson-Tovey, S., Liew, J. W., Ljung, L., Mateus, E. F., Richez, C., Santos, M. J., Schmajuk, G., Scirè, C. A., Sirotich, E., Sparks, J. A., Sufka, P., Thomas, T., Trupin, L., Wallace, Z. S., Al-Adely, S., Bachiller-Corral, J., Bhana, S., … COVID-19 Global Rheumatology Alliance (2021). Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the rheumatic diseases, 80(7), 930–942. [CrossRef]
- Papagoras, C., Fragoulis, G. E., Zioga, N., Simopoulou, T., Deftereou, K., Kalavri, E., Zampeli, E., Gerolymatou, N., Kataxaki, E., Melissaropoulos, K., Panopoulos, S., Fragiadaki, K., Evangelatos, G., Bournia, V. K., Arida, A., Karamanakos, A., Pappa, M., Panagiotopoulos, A., Koutsianas, C., Mparouta, G., … Sfikakis, P. P. (2022). Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Annals of the rheumatic diseases, 81(7), 1013–1016. [CrossRef]
- Fragoulis, G. E., Karamanakos, A., Arida, A., Bournia, V. K., Evangelatos, G., Fanouriakis, A., Fragiadaki, K., Kravvariti, E., Laskari, K., Panopoulos, S., Papazoglou, N., Pappa, M., Tektonidou, M. G., & Sfikakis, P. P. (2022). Clinical outcomes of breakthrough COVID-19 after booster vaccination in patients with systemic rheumatic diseases. RMD open, 8(1), e002279. [CrossRef]
- Patel, N. J., Wang, X., Fu, X., Kawano, Y., Cook, C., Vanni, K. M. M., Qian, G., Banasiak, E., Kowalski, E., Zhang, Y., Sparks, J. A., & Wallace, Z. S. (2023). Factors associated with COVID-19 breakthrough infection among vaccinated patients with rheumatic diseases: A cohort study. Seminars in arthritis and rheumatism, 58, 152108. [CrossRef]
| Characteristic |
Overall (N = 160)1 |
No (N = 132)1 |
Yes, (N = 28)1 |
p-value2 |
| Age (years) | 44.08 (12.81) | 44.21 (13.21) | 43.46 (10.93) | 0.9 |
| Gender [Female] | 133 (83.1%) | 110 (83.3%) | 23 (82.1%) | >0.9 |
| Self-declared white color | 97 (60.6%) | 77 (58.3%) | 20 (71.4%) | 0.2 |
| CTD/Vasculitis/Other RD | 96 (60.0%) | 78 (60%) | 18 (64%) | 0.67 |
| IJD | 62 (39%) | 52 (40%) | 10 (36%) | 0.67 |
| RD time length (years) | 9.50 (4.00 - 15.00) | 10.00 (4.00 - 15.00) | 8.50 (3.00 - 16.00) | 0.5 |
| RD moderate/high activity before breakthrough COVID-19 | 23 (14%) | 14 (11.5%) | 9 (37.5%) | 0.001 |
| Comorbidity (any) | 102 (63.8%) | 83 (62.9%) | 19 (67.9%) | 0.6 |
| Number of comorbidities | 1 (0-1) | 1 (0-1.25) | 1 (0-1) | 0.3 |
| Number of COVID-19 vaccine doses | 3 (3-4) | 3 (2-4) | 3 (2-3) | 0.028 |
| RD treatment before COVID-19 | ||||
| Glucocorticoids use | 34 (21%) | 24 (10%) | 10 (34%) | 0.04 |
| Glucocorticoids daily doses (mg/Prednisone) | 7.5 (5-10) | 8.75 (5-10) | 5 (5-7.5) | 0.5 |
| cDMARDs | 96 (60%) | 79 (60%) | 17 (60%) | 0.16 |
| bDMARDs | 19 (12%) | 15 (11.5%) | 4 (15%) | 0.4 |
| tsDMARDs | 3 (1.9%) | 1 (0.8%) | 2 (7.1%) | 0.08 |
| Rituximab | 1 (0.6%) | 1 (1%) | 0 (0%) | 0.6 |
| IS | 14 (9%) | 14 (11%) | 0 (0%) | 0.13 |
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Characteristic | N | OR (95%CI) | p-value | OR (95%CI) | p-value |
| White color | 160 | 1.83 (0.78-4.7) | 0.2 | - | - |
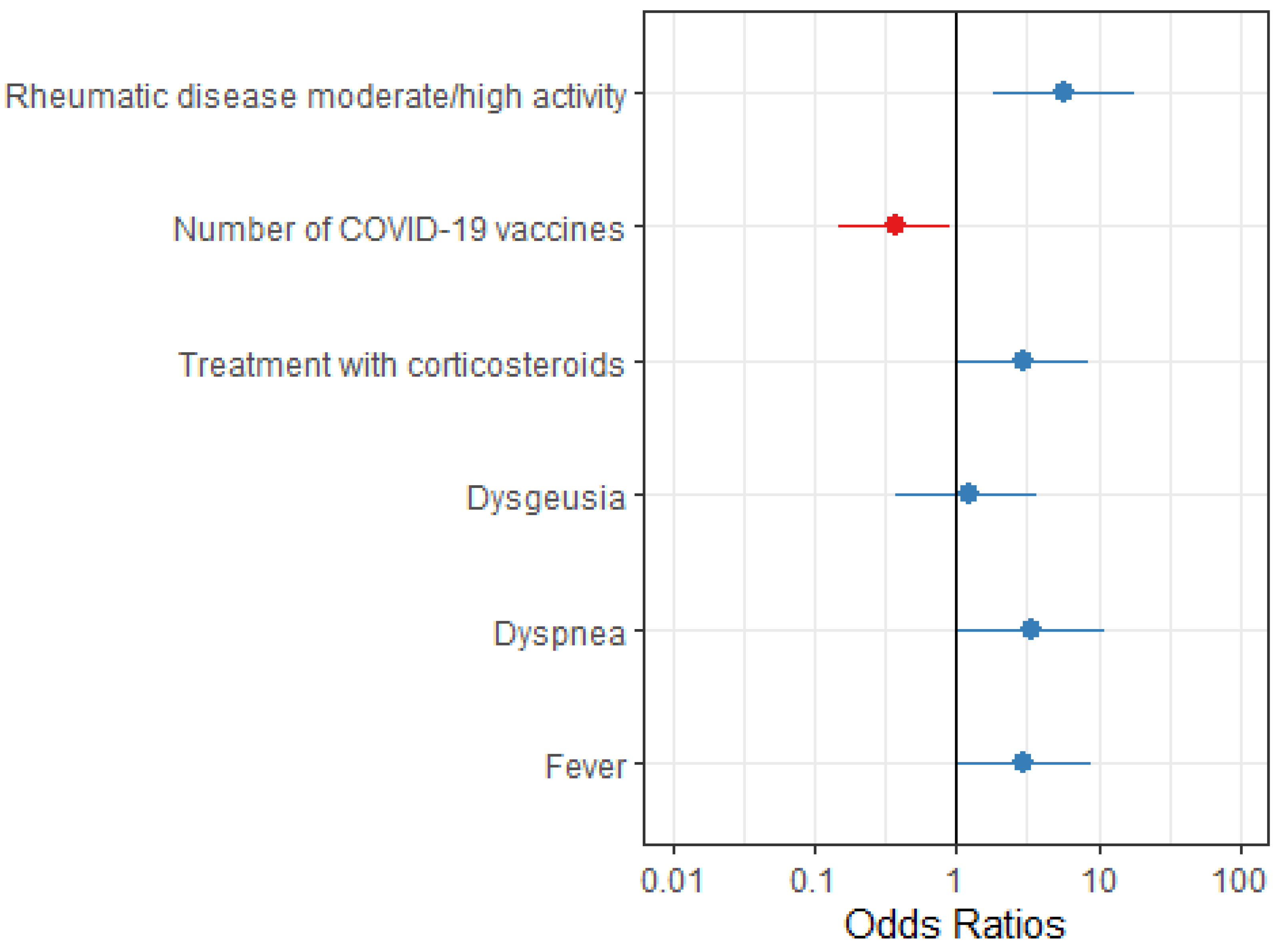
| RD moderate/high activity | 146 | 4.63 (1.68-12.6) | 0.003 | 5.73 (1.85-18.3) | 0.002 |
| Number of COVID-19 vaccine doses | 160 | 0.42 (0.19-0.92) | 0.003 | 0.38 (0.15-0.91) | 0.035 |
| Treatment with glucocorticoids before COVID-19 | 160 | 2.40 (0.95-5.86 | 0.056 | 2.92 (1.01-8.45) | 0.045 |
| Lack of treatment with antirheumatic drugs before COVID-19 | 160 | 0.46 (0.14-1.2 | 0.14 | - | - |
| COVID-19 manifestations | |||||
| Dysgeusia | 160 | 2.58 (1.03-6.27) | 0.038 | - | - |
| Dyspnea | 160 | 4.30 (1.7-10.8 | 0.002 | 3.44 (1.05-11.2) | 0.038 |
| Sore throat | 160 | 2.10 (0.92-5.06 | 0.086 | - | - |
| Fever | 160 | 2.45 (1.07-5.92) | 0.038 | 2.95 (1.05-9.08) | 0.047 |
| Hyposmia | 160 | 2.20 (0.86-5.4) | 0.090 | - | - |
| Cough | 160 | 2.42 (1.03-6.21) | 0.051 | - | - |
| Characteristic | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| SEX | Female | Male | Female |
| AGE (YEARS) | 43 | 63 | 62 |
| IMRD DIAGNOSIS | SLE | IgA-vasculitis | SLE |
| NUMBER OF COVID-19 VACCINE DOSES | 3 | 3 | 1 |
| DATES AND TYPES OF COVID-19 VACCINES ADMINISTERED | Dose 1: CoronaVac (11Sep2021) Dose 2: CoronaVac (13Oct2021) Dose 3: ChAdOx1 (13Oct2022) |
Dose 1: ChAdOx1 (05May2021) Dose 2: ChAdOx1 (27May2021) Dose 3: ChAdOx1 (27Sep2021) |
Dose 1: ChAdOx1 (05May2021) |
| COMORBIDITIES | CKD stage II, arterial hypertension, obesity (BMI: 41) | None | CKD stage II-III, arterial hypertension, type 2 diabetes mellitus |
| IMRD TREATMENT | PDN 12,5 mg + MMF | PDN 10 mg | HCQ |
| COVID-19 MANIFESTATIONS | NR |
Flu-like symptoms, fever and cough starting on 14Nov2021. Positive COVID-19 on 17Nov2021. Hospitalized on 19Nov2021. Was transferred to the ICU on 23Nov2021. Developed SARS with acute respiratory failure and the need for invasive mechanical ventilation, and AKI and died on 29Nov2021. |
Flu-like symptoms starting on 03Jun2021. Positive COVID-19 RT-PCR on 04Jun2021. Hospitalized for respiratory support. Developed SARS and died com 03Jul2021 |
| DEATH DATE | 19Nov2022 | 29Nov2021 | 03Jul2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





