Submitted:
29 July 2024
Posted:
30 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Cryptococcus Strains
2.2. Animals
2.3. Anesthesia
2.4. Infection
2.5. Cell Washes
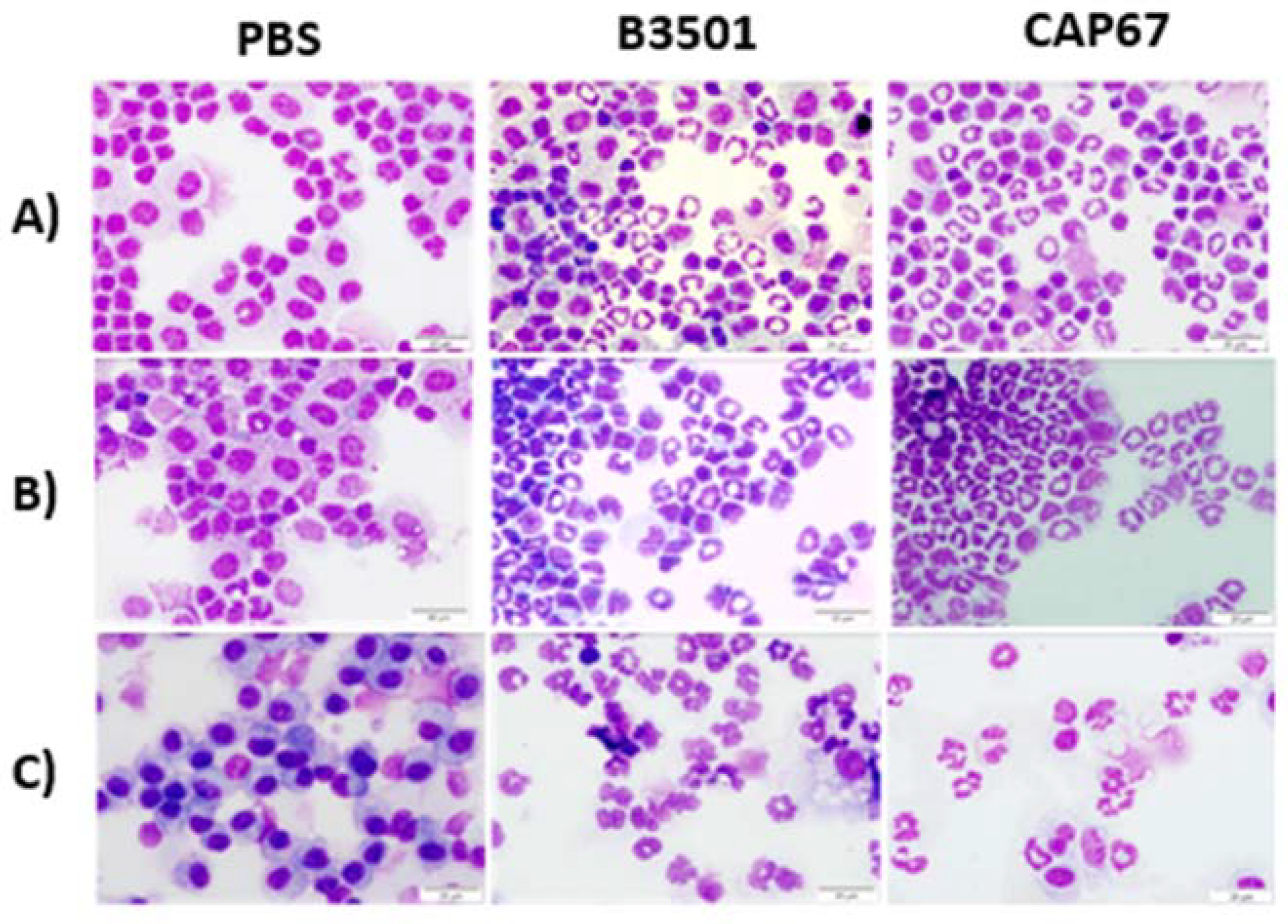
2.6. Morphological Analysis
2.7. Flow Cytometry
2.8. Cell Sorting
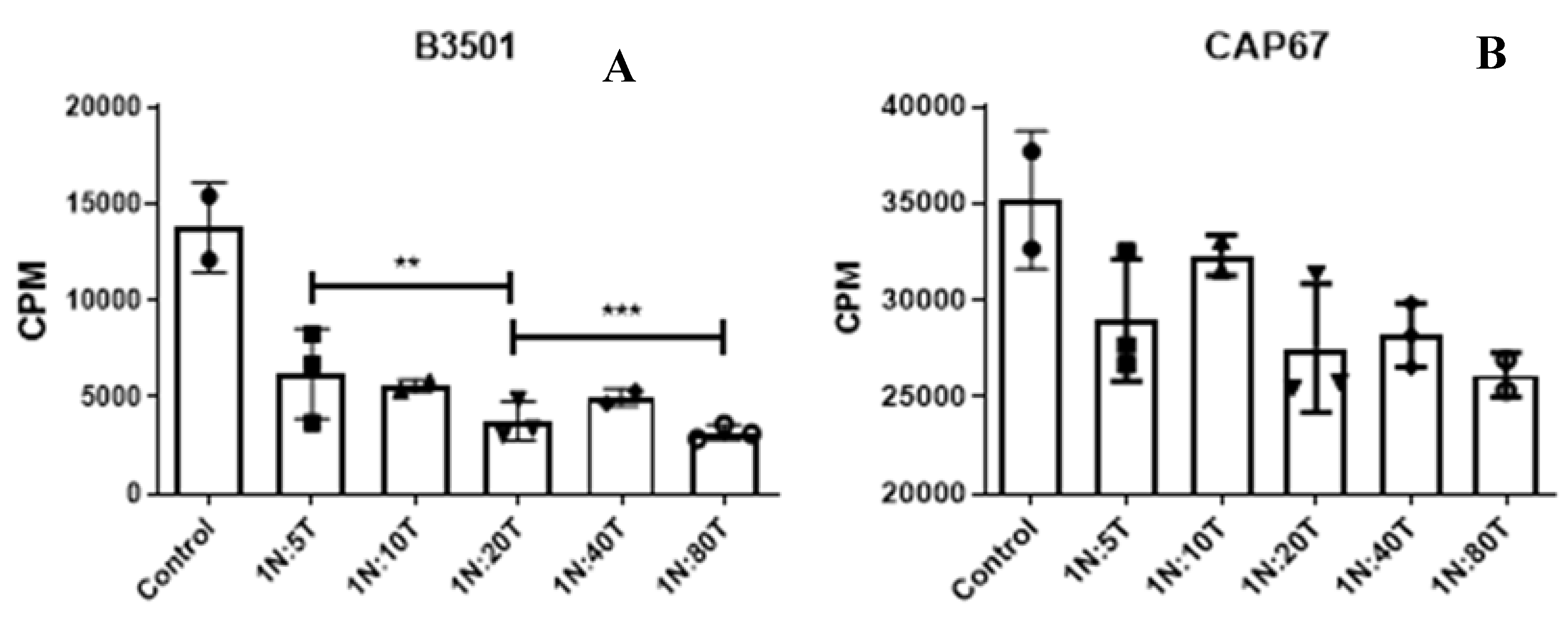
2.9. Cell Proliferation Assay
2.10. Statistical Analysis
3. Results
3.1. Observation of Precursor Cells in Peritoneal and Bronchoalveolar Lavage after Infections with C. neoformans
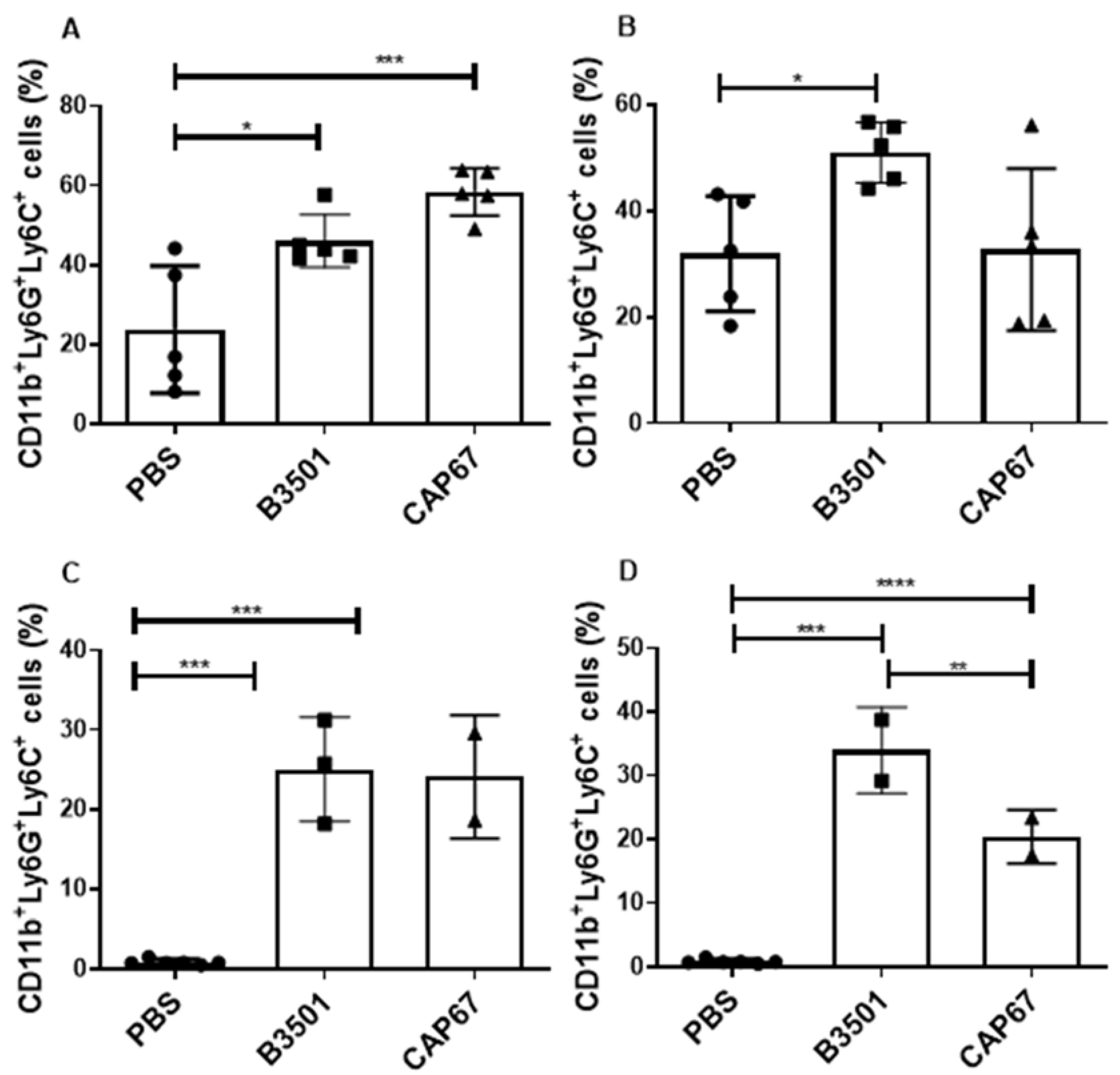
3.2. Characterization of Granulocytic MDSCs after Infection with C. neoformans
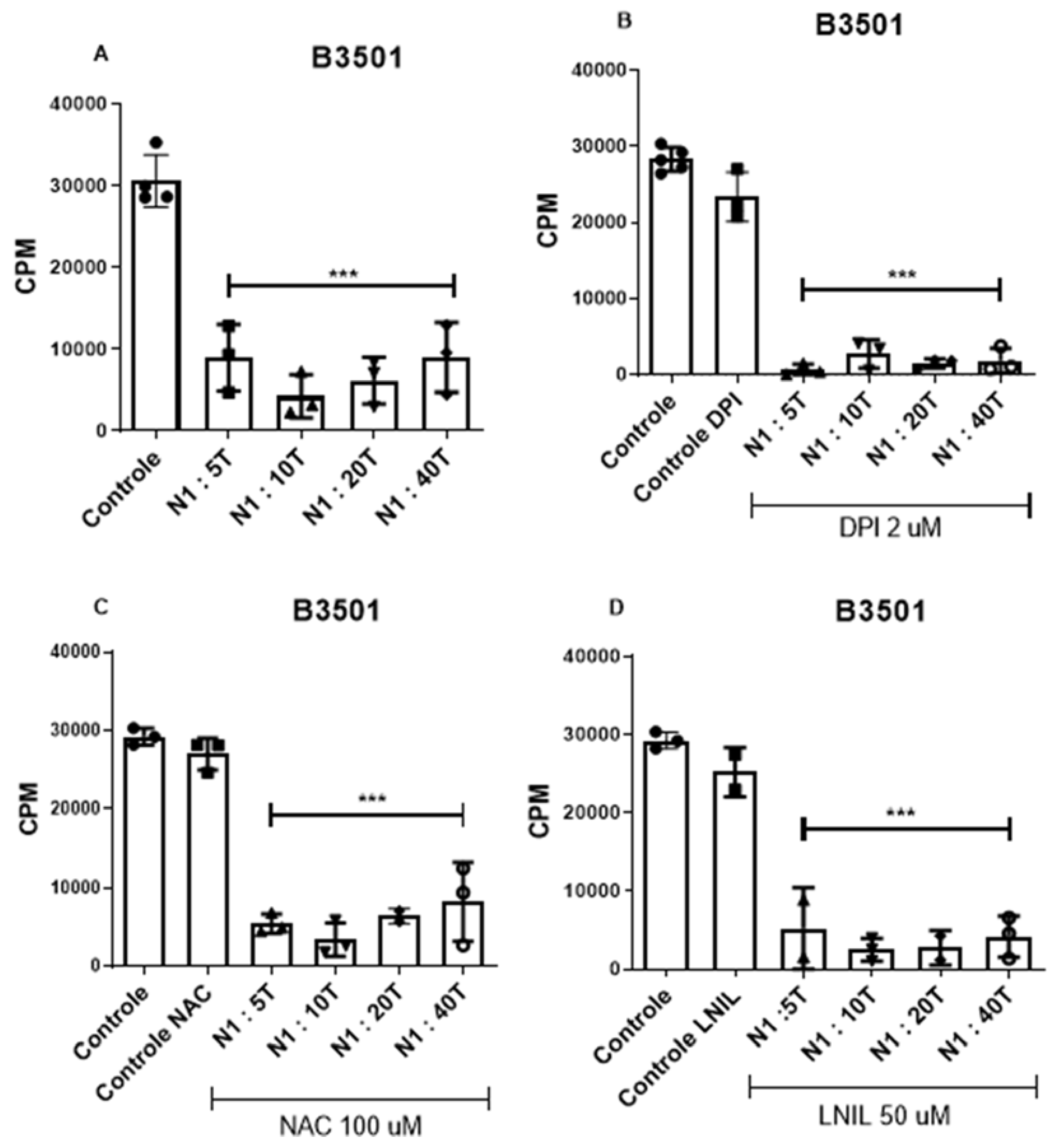
3.3. The Suppressive Effect Is Not Dependent on the Production of Reactive Oxygen Species
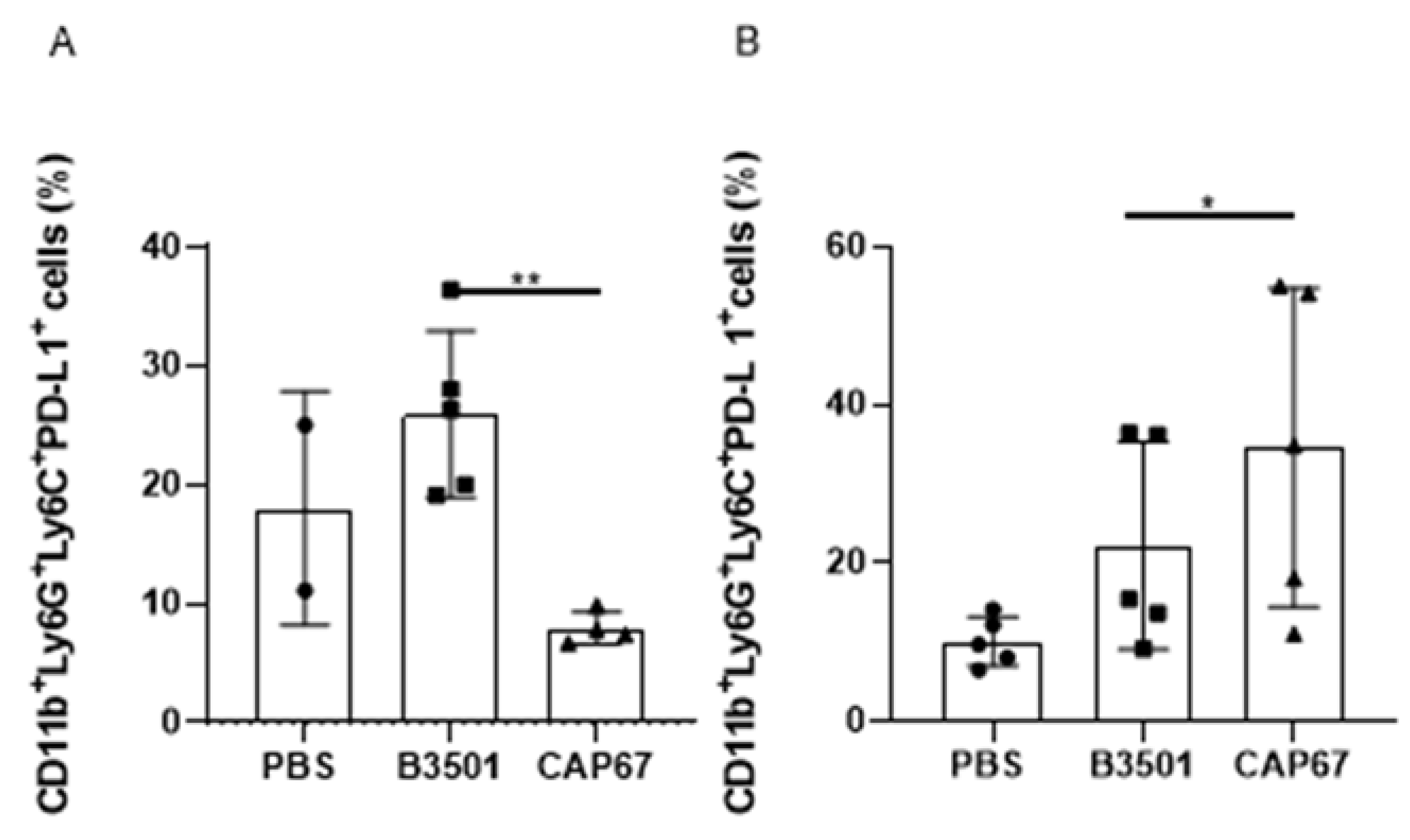
3.4. Recruited MDSCs Show PD-L1 Expression in Bronchoalveolar and Peritoneal Infection
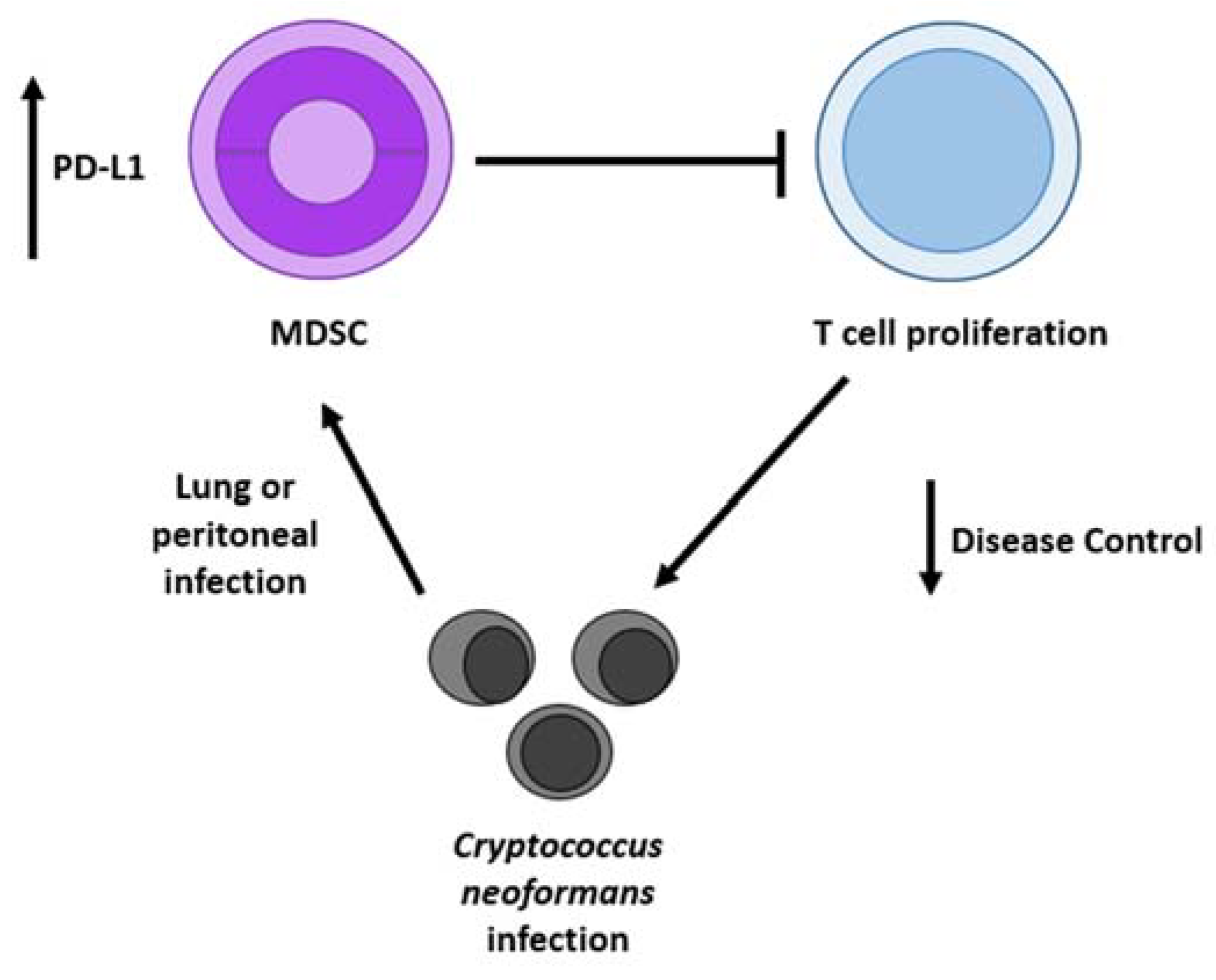
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husain, S.; Wagener, M.M.; Singh, N. Cryptococcus neoformans Infection in Organ Transplant Recipients: Variables Influencing Clinical Characteristics and Outcome. Emerg Infect Dis. 2001, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol. 2006, 60, 69–105. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.; Mai, N.H.; Phu, N.H.; Nghia, H.D.; Chuong, L.V.; Sinh, D.X.; Duong, V.A.; Diep, P.T.; Campbell, J.I.; Baker, S.; et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2015, 4, 7. [Google Scholar] [CrossRef]
- Firacative, C.; Lizarazo, J.; Illnait-Zaragozí, M.T.; Castañeda, E. The status of cryptococcosis in latin America. Mem Inst Oswaldo Cruz. 2018, 113, 7. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Cherniak, R.; Sundstrom, J.B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994, 62, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Decote-Ricardo, D.; LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nascimento, D.O.; Nunes, M.P.; Morrot, A.; Freire-de-Lima, L.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Immunomodulatory Role of Capsular Polysaccharides Constituents of Cryptococcus neoformans. Front Med 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. The Capsule of the Fungal Pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009, 68, 133–216. [Google Scholar]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect Dis Clin North Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Zhao, M.D.; Murphy, J.W. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun. 1995, 63, 2632–2644. [Google Scholar]
- Li, S.S.; Mody, C.H. Cryptococcus. Proc Am Thorac Soc. 2010, 7, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Retini, C.; Vecchiarelli, A.; Monari, C.; Bistoni, F.; Kozel, T.R. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998, 66, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Syme, R.M.; Bruno, T.F.; Kozel, T.R.; Mody, C.H. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun. 1999, 67, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- Walenkamp, A.M.; Chaka, W.S.; Verheul, A.F.; Vaishnav, V.V.; Cherniak, R.; Coenjaerts, F.E.; Hoepelman, I.M. Cryptococcus neoformans and its cell wall components induce similar cytokine profiles in human peripheral blood mononuclear cells despite differences in structure. FEMS Immunol Med Microbiol. 1999, 26, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.; Nascimento, M.T.; Decote-Ricardo, D.; Côrte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonça-Previato, L.; DosReis, G.A. , et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep. 2015, 5, 8008. [Google Scholar] [CrossRef] [PubMed]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- LaRocque-de-Freitas, I.F.; Rocha, J.D.B.; Nunes, M.P.; Oliveira, P.A.V.; Nascimento, D.O.; Freire-de-Lima, L.; Takiya, C.M.; Morrot, A.; Decote-Ricardo, D.; Previato, J.O. , et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci Rep. 2018, 8, 1. [Google Scholar]
- Rieber, N.; Singh, A.; Öz, H.; Carevic, M.; Bouzani, M.; Amich, J.; Ost, M.; Ye, Z.; Ballbach, M.; Schäfer, I. , et al. Pathogenic fungi regulate immunity by inducing neutrophilic myeloid-derived suppressor cells. Cell Host Microbe. 2015, 17, 507–514. [Google Scholar] [CrossRef]
- Mueller-Leisse, J.; Brueggemann, S.; Bouzani, M.; Schmitt, A.L.; Einsele, H.; Loeffler, J. Polymorphonuclear neutrophils and granulocytic myeloid-derived suppressor cells inhibit natural killer cell activity toward Aspergillus fumigatus. Med Mycol. 2015, 53, 622–629. [Google Scholar] [CrossRef]
- Chen, M.F.; Kuan, F.C.; Yen, T.C.; Lu, M.S.; Lin, P.Y.; Chung, Y.H.; Chen, W.C.; Lee, K.D. IL-6-stimulated CD11b+CD14+HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014, 5, 8716–8728. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells [1]. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef] [PubMed]
- Jessup, J.M.; Le Grue, S.J.; Kahan, B.D.; Pellis, N.R. Induction of suppressor cells by a tumor-derived suppressor factor. Cell Immunol. 1985, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Endicott, R.A.; Duffie, G.P.; Wepsic, H.T. Suppressor alveolar macrophages in mice bearing metastatic Lewis lung carcinoma tumors. J Leukoc Biol. 1987, 42, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Wheeler, E.; Newby, M. Macrophage-mediated suppression of natural killer cell activity in mice bearing Lewis lung carcinoma. J Natl Cancer Inst. 1986, 76, 745–750. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wu, T.; Shao, S.; Shi, B.; Zhao, Y.Y. Phenotype, development, and biological function of myeloid-derived suppressor cells. Oncoimmunology. 2016, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016, 7. [Google Scholar] [CrossRef]
- Bronte, V.; Wang, M.; Overwijk, W.W.; Surman, D.R.; Pericle, F.; Rosenberg, S.A.; Restifo, N.P. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998, 161, 5313–5320. [Google Scholar] [CrossRef]
- Youn, J.-I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Dugast, A.S.; Haudebourg, T.; Coulon, F.; Heslan, M.; Haspot, F.; Poirier, N.; Vuillefroy de Silly, R.; Usal, C.; Smit, H.; Martinet, B.; et al. Myeloid-Derived Suppressor Cells Accumulate in Kidney Allograft Tolerance and Specifically Suppress Effector T Cell Expansion. J Immunol. 2008, 180, 7898–7906. [Google Scholar] [CrossRef]
- Guan, Q.; Blankstein, A.R.; Anjos, K.; Synova, O.; Tulloch, M.; Giftakis, A.; Yang, B.; Lambert, P.; Peng, Z.; Cuvelier, G.D. , et al. Functional Myeloid-Derived Suppressor Cell Subsets Recover Rapidly after Allogeneic Hematopoietic Stem/Progenitor Cell Transplantation. Biol Blood Marrow Transplant. 2015, 21, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell suppressive activity. Blood. 2008, 111, 4233–4244. [Google Scholar] [CrossRef]
- Deshane, J.; Zmijewski, J.W.; Luther, R.; Gaggar, A.; Deshane, R.; Lai, J.F.; Xu, X.; Spell, M.; Estell, K.; Weaver, C.T.; et al. Free radical-producing myeloid-derived regulatory cells: Potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 2011, 4, 503–518. [Google Scholar] [CrossRef]
- Soler, D.C.; Young, A.B.; Fiessinger, L.; Galimberti, F.; Debanne, S.; Groft, S.; McCormick, T.S.; Cooper, K.D. Increased, but Functionally Impaired, CD14+ HLA-DR–/low Myeloid-Derived Suppressor Cells in Psoriasis: A Mechanism of Dysregulated T Cells. J Invest Dermatol. 2016, 136, 798–808. [Google Scholar] [CrossRef]
- Greifenberg, V.; Ribechini, E.; Rößner, S.; Lutz, M.B. Myeloid-derived suppressor cell activation by combined LPS and IFN-γ treatment impairs DC development. Eur J Immunol. 2009, 39, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-β1. J Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Bruchard, M.; Ghiringhelli, F. Microenvironnement tumoral. Medecine/Sciences. 2014, 30, 429–435. [Google Scholar] [CrossRef]
- Lim, H.X.; Hong, H.-J.; Cho, D.; Kim, T.S. IL-18 Enhances Immunosuppressive Responses by Promoting Differentiation into Monocytic Myeloid-Derived Suppressor Cells. J Immunol. 2014, 193, 5453–5460. [Google Scholar] [CrossRef]
- Youn, J.I.; Gabrilovich, D.I. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef]
- Egelston, C.; Kurkó, J.; Besenyei, T.; Tryniszewska, B.; Rauch, T.A.; Glant, T.T.; Mikecz, K. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum. 2012, 64, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Kurkó, J.; Vida, A.; Glant, T.T.; Scanzello, C.R.; Katz, R.S.; Nair, A.; Szekanecz, Z.; Mikecz, K. Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: A pilot study. BMC Musculoskelet Disord. 2014, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Luan, B.; Wang, X.F.; Qiao, J.Y.; Song, L.; Lei, R.R.; Gao, W.X.; Liu, Y. Peripheral Blood MDSCs, IL-10 and IL-12 in Children with Asthma and Their Importance in Asthma Development. PLoS One. 2013, 8, 5. [Google Scholar] [CrossRef]
- Pan, W.; Zhou, H.J.; Shen, Y.J.; Wang, Y.; Xu, Y.X.; Hu, Y.; Jiang, Y.Y.; Yuan, Z.Y.; Ugwu, C.E.; Cao, J.P. Surveillance on the Status of Immune Cells after Echinnococcus granulosus Protoscoleces Infection in Balb/c Mice. PLoS One. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Vollbrecht, T.; Stirner, R.; Tufman, A.; Roider, J.; Huber, R.M.; Bogner, J.R.; Lechner, A.; Bourquin, C.; Draenert, R. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. 2012, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Tamadaho, R.S.E.; Hoerauf, A.; Layland, L.E. Immunomodulatory effects of myeloid-derived suppressor cells in diseases: Role in cancer and infections. Immunobiology. 2018, 223, 432–442 [PubMed]. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Plessis, N.D. . Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol. 2018, 8, 1895. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process. Ageing Res Rev. 2018, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Brito, P.K.M.; Rezende, C.P.; Almeida, F.; Roque-Barreira, M.C.; da Silva, T.A. iNOS/Arginase-1 expression in the pulmonary tissue over time during Cryptococcus gattii infection. Innate Immun. 2020, 26, 117–129. [Google Scholar] [CrossRef]
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Müller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010, 207, 1453–1464. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H. , et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect Dis Clin North Am. 2002, 16, 837–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, G.S.; Shao, S.; Jung, H.W.; Durant, P.J.; Lee, C.H. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infect Immun. 2012, 80, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.R.; Cervi, L.A.; García, M.M.; Chiapello, L.S.; Sastre, D.A.; Masih, D.T. Involvement of nitric oxide in protecting mechanism during experimental cryptococcosis. Clin Immunol. 1999, 90, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K. Reactive Oxygen Species as Regulators of MDSC-Mediated Immune Suppression. Front Immunol. 2018, 9, 2499 [PubMed]. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Wickes, B.L.; Stockman, L.; Roberts, G.D.; Ellis, D.; Howard, D.H. Virulence, serotype, and molecular characteristics of environmental strains of Cryptococcus neoformans var. gattii. Infect Immun. 1992, 60, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.S.; Ayers, D.J.; Harrell, A.C.; Nicholas, C.C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982, 150, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Chaka, W.; Verheul, A.F.; Vaishnav, V.V.; Cherniak, R.; Scharringa, J.; Verhoef, J.; Snippe, H.; Hoepelman, I.M. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997, 65, 272–278. [Google Scholar] [CrossRef]
- James, P.G.; Cherniak, R. Galactoxylomannans of Cryptococcus neoformans. Infect Immun. 1992, 60, 1084–1088. [Google Scholar] [CrossRef]
- Ribechini, E.; Greifenberg, V.; Sandwick, S.; Lutz, M.B. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol. 2010, 199, 273–281. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.M.; Abuharfeil, N.M.; Darmani, H.; Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.J.; Malik, R.; Bartlett, K.H.; Duncan, C.G. Cryptococcosis: Update and emergence of Cryptococcus gattii. Vet Clin Pathol. 2011, 40, 4–17. [Google Scholar] [CrossRef] [PubMed]
- O'Meara, T.R.; Alspaugh, J.A. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev. 2012, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Bürgel, P.H.; Marina, C.L.; Saavedra, P.H.V.; Albuquerque, P.; de Oliveira, S.A.M.; Veloso Janior, P.H.H.; de Castro, R.A.; Heyman, H.M.; Coelho, C.; Cordero, R.J.B. , et al. Cryptococcus neoformans Secretes Small Molecules That Inhibit IL-1 β Inflammasome-Dependent Secretion. Mediators Inflamm. 2020, 2020, 3412763 [PubMed]. [Google Scholar] [CrossRef]
- Srikanta, D.; Santiago-Tirado, F.H.; Doering, T.L. Cryptococcus neoformans: Historical curiosity to modern pathogen. Yeast. 2014, 31, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: Not only in tumor immunity. Front Immunol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Raber, P.L.; Thevenot, P.; Sierra, R.; Wyczechowska, D.; Halle, D.; Ramirez, M.E.; Ochoa, A.C.; Fletcher, M.; Velasco, C.; Wilk, A. , et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer. 2014, 134, 2853–2864. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Xuejin, O.; Qizhi, M.; Yue, C.; Pei, S.; Dan, L.; Yongsheng, W. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Sig Transduct Target Ther 6. 2021, 6, 1–6. [Google Scholar] [CrossRef]
- Yu, R.; Bo, Z.; Degao, C. Type I Interferon-Mediated Tumor Immunity and Its Role in Immunotherapy. Cellular and Molecular Life Sciences 2022. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




