1. Introduction
Hematologic malignancies (HM) are a group of diseases, which are characterized by high heterogeneity, particularly regarding incidence, prognosis, and etiology [
1]. Based on the estimations of the GLOBOCAN project, these malignant neoplasms (MN) accounted for approximately 7% of all newly diagnosed cancer cases in Europe, during 2018 [
2,
3].
(1) A documented increase in the incidence burden of Non Hodgkin’s lymphomas (NHLs) was depicted in Europe, mainly during the last three decades of the 20
th century; in some parts of Europe they had almost doubled their incidence rates [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Their mortality rate was also increasing during these years, following a smoother ascending curve, while survival rates had been rising too [
18,
19,
20,
21,
22,
23].
(2) The incidence rate of multiple myeloma (MM) recorded a relatively steady trend presenting differences among countries and ethnic groups. MM’s survival rate was slowly, but consistently going up until recently, presenting a bigger raise during the 2000s [
8,
10,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27].
(3) Leukemias’ incidence and mortality had a mixed trend among its different subtypes and among different age groups, but their overall rate was relatively steady for many European countries. In general, leukemias’ survival has been continuously increasing [
8,
10,
14,
15,
18,
19,
20,
21,
22,
23,
24].
(4) Hodgkin’s lymphoma’s (HL) incidence was relatively stable for many areas of Europe, while it was slightly decreasing or increasing for others. Its mortality rate has shown an annual periodic decrease, starting at about the 1980s, while its survival rate has mostly risen through these years, mainly because HL had been marking a significant improvement in their treatment and prognosis [
4,
7,
8,
9,
10,
18,
19,
20,
21,
22,
23,
27,
28,
29].
It is important to note that there exist significant differences in the incidence, the mortality and the survival rates of all these MN, among their subtypes, among age groups, among ethnic groups, and among countries and areas of Europe; classification changes and modifications in the registration and diagnostic procedures during the recent years: a) in some cases may partly explain the rise in the incidence of some of these MN in some areas of Europe, and b) also make it difficult to make comparisons between different registries of Europe [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
Regarding Greece, there is an information gap, as the available recent published population-based rates for HM are rare: (a) two studies for children [
30,
31], and (b) one study for myelodysplastic syndromes in Western Greece regarding adults [
32]. There has been no article published for adults (≥18 years old) for any of the 4 main groups of HM for more than two decades. A previous report of the Cancer Registry of Crete (CRC) published back in 1998, had shed some light on the frequency of leukemias in Crete, analyzing data for adults for the years 1992-1993 [
33]. This study reported a high burden of leukemias and generated the following research questions: (a) whether the rates of all hematologic MN are high; (b) whether their rates are inter-temporally high in Crete; and (c) whether there exist areas in Crete with a higher burden than others; Due to lack of data at a national level, acquisition of population-based data from a large region like Crete is important. The aforementioned research questions motivated the authors to conduct the current research.
The aim of this study was to assess the overall burden of hematologic MN for 22 years, regarding the adult population of Crete, by setting the following objectives:
(a) Assess age standardized morbidity and mortality rates per (ASIRs and ASMRs/100.000/year);
(b) Report on the demographic and clinical profile of patients;
(c) Depict the temporal trends and the spatial distribution of the age standardized incidence and mortality rates;
(d) Assess the 10 year net-survival curves of patients diagnosed throughout the years of the study.
2. Materials and Methods
Crete is a naturally bounded region of Greece, as a big island in the South, separated for administrative reasons to four counties and nineteen municipalities (23 in total). Its whole population consisted of approximately 620,000 permanent residents in 2011. The composition of the Cretan population comprises an interesting context for conducting research on chronic diseases, due to the fact that most of its permanent residents have a relatively homogeneous socio-demographic profile, presenting no significant diversities [
32]. The vast majority of the Cretan population resides in urban, rural and semi urban areas. The Cretans are mainly occupied in the tourism service, and the business and agricultural sectors.
The Cancer Registry of Crete (CRC) is a population based registry, unique for Greece (
https://www.linkedin.com/in/crc-registry-88993a99/?originalSubdomain=gr), which monitors the epidemiology of cancer in the region of Crete and has recorded both cases and deaths regarding adults (≥18 years old), covering the island of Crete, for the time period from 1/1/1992 to 31/12/2013. It was founded in 1992, by the School of Medicine of the University of Crete and operated under a small fund from the Region of Crete (
https://www.crete.gov.gr/), which just covered the travel expenses that were needed for the data collection (for 2005-2013). It worked under the license of the Hellenic Data Protection Authority (Protocol number: 960/11-08-2009) and had adopted all the International Standards, which were prerequisite for recording, managing and processing sensitive and personal data. All information and data were recorded using a cryptographic coding system, in accordance with the Greek and the International Laws and Principles.
Primary data on HM were obtained from the CRC’s database for the 22-year study period (1/1/1992 to 31/12/2013). All information on diagnosed cases and deaths were retrospectively collected by trained registrars, who extracted data from clinical records, which derived from all eight public hospitals of Crete. Data on deaths were additionally captured by the death certificates, from all the registry offices of the island. The data was coded and reported according to the International Classification of Diseases for Oncology, 2nd version (ICD-O-2) [
34], using the new Cancer Monitoring System (CMS).
Each registration included information regarding demographic characteristics, age at diagnosis (and age at death for cases that had passed away due to these MN), site of the tumor, ICD10 code of the malignant neoplasm, confirmation of diagnosis by [a histological biopsy (nodal and/or extra-nodal) and/or a bone marrow biopsy at the time of diagnosis], stage at diagnosis, personal medical history, family history (cancer and/or other diseases), clinical characteristics and two lifestyle risk factors (smoking and alcohol use).
From all cancer data of the CRC, subset analyses on HM were conducted; all records were classified into the four main categories of hematologic MN based on their ICD10 code and the ICDO-2 classification for cancer registries: (1) C81: HL; (2) C82 to C88: NHLs; (3) C90: MM; (4) C91 to C95: All leukemias: [(a) C91: Lymphoid (chronic and acute); (b) C92: Myeloid (chronic and acute); (c) C93: Monocytic (chronic and acute); (d) C94: Other leukemias of specified cell type; (e) C95: Leukemia of unspecified cell type].
In order to add a new case into the analysis, an identification of a positive bone marrow biopsy and/or a positive histological biopsy (nodal and/or extra-nodal) was prerequisite as inclusion criteria. The histological report of each case indicated its type and subtype and based on its corresponding ICD-10 code, all cases were classified as aforementioned, based on the ICD-O-2 [
34].
Data mining techniques were performed automatically (by the CMS) and manually (by the administrator of the CMS) in order to proofread the quality of the data. Duplicate and multiple records were subsequently corrected and merged. Additionally, quality controls were conducted by oncologists, who randomly inspected half of all entries (50%) recorded by each registrar. After 118 (2.2%) registrations were excluded, due to inclusion, and/or exclusion criteria, and/or missing data, and/or invalid information, a total of 5432 records (3380 new cases and 2052 deaths) were included in the final analyses.
The direct standardization method was utilized, based on the population pyramids of: [(1) the Cretan population (2011), and (2) the European (2011) standard population]. Annual, gender specific, age standardized incidence and age standardized mortality rates per 100.000 (ASIR/100,000/year; ASMR/100,000/year) were extracted for each one of the four main disease categories and for all MN in total. Spatial analysis depicted the ASIRs and ASMRs, for each municipality, and overall for all the region of Crete.
Additionally, survival analysis was performed for each one of the four disease groups, using the Kaplan-Meier estimator, which calculated ten-year net-survival (NS) curves. All statistical analyses and spatial tests were performed using STATA (version 12) and ArcGIS (version 10.3.1.) softwares, and had the level of significance set at 95%.
3. Results
3.1. Demographic and Clinical Characteristics of Patients Diagnosed with HM in Crete
Demographic and clinical characteristics of all 3380 confirmed cases of the study [(1) NHL: n=1,152; (2) HL: n=254; (3) MM: n=496; (4) Leukemias: n=1,478] are summarized in
Table 1. A male predominance was highlighted in all four categories (overall n for men=1990; 58.8% of all cases). Mean age at diagnosis was: (1) 73 years (SD±12.7) for NHLs; (2) 46 years for HL (SD±8.1); (3) 73 years (SD±7.6) for MM; (4) 72 years (SD±9.4) for leukemias. Most cases had a positive family history (1) 75% of NHLs; (2) 74% of HL; (3) 78% of MM; (4) 80% of leukemias), had one (or more) 1
st and/or 2
nd degree relative diagnosed with any malignancy and/or any disease of the hematopoietic system.
3.2. Incidence (ASIRs) and Mortality (ASMRs) Statistics for HM in Crete
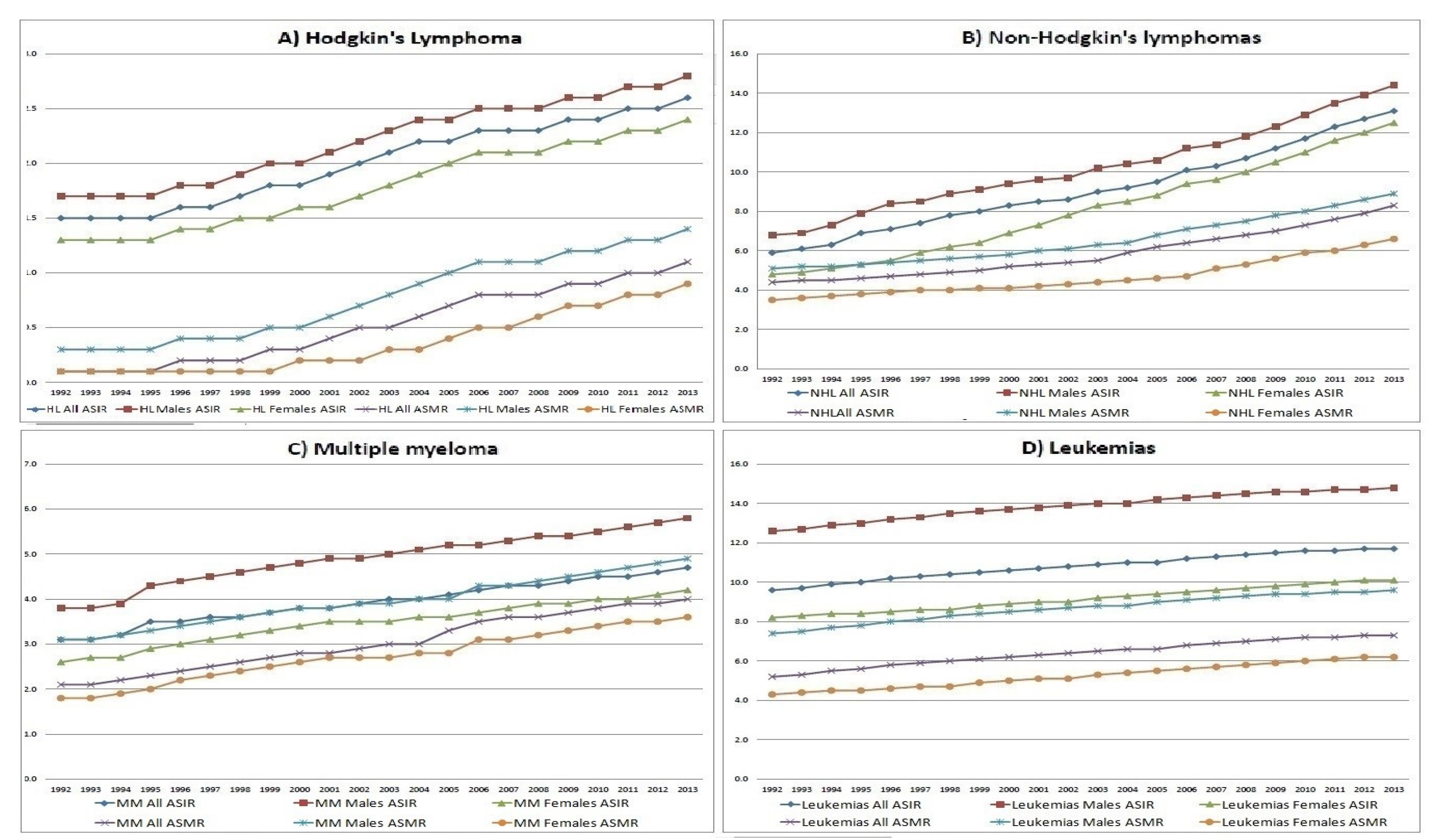
Extracted time trends of ASIRs for each group of diseases are illustrated in
Figure 1. Gradual continuous increase was documented for all ASIRs (overall, HL, NHLs, MM and leukemias) for both genders, starting at their lowest rate (20.1, 1.5, 5.9, 3.1 and 9.6 new cases/100,000, respectively) in 1992, and reaching their peak value (32.1, 2.6, 13.1, 4.7 and 11.7 new cases/100,000, respectively), in 2013. ASIRs for males were inter-temporally higher for all four groups of diseases.
All deaths, due to these MN were 2,052 [of which 1,257 (61.2%) were males]. All four ASMRs showed statistically significant increasing time trends (p-value<0.05), from 1992 to 2013 (
Figure 1). ASMRs for HL, NHLs, MM, leukemias and for all four MN were (0.1, 4.4, 2.1, 5.2 and 11.8 deaths/100,000 for both genders, respectively), in 1992 and (1.1, 8.3, 4.0, 7.2 and 20.7 deaths/100,000, respectively) in 2013.
3.3. Geographical Variation of ASIRs and ASMRs among Municipalities of Crete
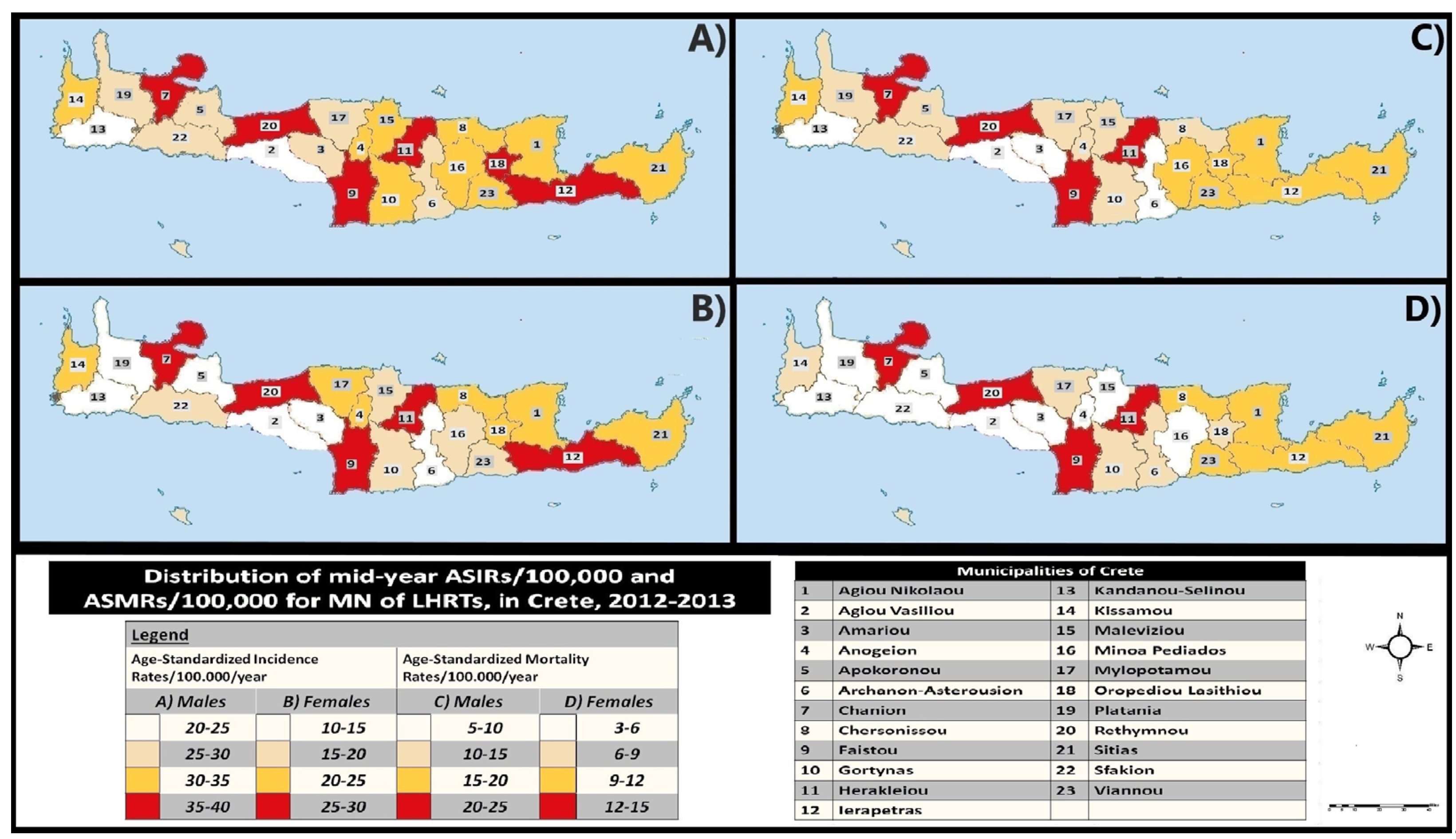
Overall mid-year ASIRs for 2012-2013 per municipality are depicted geographically in
Figure 2A for males and 2B for females. The highest ASIRs of the study [males (35-40 new cases/100,000) and females (25-30/100,000)] were observed in five municipalities (codes no: 7, 9, 11, 12 and 20), with the addition of code no 18 for males. On the contrary, the lowest ASIRs were found in two municipalities for males (codes no: 2 and 13; ASIR<25 new cases/100,000/year) and six for females (codes no: 2, 3, 5, 6, 13 and 19; ASIR<15 new cases/100,000/year).
Moreover, the spatial distribution for the overall mid-year ASMRs (2012-2013), among Crete’s municipalities is illustrated in
Figure 2C (males) and
Figure 2D (females). The highest ASMRs [males (20-25/100,000/year); females (12-15/100,000/year)] were seen in four municipalities (codes no 7, 9, 11 and 20) for both genders. On the other hand, the lowest mid-year ASMRs (2012-2013) were recorded in four municipalities for males (codes no: 2, 3, 6 and 13; ASMR<10/100,000/year) and in nine for females (codes no: 2, 3, 4, 5, 13, 15, 16, 19 and 22; ASMR<6/100,000/year).
3.4. Ten-Year Net-Survival Curves
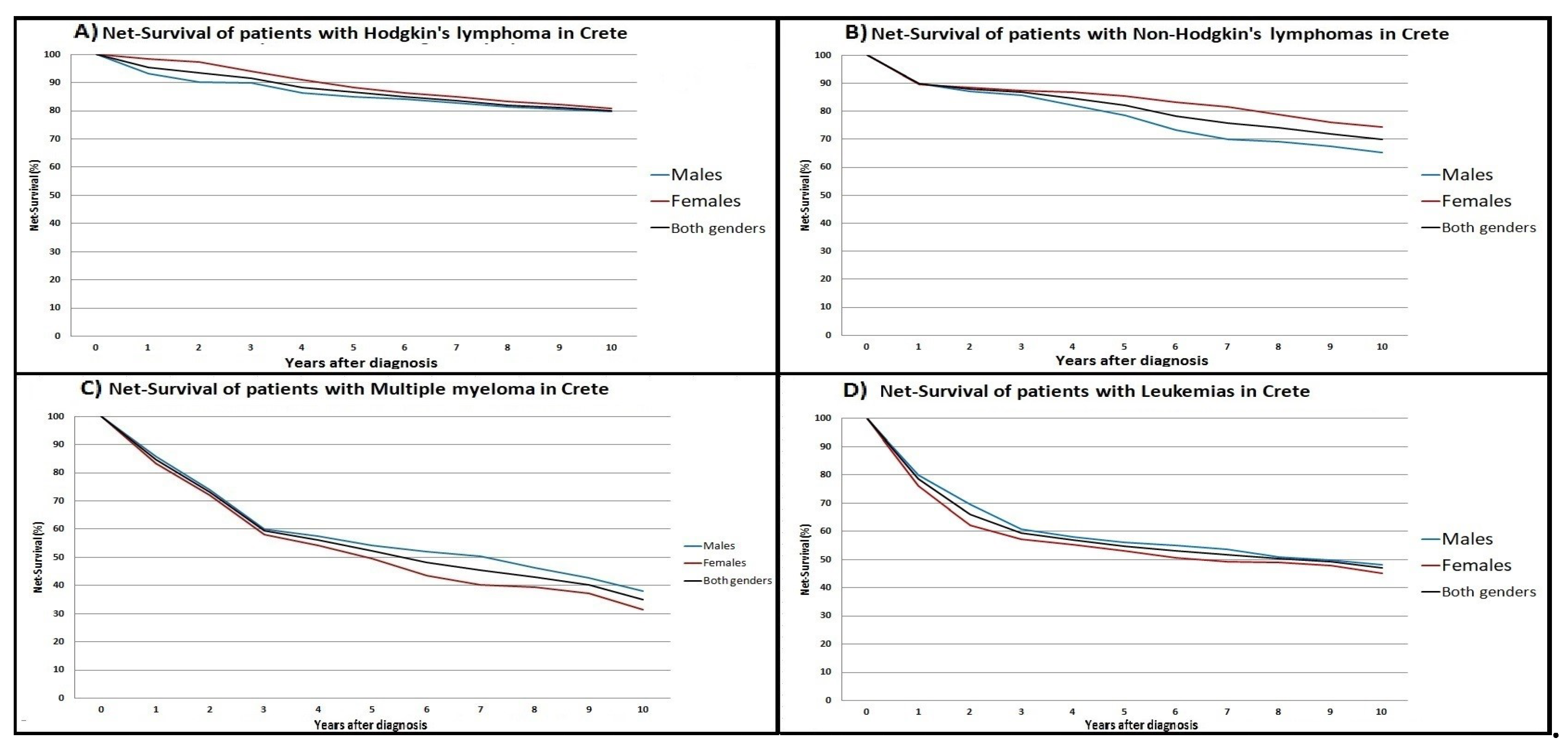
Ten-year net-survival (NS) curves for each one of the four disease categories are illustrated in
Figure 3. NS for NHLs, HL, MM and leukemias in Crete were: [5-year NS (79%, 85%, 54% and 56%) and 10-year NS (65%, 80%, 38% and 48%) for males]; [5-year NS (85%, 89%, 50% and 53%) and 10-year NS (75%, 81%, 31% and 45%) for females], respectively.
4. Discussion
4.1. Three Major Key Findings of the Study
Three key points need to be discussed: (1) The statistical significant increase, which was depicted for all 4 ASIRs and all 4 ASMRs throughout all the study period, (2) The geographical variation, which was recorded highlighting differences in the distribution of the incidence and the mortality rates among municipalities of Crete (3) The higher rates, which were documented for leukemias in Crete, in comparison with Catalonia-Spain (2003-2007) and Italy (2005) [
35,
36,
37].
4.2. Comparisons with Evidence from the Literature
A male predominance was observed for all four groups of ASIR in accordance with data from France, United Kingdom and Europe [
3,
4,
9,
14,
16,
38,
39,
40,
41]. Furthermore, the rising trends in the burden of these MN in Crete are consistent: a) with rates from the USA: for NHLs (which were rising from 1970 to 2000, but not for 2000 to 2021), for MM (which were rising for 2000 to 2021, but not for 1970 to 2000), but not for leukemias (which were stable from 2000 to 2021), and also not for HL (which were mostly stable from 1970 to 2021) [
24,
42,
43], and b) with Europe mainly regarding the data for NHL’s patients, which were showing increasing trends in Europe and Eastern Europe [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
The main differences are the following two: a) the incidence rates for NHL in Crete were still increasing, at least until the end of the study period (2013), in contrast with rates of most European countries, which seem to have leveled off for some years, and also b) the three ASIRs (except of those for NHLs) of the study which were rising for all the 22 years of the study, while rates in most European countries were mostly relatively stable through these years [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
Furthermore, comparisons were conducted between the ASIRs of Crete, and the corresponding rates from other countries (
Table 2) [
35,
36,
37]. Higher rates were documented for leukemias in Crete, in comparison with Catalonia-Spain (2003-2007) and Italy (2005) [
35,
36,
37]. All other comparisons showed relatively equal or lower rates for Crete, except of the ASIR for MM, which was higher in this study, than in Catalonia-Spain (2003-2007). All the rates of Crete, Italy, Catalonia and Spain were standardized based on the European Standard population.
4.3. Strengths and Limitations
First of all, this study is offering valuable data for the Cretan and the Greek health authorities and scientific community. All data have undergone several quality controls (reliability=97.5%: completeness=97%; continuity=98%) and follow the International Standards and Laws on disease coding, data collection, data protection and data analysis and interpretation.
On the other hand the following study limitations should be discussed. Data derived from all eight public hospitals of the island and all registry offices of the 23 municipalities, covering approximately the 95% of the real burden of these MN in Crete. It is expected that the missing cases do not exceed a 5%, which is the estimated number of patients who are managed in private clinics in Crete or in medical centers outside of Crete. Furthermore, we may have overestimated the disease burden, due to the small population in some Cretan municipalities. The authors used standardization techniques in order to assess and manage such diversities.
Also, two important limitations of the study are: (1) on the one hand we used the ICD-O-2 coding, and (2) on the other hand that we did not at least separate the 4 different types of leukemias in our analyses (we studied them all together); The aforementioned was conducted this way because a great part of the data was already classified and coded by the means of this classification (regarding the time period: 1992-2004). Although the use of the ICD-O-2 is a methodologically correct approach, it has 3 major problems: a) it is difficult to have a clear picture of the real burden of these MN, because different diseases are studied together, b) it does not study all myeloproliferative syndromes (MPS), all myelodysplastic syndromes (MDS) and some myelodysplastic/myeloproliferative neoplasms (MDS/MPN), because they were not considered as malignant based on the ICD-O-2 [
44], and c) it is incompatible to make comparisons with most of the other registries in Europe, which use the ICD-O-3. Future data analyses and upcoming reports from the CRC will be conducted using the ICD-O-3, which is a classification system for diseases for cancer registries, which extracts a detailed view of the real burden and studies only similar disease entities together (which are divided mainly to Lymphoid and Myeloid lineage).
Last, it is important to note that this research although pioneering for Greece, does not have a novelty at the European level, because cancer registration has been established in most European countries already; although the cancer burden is not 100% covered in all countries of Europe [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. In fact, Greece is among the last countries in Europe to have a consistent recording mechanism, which studies population-based rates for adults for approximately the 6% of its population (the Cretan population). However, it is of paramount importance to monitor the burden of cancer in every country and every area of the world; at the turn of the previous decade (2010), the 24% of the global cancer burden was being recorded and monitored, using a population based registration system [
2,
3]. Therefore, this study adds a small step towards the gradual increasing coverage and monitoring of the global cancer burden.
4.4. Implications of the Study
Our study depicted the overall burden, the time trends and the geographical distribution of these MN for a relatively long time period in Crete, covering an important information gap for the adult population. These population-based data are expected to add knowledge to researchers of cancer and to inform clinical medical doctors, public health authorities and the Greek Ministry of Health, by providing detailed data on the cancer burden of the area. Also, this article may contribute to the enhancement of cancer registration and cancer research in Crete and Greece. Our findings may guide the implementation of targeted preventive interventions and health promotion policies towards cancer control.
Additionally, the fact that many patients with NHL and HL and MM were diagnosed at an advanced stage of disease highlights the need for educational and screening programs for at least the at risk populations; this approach may lead to earlier diagnosis and consequently to better management of cases, increased survival and cost reduction.
4.5. The context of Crete Future Research Hypotheses
The context of Crete and its daily lifestyle, which were characterized by the Mediterranean diet, had a gradual transition to a Western pattern of life, during the last decades. This transition may have affected the cancer burden by increasing the exposure to risk factors. For example, there occurred many changes in Crete: (a) substitution of the Cretan-Mediterranean diet and lifestyle by a more Westernized pattern, (b) which is associated with higher rates of consumption of red meat, fast food and sweets, (c) contributing to high prevalence of obesity, (d) which are combined with high rates of smoking/passive smoking, (e) physical inactivity and (f) in some cases heavy alcohol consumption from an early age [
45,
46,
47,
48,
49,
50,
51,
52]. These lifestyle exposures may represent probable risk factors, which may are associated with the aforementioned three main key points of the study and may partly explain the rise of the rates [
53,
54,
55,
56,
57,
58,
59,
60].
5. Conclusions
The major findings and the key points, in combination with the changes in the lifestyle of the Cretans during the last decades may indicate the existence of potential risk factors, which may are associated with these disease entities in Crete. The identification of these potential risk factors and mechanisms, which may partly explain the spatio-temporal variation found in this research on hematologic MN in Crete, is an open field for research and needs to be studied in the future.
Author Contributions
The idea was conceived by S.L., D.SP. and C.L.; S.L., D.SP. and C.L. defined and developed the study design; conceptualization and methodology, S.L., D.SP. and C.L.; statistical analysis was performed by D.SP.; writing—original draft preparation, S.L. and D.SP.; writing—review and editing, S.L., D.SP., H.A.P., D.M. and C.L.; supervision, C.L., H.A.P. and D.M.; All authors have read and agreed to the published version of the manuscript.
Funding
The current study was not funded. Nevertheless, a small fund from the Region of Crete covered the travel expenses that were needed for the data collection process (for data from 2005 to 2013).
Institutional Review Board Statement
The Cancer Registry of Crete (CRC) holds a license from the Hellenic Data Protection Authority (Protocol number: 960/11-08-2009) and has adopted the rules for collecting, managing, and processing sensitive and personal data. All cancer-related information was recorded using a cryptographic coding system in accordance with the federal law principles and stored in the CMS server. No personal or individual-level data are or will be published, while no additional license was required for this study.
Informed Consent Statement
The Cancer Registry of Crete holds a license from the Hellenic Data Protection Authority (Protocol number: 960/11-08-2009) and has adopted the rules for collecting, managing, and processing sensitive and personal data. All information was recorded using a cryptographic coding system in accordance with the federal law principles and stored in the CMS server. No personal or individual level data are or will be published.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Raw data are not available due to personal/private data regulations. Further results could be shared for research purposes, upon request.
Acknowledgments
The authors would like to thank all participants of the expert panel, as well as the scientific associates of the Cancer Registry of Crete (CRC). Also, the authors would like to acknowledge the significant contribution of all the registrars of the CRC to the data collection, the data classification, and the performance of quality controls, namely: E. Frouzi, E. Vassilaki, M. Kouroupi, and T. Romanidou. Additionally, they also appreciate the support of the Region of Crete and the Heads and Directors of each hospital department and death registry in Crete.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maynadie, M.; Girodon, F.; Manivet-Janoray, I.; Mounier, M.; Mugneret, F.; Bailly, F.; Favre, B.; Caillot, D.; Petrella, T.; Flesch, M.; Carli, P. M. Twenty-five years of epidemiological recording on myeloid malignancies: data from the specialized registry of hematologic malignancies of Cote d’Or (Burgundy, France). Haematologica 2011, 96(1), 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R. L.; Torre, L. A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 2018; 68, (6), 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. , Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. European journal of cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.; Bray, F.; Costantini, A. S.; Tao, M. H.; Weiderpass, E.; Roman, E. , Time trends in the registration of Hodgkin and non-Hodgkin lymphomas in Europe. European journal of cancer 2007, 43(2), 391–401. [Google Scholar] [CrossRef] [PubMed]
- osetti, C.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Chatenoud, L.; Negri, E.; La Vecchia, C., Cancer mortality in Europe, 2005-2009, and an overview of trends since 1980. Annals of oncology: official journal of the European Society for Medical Oncology 2013, 24 (10), 2657-2671.
- Bosetti, C.; Levi, F.; Ferlay, J.; Lucchini, F.; Negri, E.; La Vecchia, C. Incidence and mortality from non-Hodgkin lymphoma in Europe: the end of an epidemic? International journal of cancer 2008, 123(8), 1917–23. [Google Scholar] [CrossRef] [PubMed]
- Broccia, G.; Cocco, P.; Casula, P.; Research Group on the Epidemiology of Lymphomas in, S. Incidence of non-Hodgkin’s lymphoma and Hodgkin’s disease in Sardinia, Italy: 1974-1993. Haematologica 2001, 86(1), 58–63. [Google Scholar]
- Carli, P. M.; Coebergh, J. W.; Verdecchia, A. Variation in survival of adult patients with haematological malignancies in Europe since 1978. EUROCARE Working Group. European journal of cancer 1998, 34 (14 Spec No), 2253–63. [Google Scholar] [CrossRef]
- Cartwright, R.; Brincker, H.; Carli, P. M.; Clayden, D.; Coebergh, J. W.; Jack, A.; McNally, R.; Morgan, G.; de Sanjose, S.; Tumino, R.; Vornanen, M. The rise in incidence of lymphomas in Europe 1985-1992. European journal of cancer 1999, 35(4), 627–33. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, R. A.; Gilman, E. A.; Gurney, K. A. Time trends in incidence of haematological malignancies and related conditions. British journal of haematology 1999, 106(2), 281–95. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Minicozzi, P.; Sant, M.; Dal Maso, L.; Brewster, D. H.; Osca-Gelis, G.; Visser, O.; Maynadie, M.; Marcos-Gragera, R.; Troussard, X.; Agius, D.; Roazzi, P.; Meneghini, E.; Monnereau, A.; Group, E.-W. Survival variations by country and age for lymphoid and myeloid malignancies in Europe 2000-2007: Results of EUROCARE-5 population-based study. European journal of cancer 2015, 51(15), 2254–2268. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Sant, M.; Coleman, M. P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; Bielska-Lasota, M.; Engholm, G.; Nennecke, A.; Siesling, S.; Berrino, F.; Capocaccia, R.; Group, E.-W. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. The Lancet. Oncology 2014, 15(1), 23–34. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T. C.; Medeiros, L. J.; Jaffe, E. S. Non-Hodgkin’s lymphoma. Cancer 1995, 75 (1 Suppl), 370–80. [Google Scholar] [CrossRef]
- Le Guyader-Peyrou, S.; Belot, A.; Maynadie, M.; Binder-Foucard, F.; Remontet, L.; Troussard, X.; Bossard, N.; Monnereau, A.; French network of cancer, r., Cancer incidence in France over the 1980-2012 period: Hematological malignancies. Revue d’epidemiologie et de sante publique 2016, 64 (2), 103-12. [CrossRef]
- Novak, I.; Jaksic, O.; Kulis, T.; Batinjan, K.; Znaor, A. Incidence and mortality trends of leukemia and lymphoma in Croatia, 1988-2009. Croatian medical journal 2012, 53(2), 115–23. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Hjalgrim, H.; Glimelius, B.; Rostgaard, K.; Pukkala, E.; Askling, J., Incidence of non-Hodgkin’s lymphoma in Sweden, Denmark, and Finland from 1960 through 2003: an epidemic that was. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2006, 15 (7), 1295-300.
- Viel, J. F.; Fournier, E.; Danzon, A., Age-period-cohort modelling of non-Hodgkin’s lymphoma incidence in a French region: a period effect compatible with an environmental exposure. Environmental health: a global access science source 2010, 9, 47.
- Sant, M.; Aareleid, T.; Berrino, F.; Bielska Lasota, M.; Carli, P. M.; Faivre, J.; Grosclaude, P.; Hedelin, G.; Matsuda, T.; Moller, H.; Moller, T.; Verdecchia, A.; Capocaccia, R.; Gatta, G.; Micheli, A.; Santaquilani, M.; Roazzi, P.; Lisi, D.; Group, E. W., EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Annals of oncology: official journal of the European Society for Medical Oncology 2003, 14 Suppl 5, v61-118.
- Sant, M.; Allemani, C.; Santaquilani, M.; Knijn, A.; Marchesi, F.; Capocaccia, R.; Group, E. W., EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. European journal of cancer 2009, 45 (6), 931-91.
- Sant, M.; Capocaccia, R.; Coleman, M. P.; Berrino, F.; Gatta, G.; Micheli, A.; Verdecchia, A.; Faivre, J.; Hakulinen, T.; Coebergh, J. W.; Martinez-Garcia, C.; Forman, D.; Zappone, A.; Group, E. W. Cancer survival increases in Europe, but international differences remain wide. European journal of cancer 2001, 37(13), 1659–67. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Minicozzi, P.; Mounier, M.; Anderson, L. A.; Brenner, H.; Holleczek, B.; Marcos-Gragera, R.; Maynadie, M.; Monnereau, A.; Osca-Gelis, G.; Visser, O.; De Angelis, R.; Group, E.-W. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. The Lancet. Oncology 2014, 15(9), 931–42. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, A.; Francisci, S.; Brenner, H.; Gatta, G.; Micheli, A.; Mangone, L.; Kunkler, I.; Group, E.-W. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. The Lancet. Oncology 2007, 8(9), 784–96. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, A.; Guzzinati, S.; Francisci, S.; De Angelis, R.; Bray, F.; Allemani, C.; Tavilla, A.; Santaquilani, M.; Sant, M.; Group, E. W. Survival trends in European cancer patients diagnosed from 1988 to 1999. European journal of cancer 2009, 45(6), 1042–66. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J. A.; Land, K. J.; McKenna, R. W. Leukemias, myeloma, and other lymphoreticular neoplasms. Cancer 1995, 75 (1 Suppl), 381–94. [Google Scholar] [CrossRef]
- Pulte, D.; Jansen, L.; Castro, F. A.; Emrich, K.; Katalinic, A.; Holleczek, B.; Brenner, H.; Group, G. C. S. W. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. British journal of haematology 2015, 171(2), 189–196; [Google Scholar] [CrossRef] [PubMed]
- Pulte, D.; Redaniel, M. T.; Brenner, H.; Jansen, L.; Jeffreys, M., Recent improvement in survival of patients with multiple myeloma: variation by ethnicity. Leukemia & lymphoma 2014, 55 (5), 1083-9. [CrossRef]
- Karim-Kos, H. E.; de Vries, E.; Soerjomataram, I.; Lemmens, V.; Siesling, S.; Coebergh, J. W. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. European journal of cancer 2008, 44(10), 1345–89. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Serra, L.; Renart, G.; Osca-Gelis, G.; Comas, R.; Vilardell, L.; Gallardo, D.; Marcos-Gragera, R. Incidence and survival of Hodgkin lymphoma patients in Girona (Spain) over three decades: a population-based study. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation 2017, 26 Joining forces for better cancer registration in Europe, S164-S169. [CrossRef]
- Berrino, F.; De Angelis, R.; Sant, M.; Rosso, S.; Bielska-Lasota, M.; Coebergh, J. W.; Santaquilani, M.; group, E. W. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. The Lancet. Oncology 2007, 8(9), 773–83. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Andrie, E.; Dessypris, N.; Dikalioti, S. K.; Trichopoulos, D.; Childhood Hematology-Oncology, G. Incidence and characteristics of childhood Hodgkin’s lymphoma in Greece: a nationwide study (Greece). Cancer causes & control: CCC 2006, 17(2), 209–15. [Google Scholar] [CrossRef]
- Petridou, E. T.; Pourtsidis, A.; Dessypris, N.; Katsiardanis, K.; Baka, M.; Moschovi, M.; Polychronopoulou, S.; Koliouskas, D.; Sidi, V.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; Belechri, M.; La Vecchia, C.; Curado, M. P.; Skalkidis, I. Childhood leukaemias and lymphomas in Greece (1996-2006): a nationwide registration study. Archives of disease in childhood 2008, 93(12), 1027–32. [Google Scholar] [CrossRef] [PubMed]
- Avgerinou, C.; Alamanos, Y.; Zikos, P.; Lampropoulou, P.; Melachrinou, M.; Labropoulou, V.; Tavernarakis, I.; Aktypi, A.; Kaiafas, P.; Raptis, C.; Kouraklis, A.; Karakantza, M.; Symeonidis, A. The incidence of myelodysplastic syndromes in Western Greece is increasing. Annals of hematology 2013, 92(7), 877–87. [Google Scholar] [CrossRef] [PubMed]
- Vlachonikolis, I. G.; Philalithis, A. E.; Brittan, Y.; Georgoulias, V. Mortality from malignant neoplasms in Crete, 1992-1993. Journal of epidemiology and community health 1998, 52(2), 126–7; [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International classification of diseases (oncology). WHO. Available online: https://icd.who.int/browse10/2019/en#/C81-C96 (accessed on 8 October 2023).
- Cleries, R.; Esteban, L.; Borras, J.; Marcos-Gragera, R.; Freitas, A.; Carulla, M.; Buxo, M.; Puigdefabregas, A.; Izquierdo, A.; Gispert, R.; Galceran, J.; Ribes, J. Time trends of cancer incidence and mortality in Catalonia during 1993-2007. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2014, 16(1), 18–28; [Google Scholar]
- Airtum Working Group, Italian cancer figures, report 2009: Cancer trend (1998-2005). Epidemiol Prev 2009, 33 (4-5 Suppl 1), 1-168.
- Galceran, J.; Ameijide, A.; Carulla, M.; Mateos, A.; Quiros, J. R.; Rojas, D.; Aleman, A.; Torrella, A.; Chico, M.; Vicente, M.; Diaz, J. M.; Larranaga, N.; Marcos-Gragera, R.; Sanchez, M. J.; Perucha, J.; Franch, P.; Navarro, C.; Ardanaz, E.; Bigorra, J.; Rodrigo, P.; Bonet, R. P.; Group, R. W., Cancer incidence in Spain, 2015. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2017, 19 (7), 799-825.
- Renshaw, C.; Ketley, N.; Moller, H.; Davies, E. A. Trends in the incidence and survival of multiple myeloma in South East England 1985-2004. BMC cancer 2010, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Allemani, C.; Tereanu, C.; De Angelis, R.; Capocaccia, R.; Visser, O.; Marcos-Gragera, R.; Maynadie, M.; Simonetti, A.; Lutz, J. M.; Berrino, F.; Group, H. W. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010, 116(19), 3724–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. British journal of cancer 2011, 105(11), 1684–92. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Fabrega, A.; Morea, D.; Aunon-Sanz, C.; Granada, I.; Roncero, J. M.; Blanco, A.; Kelleher, N.; Buch, J.; Saez, M.; Marcos-Gragera, R. Population-based incidence of lymphoid neoplasms: Twenty years of epidemiological data in the Girona province, Spain. Cancer epidemiology 2019, 58, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program. SEER, Cancer Statistics. Available online: https://seer.cancer.gov/ (accessed on 1 September 2023).
- Morton, L. M.; Wang, S. S.; Devesa, S. S.; Hartge, P.; Weisenburger, D. D.; Linet, M. S. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006, 107(1), 265–76; [Google Scholar] [CrossRef] [PubMed]
- Maynadie, M.; De Angelis, R.; Marcos-Gragera, R.; Visser, O.; Allemani, C.; Tereanu, C.; Capocaccia, R.; Giacomin, A.; Lutz, J. M.; Martos, C.; Sankila, R.; Johannesen, T. B.; Simonetti, A.; Sant, M.; Group, H. W. Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica 2013, 98(2), 230–8. [Google Scholar] [CrossRef]
- Roditis, M. L.; Parlapani, E. S.; Tzotzas, T.; Hassapidou, M.; Krassas, G. E. Epidemiology and predisposing factors of obesity in Greece: from the Second World War until today. Journal of pediatric endocrinology & metabolism: JPEM 2009, 22(5), 389–405. [Google Scholar] [CrossRef]
- Filippidis, F. T.; Vardavas, C. I.; Loukopoulou, A.; Behrakis, P.; Connolly, G. N.; Tountas, Y. Prevalence and determinants of tobacco use among adults in Greece: 4 year trends. European journal of public health 2013, 23(5), 772–6; [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E. K. The protective effect of the Mediterranean diet: focus on cancer and cardiovascular risk. Medical principles and practice: international journal of the Kuwait University, Health Science Centre 2011, 20(2), 103–11; [Google Scholar] [CrossRef] [PubMed]
- Sifaki-Pistolla, D.; Lionis, C.; Georgoulias, V.; Kyriakidis, P.; Koinis, F.; Aggelaki, S.; Tzanakis, N. Lung cancer and tobacco smoking in Crete, Greece: reflections from a population-based cancer registry from 1992 to 2013. Tobacco induced diseases 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Papandreou, M.; Galanos, A.; Kortianou, E.; Tsepis, E.; Kalfakakou, V.; Evangelou, A. Smoking and physical activity interrelations in health science students. Is smoking associated with physical inactivity in young adults? Hellenic journal of cardiology: HJC = Hellenike kardiologike epitheorese 2012, 53(1), 17–25; [Google Scholar] [PubMed]
- Hatzis, C. M.; Papandreou, C.; Patelarou, E.; Vardavas, C. I.; Kimioni, E.; Sifaki-Pistolla, D.; Vergetaki, A.; Kafatos, A. G. A 50-year follow-up of the Seven Countries Study: Prevalence of cardiovascular risk factors, food and nutrient intakes among Cretans. Hormones 2013, 12(3), 379–85. [Google Scholar] [CrossRef] [PubMed]
- Tourlouki, E.; Matalas, A. L.; Bountziouka, V.; Tyrovolas, S.; Zeimbekis, A.; Gotsis, E.; Tsiligianni, I.; Protopapa, I.; Protopapas, C.; Metallinos, G.; Lionis, C.; Piscopo, S.; Polychronopoulos, E.; Panagiotakos, D. B. Are current dietary habits in Mediterranean islands a reflection of the past? Results from the MEDIS study. Ecology of food and nutrition 2013, 52(5), 371–86; [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Magkos, F.; Christakis, G.; Kafatos, A. G. Twenty-year dynamics in adiposity and blood lipids of Greek children: regional differences in Crete persist. Acta paediatrica 2005, 94(7), 859–65; [Google Scholar] [CrossRef] [PubMed]
- Casey, R.; Piazzon-Fevre, K.; Raverdy, N.; Forzy, M. L.; Tretare, B.; Carli, P. M.; Maynadie, M. Case-control study of lymphoid neoplasm in three French areas: description, alcohol and tobacco consumption. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation 2007, 16(2), 142–50; [Google Scholar] [CrossRef] [PubMed]
- Diver, W. R.; Patel, A. V.; Thun, M. J.; Teras, L. R.; Gapstur, S. M. The association between cigarette smoking and non-Hodgkin lymphoid neoplasms in a large US cohort study. Cancer causes & control: CCC 2012, 23(8), 1231–40; [Google Scholar] [CrossRef] [PubMed]
- Kamper-Jorgensen, M.; Rostgaard, K.; Glaser, S. L.; Zahm, S. H.; Cozen, W.; Smedby, K. E.; Sanjose, S.; Chang, E. T.; Zheng, T.; La Vecchia, C.; Serraino, D.; Monnereau, A.; Kane, E. V.; Miligi, L.; Vineis, P.; Spinelli, J. J.; McLaughlin, J. R.; Pahwa, P.; Dosman, J. A.; Vornanen, M.; Foretova, L.; Maynadie, M.; Staines, A.; Becker, N.; Nieters, A.; Brennan, P.; Boffetta, P.; Cocco, P.; Hjalgrim, H., Cigarette smoking and risk of Hodgkin lymphoma and its subtypes: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph). Annals of oncology: official journal of the European Society for Medical Oncology 2013, 24 (9), 2245-55. [CrossRef]
- Murphy, F.; Kroll, M. E.; Pirie, K.; Reeves, G.; Green, J.; Beral, V. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. British journal of cancer 2013, 108(11), 2390–8; [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Stocks, T.; Spath, D.; Hjartaker, A.; Lindkvist, B.; Hallmans, G.; Jonsson, H.; Bjorge, T.; Manjer, J.; Haggstrom, C.; Engeland, A.; Ulmer, H.; Selmer, R.; Concin, H.; Stattin, P.; Schlenk, R. F. Metabolic factors and blood cancers among 578,000 adults in the metabolic syndrome and cancer project (Me-Can). Annals of hematology 2012, 91(10), 1519–31. [Google Scholar] [CrossRef] [PubMed]
- S Sergentanis, T. N.; Kanavidis, P.; Michelakos, T.; Petridou, E. T. Cigarette smoking and risk of lymphoma in adults: a comprehensive meta-analysis on Hodgkin and non-Hodgkin disease. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation 2013, 22(2), 131–50; [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T. N.; Zagouri, F.; Tsilimidos, G.; Tsagianni, A.; Tseliou, M.; Dimopoulos, M. A.; Psaltopoulou, T., Risk Factors for Multiple Myeloma: A Systematic Review of Meta-Analyses. Clinical lymphoma, myeloma & leukemia 2015, 15 (10), 563-77 e1-3. [CrossRef]
- Yang, T. O.; Cairns, B. J.; Kroll, M. E.; Reeves, G. K.; Green, J.; Beral, V.; Million Women Study, C. Body size in early life and risk of lymphoid malignancies and histological subtypes in adulthood. International journal of cancer 2016, 139(1), 42–9. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).