Introduction
The last two decades the initial Dresden CXL protocol has changed the treatment paradigm for the management of keratoconus and corneal ectasia.[1–10]
The technique has evolved to include higher fluence CXL and the use of a therapeutic customized surface ablation, to facilitate riboflavin absorption into the corneal stroma as well as the actual CXL process, as UV light in a cornea that has undergone even the slightest surface ablation is absorbed in the anterior cornea stroma unobstructed.
Our investigative team has introduced the technique called The Athens Protocol CXL,[
11,
12,
13,
14] subsequently many other clinicians globally, have applied the same or modified techniques with comparable outcomes.[
15,
16,
17,
18,
19,
20,
21] Globally, outside the US, the first and most common technology that has been employed as an adjunct to CXL is based on the topography-guided platform by the excimer lasers initially by Alcon/WaveLight (Erlagen, Germany) and later additionally by Schwind Amaris (Kleinostheim, Sweden), IVIS (Taranto, Italy) and Nidek (Gamagori, Aichi, Japan).
Our clinical team has had the opportunity, to employ CXL for keratoconus and corneal ectasia both in Europe as noted above, commencing 2 decades ago, and subsequently in the United States, once CXL became approved in 2013, the same year topography-guided treatments for primary myopic eyes became FDA approved as well. Keratoconus and Ectasia patients advised in the past to be treated with the Athens Protocol outside the US-usually in Canada, or even travelled to Europe-were now able to have this treatment as an off-label excimer laser and CXL application, in the United States.
Pivotal to the clinical work described herein, are the practical limitations-in existence even currently-regarding the application of the Athens Protocol excimer surface ablation in the United States. Of note here is that the Alcon/Wavelight excimer laser is the only current commercially available and FDA approved device to offer topography-guided treatments within the United States. The device, within the US approved specifications, is allowing only treatments of minimal 6mm optical zone in its topography-guided platform (Contura, Alcon, Fort Worth, TX). The spectrum of refraction treatment along with the topography guided normalization is limited to: zero refraction, myopic or myopic-astigmatic spherocylindrical treatment. No hyperopic or hyperopic-astigmatic treatments are feasible within the US specifications for the device. Additionally, there is no option for a PTK mode, to be used to remove the epithelium with the excimer laser, an integral step of the Athens Protocol procedure. A review of the technique and technology that our clinical team and other global clinicians have employed are described and summarized in our editorial by invitation in the Journal of Cornea.[
22]
In this study we report a consecutive case series of this modified technique of the original Athens Protocol CXL, within the excimer laser treatment specifications available in the United States currently.
Methods
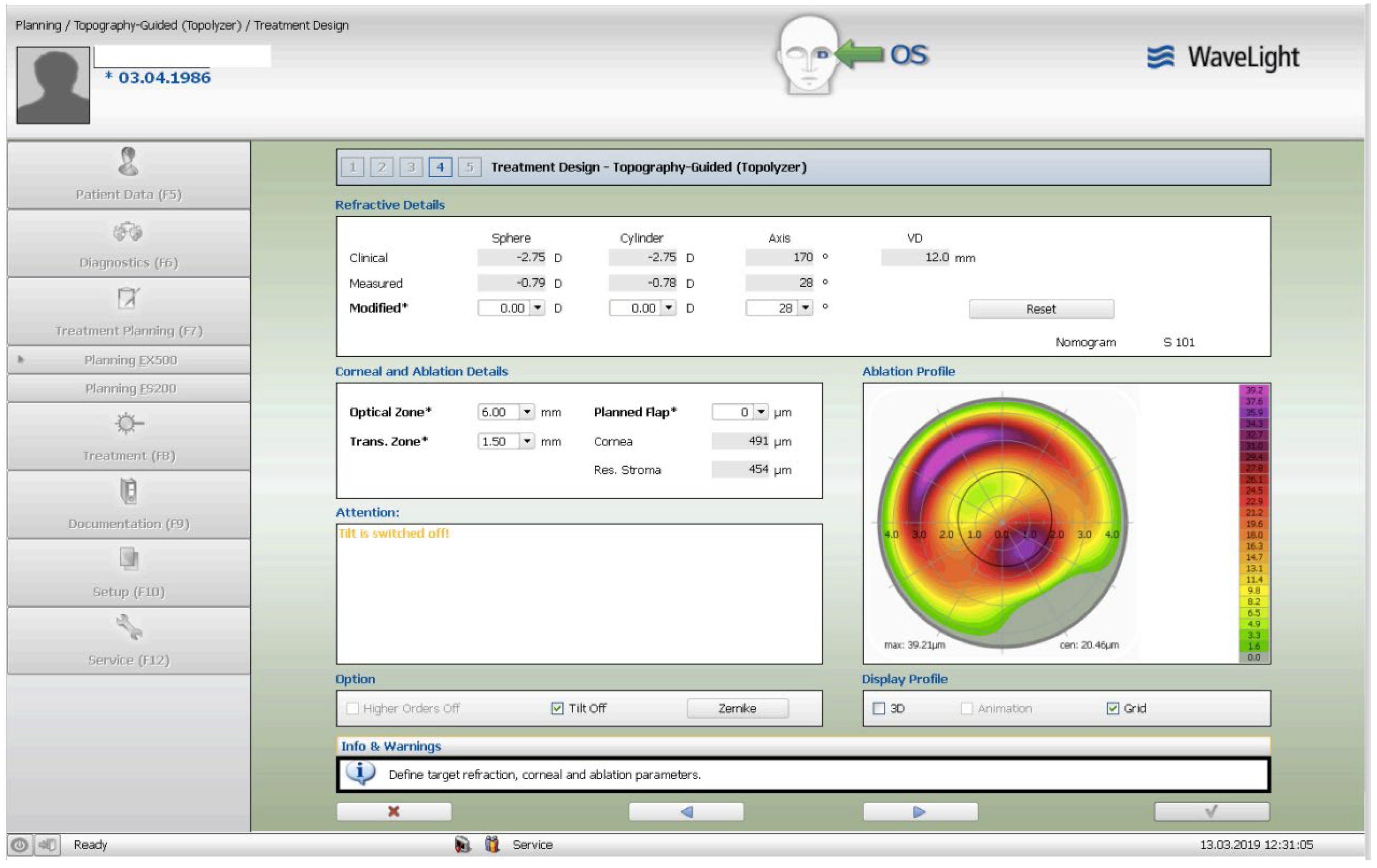
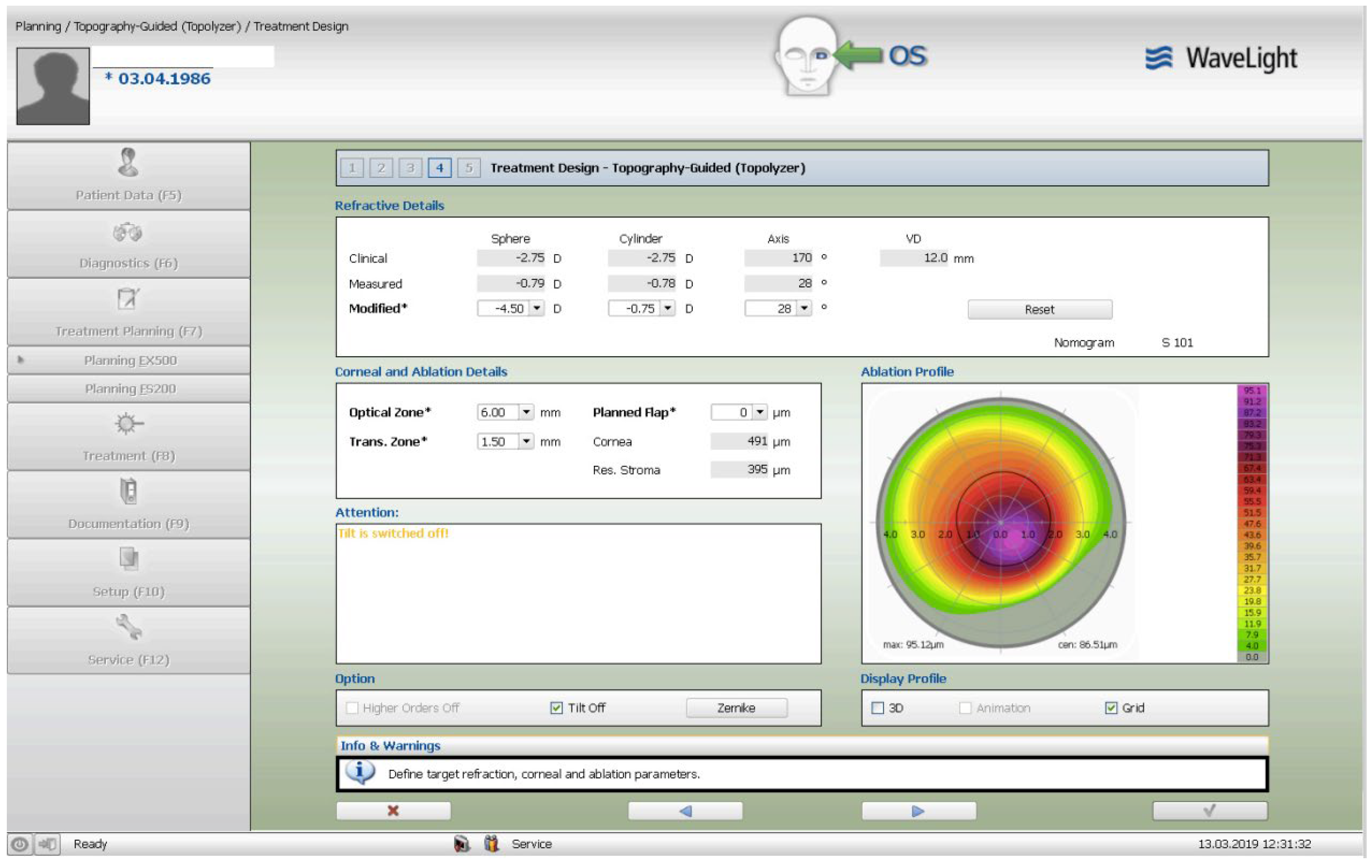
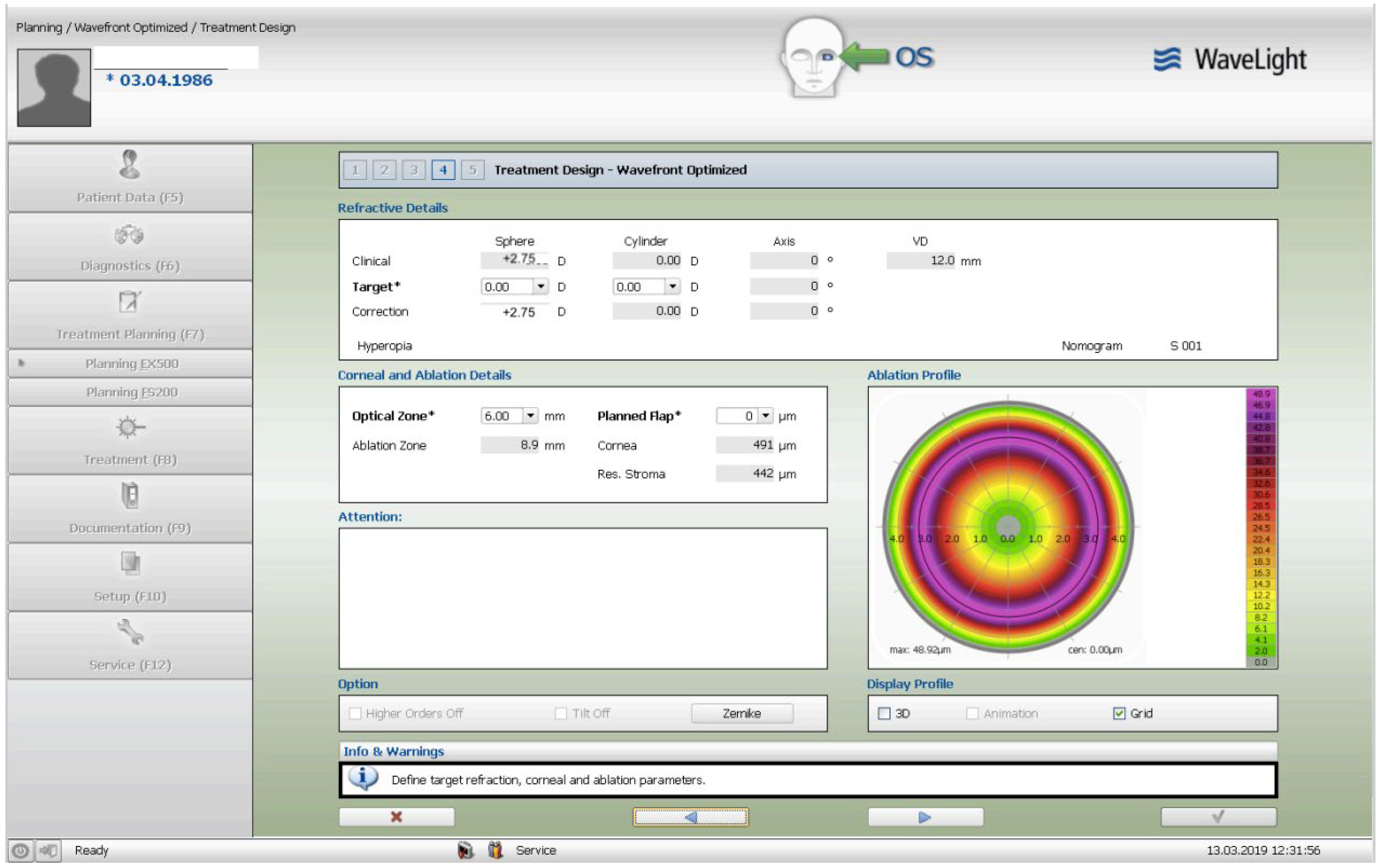
The treatment consisted of a corneal surface excimer laser ablation normalization using the topography-guided (Contura-Alcon/Wavelight, Ft. Worth, Texas, USA) myopic ablation for customized corneal re-shaping using a 6mm optical zone. The epithelial removal was accounted for by adding a -2.75 diopters correction in the topography-guided normalizing surface ablation followed by, a second, wavefront-optimized hyperopic excimer treatment of + 2.75 diopters at 6mm optical zone. The 2 sequential excimer ablations were followed by the corneal crosslinking (CXL). This revised treatment-protocol negated the use of two treatment cards-an excimer treatment necessity for all excimer lasers in the USA-one for the Contura topography-guided platform treatment, FDA approved for myopic treatments and used off-label in our US Athens Protocol described herein, and a second one, using a wavefront-optimized hyperopic treatment (WFO) with the same optical zone of 6 mm.
This is a retrospective review of consecutive case series, all patients had also initially provided in their informed consent, approval for clinical data analysis. This study is adherent to the declaration of Helsinki. The retrospective review of data was approved as a study by our Institutions Ethics committee (Laservision ASU EC). This consecutive case-series included patients with established keratoconus that was found to be progressive, and intolerant to contact lens use. All patients received detailed informed consent in regard to the advantages and potential disadvantages that combining a therapeutic topography-guided surface ablation with higher fluence CXL may have. We followed this cohort of patients for up to three years.
Once the Contura treatment was designed in a similar fashion with our Athens Protocol cases outside the US, an additional -2.75 diopters of myopia was added to that refractive error planned to be included with topography-guided platform-usually 1 to 2 diopters of myopia and up to 2.5 diopters of astigmatism-then the same absolute number of +2.75 was used for the WFO hyperopic treatment to follow with the same optical zone of 6mm. The goal-as noted above-was to account for epithelial removal along with the therapeutic topography-guided surface ablation. Following the two sequential ablations, we used saline-diluted riboflavin 1% for soaking for five minutes, and then 6mW/cm2 of UV light were used with the PXL Platinum 330 device (Peschke MedTech, Germany), in continuous mode (without pulsing) and without supplemental oxygen. We followed for 36 months the refractive error, visual function, and all the other parameters, such as visual acuity, cornea clarity, keratometry, topography, pachymetry, as well as endothelial cell density.
Discussion
We have reported [
11,
12,
13,
14,
22] and many other investigators [
15,
16,
17,
18,
19,
20,
21] have used the Athens Protocol-or subsequent modifications of it-globally, with comparable results summarized in our recent publication in the Journal of Cornea.[
21]
In recent ophthalmic practice globally, corneal crosslinking (CXL)-sometimes called with its older term: “collagen” crosslinking- emerged and has been established as a mainstream surgical intervention to stabilize progressive keratoconus and corneal ectasia.[
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]
Cornea stabilization has been reported to have high efficacy and safety, nevertheless visual function even when cornea ectasia has been stabilized can be challenging especially in patient’s intolerance to contact lens use.[
12]
We investigated early-on the speculation of whether: in a previously stabilized corneal ectasia, a therapeutic excimer laser surface ablation can be employed to normalize the cornea irregularity in the most central part of the cornea and improve visual function.[
11,
12,
13,
14] This treatment carries the disadvantage of stromal tissue removal, so in essence further stromal thinning, a key negative pathophysiology feature in corneal ectasia. We initially applied the Athens protocol [
11,
12,
13] in cases that were essentially scheduled to undergo penetrating keratoplasty-as a last resort for visual rehabilitation. The protocol as reported comprised of a surface topography-guided excimer ablation, employing-at the time-the Wavelight 200Hz Allegretto excimer laser (Wavelight, Erlagen, Germany, later acquired by Alcon), described initially as “partial” PRK (photorefractive keratectomy) referring to “partial” in regard to the manifest subjective refraction of each case, as in essence only 1 to 2 diopters of spherocylindrical were included, but in essence served more as a phototherapeutic keratectomy (PTK), customized in diameter, transition zone and refractive error -if any was added- to regularize the ectasia induced irregular corneal power refractive changes, that were associated with respective loss of functional visual acuity and visual function.
This approach carried the obvious disadvantage when applied after the CXL procedure at a later time-besides the stromal thinning noted above-the actual removal of cross-linked stroma. in CXL for ectasia the anterior stroma appears to become stronger, and this biomechanical effect diminishes in inner-deeper stromal layers.[
1,
2,
3,
4]
The initial outcomes that we reported were compelling: keratoplasty was essentially not anymore indicated as robust visual rehabilitation was achieved, in some cases even without refractive correction.[
11,
12,
13,
14]
The next evolution step of our investigative and clinical work was the use of higher fluence UV light to establish accelerated shorted duration CXL intervention was to combine higher fluence CXL with a surface excimer laser corneal reshaping intervention, customized by topography in the same treatment session. Our outcomes were first reported in 2005 at ARVO, with robust data not only in corneal stabilization, but also in enhancement of the cornea normalization and recorded depth of the CXL effect, documenting strong synergy when the 2 procedures were combined.[
11] The retrospective comparison data of the sequential approach-CXL first and topography-guided PTK at least 6 months later-compared to: same-day, combined PTK and CXL were reported in 2009.[
12] A plethora of subsequent reports by our group,[
13,
14,
22] along with many other investigators[
15,
16,
17,
18,
19,
20,
21] documented basically similar results confirming these findings.
In essence the same-day combined CXL-therapeutic PRK technique, termed “the Athens Protocol”, targeted the structural stabilization of cornea ectasia while augmenting in multiplicity the refractive normalization and function by significantly optimizing the central 5 mm anterior corneal curvature. This approach was unable-since the ablation was mainly based on surface reflection topography data-to address posterior corneal irregularity, mainly coma.
Therefore, the common primary objective among all clinicians attempting this combined customized surface ablation with CXL, was also to minimize excimer stromal tissue ablation-by customizing the therapeutic surface ablation to mainly address to irregular astigmatism and central refractive coma.
All reports support that these corneal changes have been pivotal in improvement of uncorrected and corrected distance visual acuity (UDVA & CDVA, respectively), as well as reduction of keratometry asymmetry indices.[
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]
Summary of Athens Protocol Technique Principles and Potential Advantages
As introduced: a limit of stromal tissue removal by the excimer laser ablation from the thinnest cornea area was initially set at 50um, as measured initially by scanning-slit tomography and later by Scheimpflug and/or OCT tomography, a limit that has been adapted by most subsequent technique modifications.[
11]
The customized corneal surface ablation was topography-guided by most investigators, while wavefront-guided by few others.[
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]
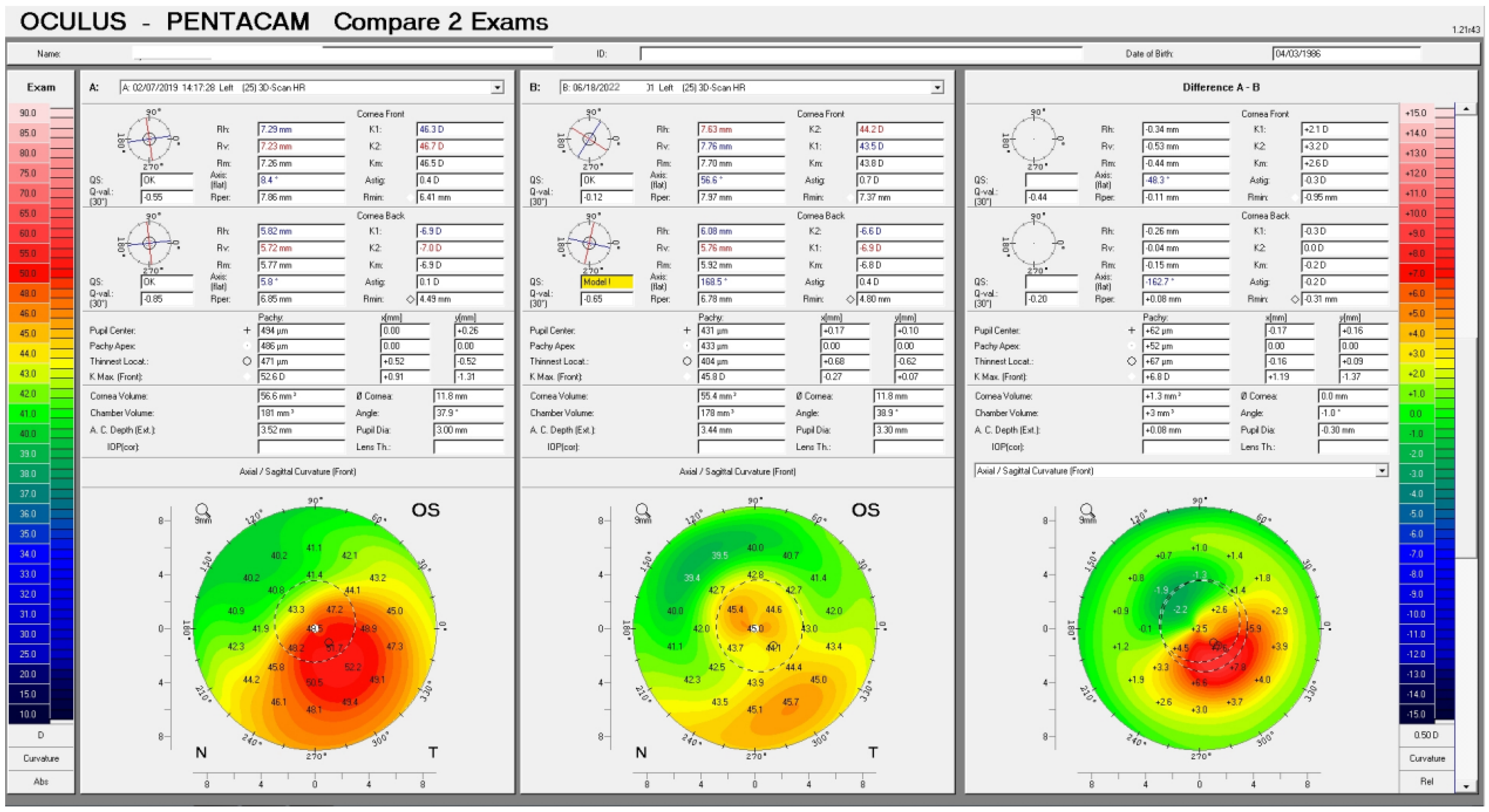
Topography-guided customization of the excimer ablation is driven by topography captured data, and even when set to correct no refractive error, it provides an ablation algorithm attempting to “flatten” the most steepened by ectasia, corneal area, and “steepen” the adjacent-usually superior-most flattened corneal area within the central 5 to 6.5mm of the cornea. This concept is best illustrated in
Figure 1.
Most such “therapeutic” excimer surface ablations combined in the same session with CXL employ higher fluence UV irradiance for a shorter time exposure, commonly termed “accelerated” CXL, for usually total energy delivered 5.5 to 7 Joules. It is therefore invariably “epithelium-off”, as even Bowman’s membrane has been ablated by the surface ablation, prior to the riboflavin solution soaking and the UV light exposure. A minimum stromal thickness of 400 um is pursued, although we have reported in advanced cases, effective treatment up to a 380 m minimum total corneal thickness, measured prior to epithelial removal.[
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]
.
Apart from the obvious advantages of combined CXL + PRK of the Athens Protocol technique and its modifications having the efficiency of completing the operative procedure in one day with a single postoperative period, other basic reported benefits include:
1-Higher efficacy of central cone flattening combined with respective superior cornea steepening, surpassing the overall central corneal curvature normalization as would be predicted by sequential CXL and PRK, achieving sustained stability even in over 10 years follow-up.[
12]
2-A uniform, “deeper” CXL effect extending both deeper to 60-80% residual stromal thickness and wider, up to 9mm corneal diameter, as evident on slit lamp biomicroscopic as well as by anterior segment OCT “CXL demarcation line”.
3-Less post-operative scarring associated with combined vs. sequential protocol cases- we found this to be significantly reduced by maximizing the transition zone of the topography-guided ablation.[
22]
Excimer ablation of a thinned cornea undergoing ectasia, remains a serious concern for the potential risk of biomechanically weakening the residual stream bed and thereby increase the potential risk for yet further ectasia. Yet the long-term combined procedure results fortunately do not identify such concerns as the cornea curvatures remain flattened and normalized (reductions ranging from 8 to 20 Diopters), suggesting synergy of “cone-flattening” and corneal curvature normalization, presumably consequent to the “deeper” and “broader” CXL effect. The minimum stromal thickness of 350 um, was suggested as the lower limit of performing this approach,[
11] necessitating respective adjustment of the “partial” -in regard to refractive error targeted -corneal surface ablation, not to exceed this planned lower limit.
Several long-term reports review of the technique outcomes, and further elaborate on the key technique steps.[
14,
15,
22,
23,
24,
25,
27,
28,
29,
30]
In summary, the preponderance of published evidence supports combined CXL, “plus” customized minimal tissue ablation PRK, to be a safe and effective strategy, long term, for anatomical and visual management of keratoconus and post-LASIK ectasia.
Although almost half of treated eyes remain variably ametropic-as the ablation limitations described above, cannot address the full refractive error-most cases have increased ability to employ visual rehabilitation with spectacles and/or contact lenses, even subsequent phakic IOL implantation.[
28] Additionally reported, improved soft contact lens use comfort tolerance and even in some cases rigid gas-permeable contact lens comfort tolerance following CXL, as CDVA demonstrates significant visual improvement.
Complications associated with the combined CXL + PRK technique and the management thereof have been reported.[
29]
1-Delayed corneal re-epithelization beyond that anticipated for a PRK procedure. To promote more efficient re-epithelialization, reduction of topical corticosteroids and addition of autologous platelet rich plasma[
20] are beneficial.
2-Cornea stromal haze over the ablation areas can develop as late as a year post-procedure in pediatric patients, possibly in response to intense natural UV light exposure.
3-Potential deep stromal scarring associated with the CXL as evident by slit-lamp biomicroscopic and anterior segment OCT, is not specific to Athens Protocol procedures, rather should be carefully observed and managed during the first 2 months following the procedure.
4-Potential progressive additional flattening effect developing years after treatment has been reported in <2% of cases. This may result in significant hyperopic shift requiring revision of optical visual correction and/or additional refractive surgical intervention.
Residual stromal pachymetry is a principle of precaution and concern:
The strongest limitation and safety consideration is the limit of PRK tissue removal in an already thinned cornea that as mentioned above we have desired to be over 400um total corneal thickness at the thinnest point prior to the partial in thickness surface ablation, a concern which also merits informed consent discussion of risks, benefits, and alternatives. Based on this concern, we sometimes limit the surface ablation to solely epithelial removal by the excimer laser (50um depth, 7mm diameter) that in areas of epithelial thinning to <50 um there is also some Bowman’s layer and underlying stroma selective ablation that can enhance the effect of CXL-for example if at the “peak’ of the cone the epithelium has remodeled to 30 um, a 50 um PTK will remove the 30 um of epithelium, the respective 10 um of Bowman’s-although in these advanced cases it may be significantly thinner and the residual ablation thickness to 50 Um from the corresponding anterior stroma. As noted above a minimum residual stromal thickness of 350 um is targeted following the partial thickness surface ablation and in advanced cases up to 330 um,[
13] as CXL in thinner stromal situations may not stabilize enough corneal stromal “volume” and as a second concern the UV penetrance during the procedure may become cytotoxic to the corneal endothelium.
Another potential CXL modification is the customized application of the UV irradiation at variable fluence and variable pattern profile, thereby utilizing CXL as an enhanced flattening tool when compared to standard CXL with uniform UV light application
.[
22]
Continued eye-rubbing appears to be a pivotal factor in the mechanism of ectasia development: It is now likely that eye rubbing, even during sleep, is one of the pivotal activities that contribute to development and progression of keratoconus and cornea ectasia. Thus, proper education and continued reinforcement of eye-rubbing avoidance can be highly beneficial in ectasia stabilization and long-term prognosis.[
22,
31,
32]
The most studied and utilized platform for topography-guided corneal reshaping addresses the anterior corneal curvature. Thus, visual rehabilitation is strongly influenced by the actual cone location regarding the cornea center. Severely oblique cones will significantly normalize with this technique regarding anterior cornea curvature but will still retain irregular posterior curvature that can functionally limit vision. Wavefront-guided or ray-tracing customization of the therapeutic surface ablation used in the Athens Protocol CXL, and modified techniques have been reported to address this point. Potential advantages noted is the measurement and potential normalization of the total corneal high order aberrations with less tissue removal planned over the thinnest cone area as raytracing appears to address a typical inferior corneal ectasia as cornea tilt about the total eye refractive system, additionally addressing the refractive role of both the anterior and posterior corneal curvature.[
22]
As cornea cross-linking has changed the management paradigm for keratoconus, revisiting the keratoconus diagnostic criteria has become increasingly crucial.
Utilizing modern corneal diagnostics such as Scheimpflug corneal tomography and anterior segment OCT corneal tomography and epithelial mapping, as well as high frequency ultrasound corneal epithelial mapping, early signs of ectasia become detectable, even when visual function, slit lamp biomicroscopic and traditional corneal topography appear normal.[
22] Cornea epithelial remodeling may be able to mask traditional topographic imaging signs in early corneal ectasia, as well as explain corneal topographic steepening that is not associated with ectasia progression but rather local epithelial thickening of the cone apex.
Recent reports support the consideration of keratoconus screening in first- and second-degree family members as becoming crucial in early diagnosis.As prevention is-in so many medical contexts-the best medicine, early detection of keratoconus and other ectasias affords the best opportunity for arresting progression and optimizing vision in the earliest stages of these important corneal conditions.[
22]
We introduce herein, this novel technique utilizing the approved and available parameters for the specific excimer laser within the United States. As noted above, it is an off-label application for the US, enabling similar treatment protocol for the Athens Protocol. No postoperative problems were encountered within this small group of cases. All cases had the same post-operative treatment: Prednisolone acetate (Pred Forte, Allergan, Irvine, CA, USA) four times a day, Bromfernac ophthalmic solution (Prolensa-Bausch and Lomb, USA) for 1-2 days, depending on post-operative discomfort, up to four times a day one drop per eye, per need, and ofloxacin topical antibiotic (Ocuflox-Allergan, Irvine, CA, USA) used four times a day until epithelialization usually by day 5. The treatment with Pred Forte QID for a month, followed by Loteprednol ophthalmic ointment (Lotemax ointment, Bausch and Lomb, USA) used at bedtime once a day in the treated eye for an additional -the second postoperative- month.
These data essentially “mirror” the European experience with the Athens Protocol, as well as data published with similar and modified techniques globally[
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22] offering clinical reference for fellow surgeons in the United States, taken that the appropriate informed consent is provided for each patient as this application is “off-label”.
Although several clinicians still opt to perform cross-linking first, to follow with a topography-guided surface ablation as a therapeutic normalization process later post-operatively-despite the fact that we have established in the literature the advantage of both being used may offer a synergistic effect that is quite the multitude in cornea normalization than if you use the two techniques at separate times-the potential disadvantage of performing a surface ablation after the initial CXL procedure, is the removal of some of the most “cross-linked” anterior stromal tissue by the initial CXL procedure
.[
11,
12]
Despite the similar clinical data, one of the limitations of the US allowed parameters, is the lower limit of a 6mm optical ablation zone, that cannot be decreased to 5mm-the minimum available with this technology outside the US- thus posing a limitation in the spectrum of some advanced ectasia cases, that due to significant thinning prohibit the application of this type of treatment.
Theoretically, when corneal curvature normalization is the priority, and not necessarily reducing the amount of myopia in these corneas with advanced ectasia, utilizing the hyperopic WFO treatment aimed for epithelial removal only, could bypass this tissue limitation, at the cost of inducing a more myopic refractive error. For example, if a 400 micron cornea is encountered as the minimum cornea thickness, and the Athens Protocol CXL treatment has decided-or is speculated to be applied-then the actual topography-guided treatment can be performed on intact epithelium without adding the -2.75D factor for epithelial removal as described above. Then the hyperopic WFO treatment for epithelial removal can follow. As a result the cornea will be normalized, but the actual end-refractive error will have a myopic-shift.
In essence the postoperative refraction will be expected to become more myopic that pre-operatively, nevertheless with parallel expected improvement for the CDVA that may be corrected with spectacles, contact lenses and even phakic IOL at a later time.
Further studies of this concept may further validate these initial clinical data.