Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
Importance of the Study
Introduction
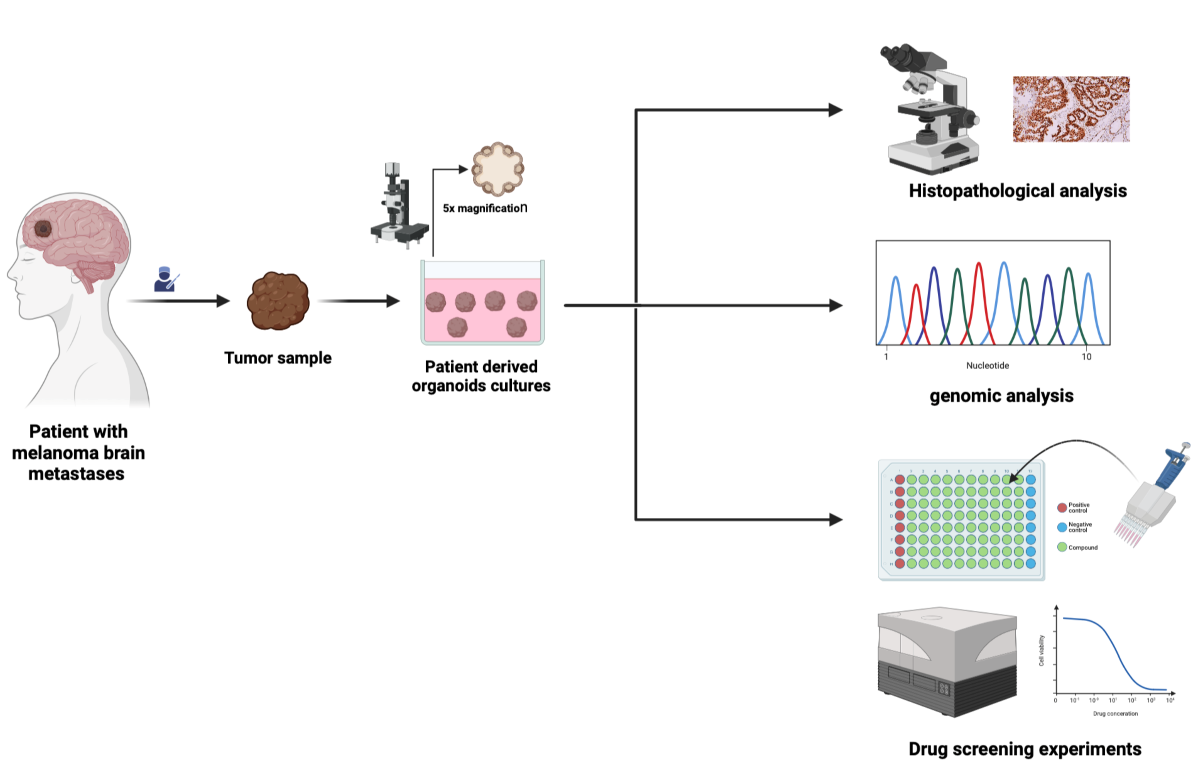
Material and Methods
Human Specimens
Human Tumor Collection
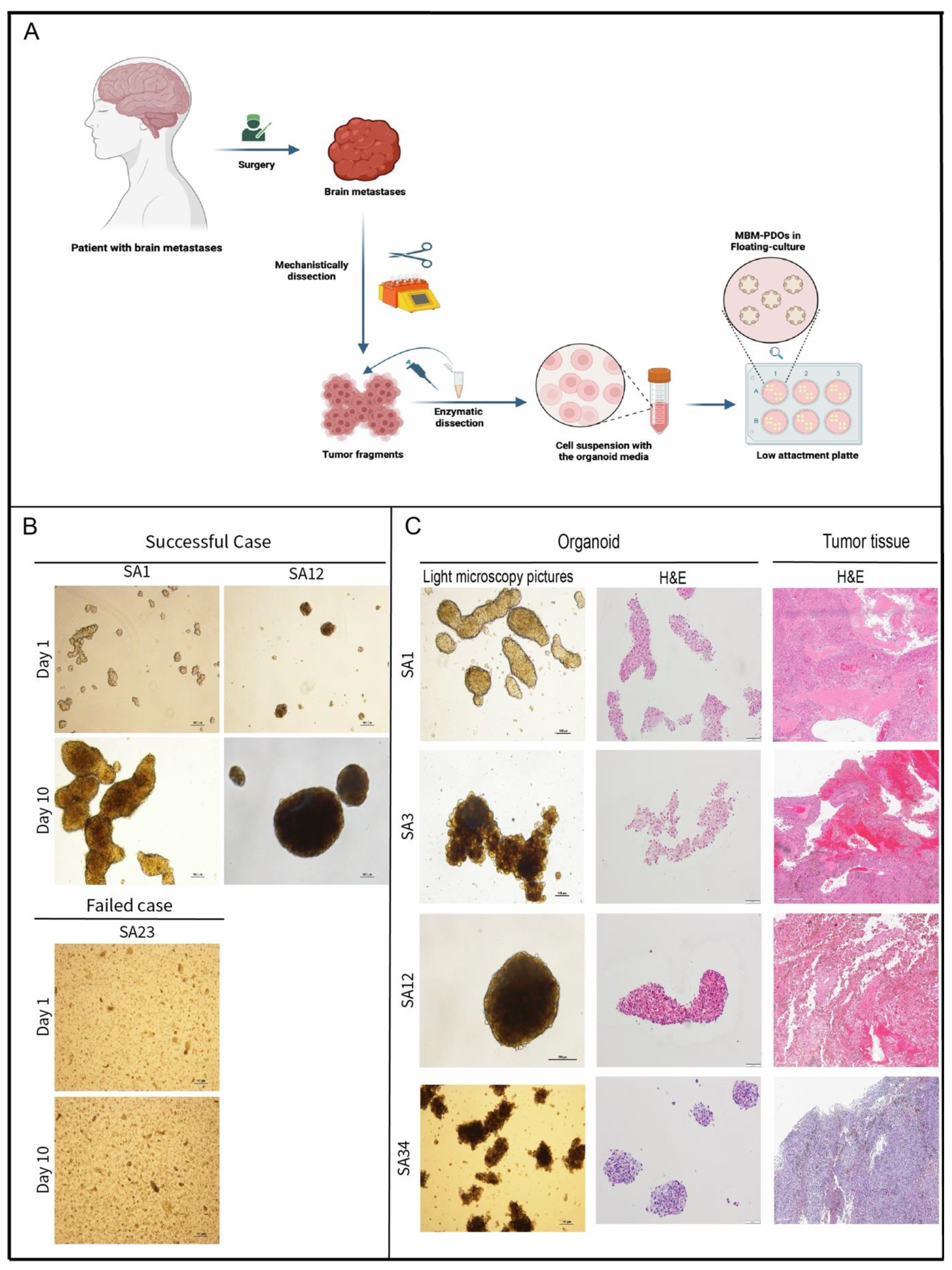
MBM-PDOs Floating Culture
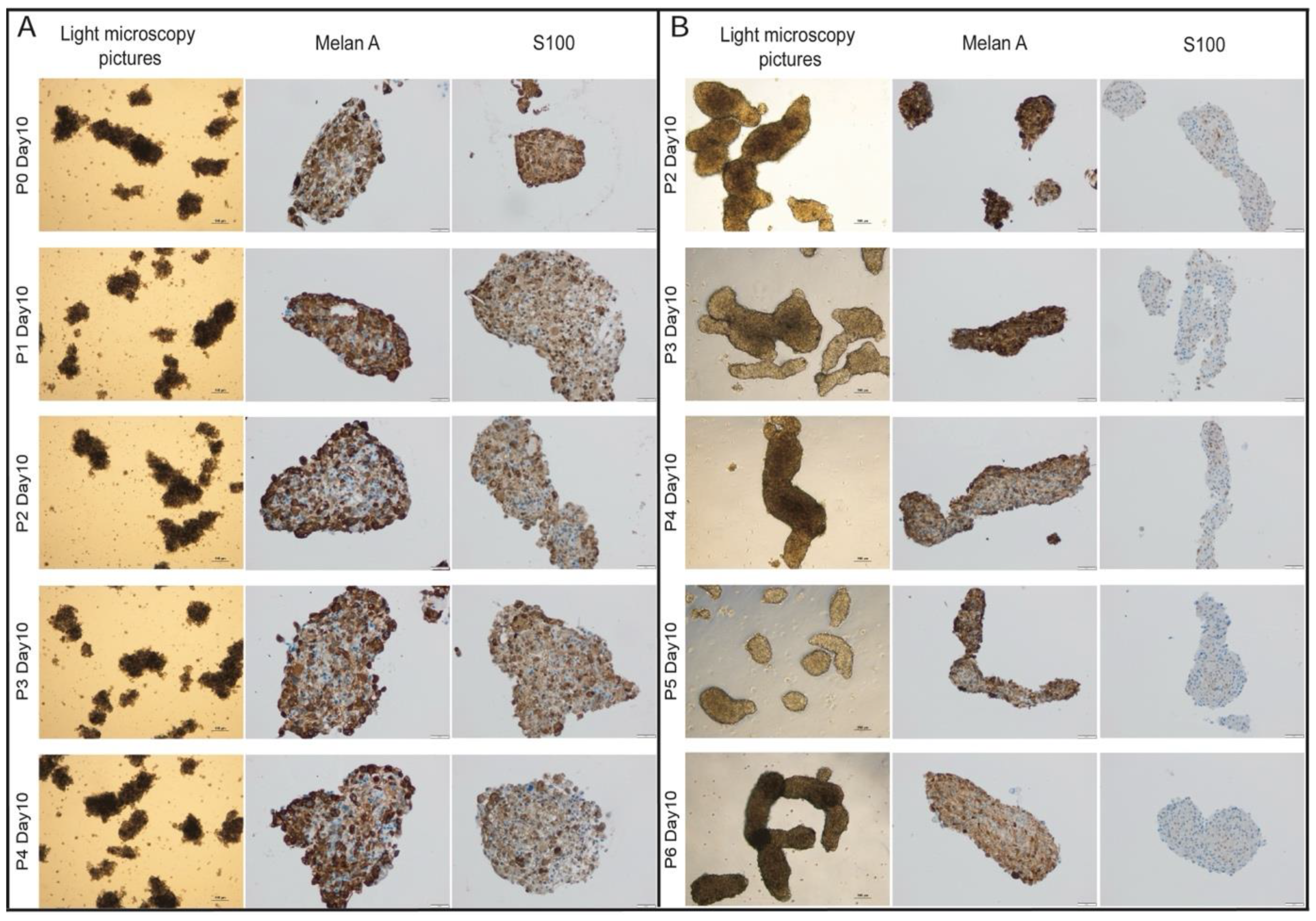
Splitting of PDO-Cultures
Embedding of PDO-Cultures
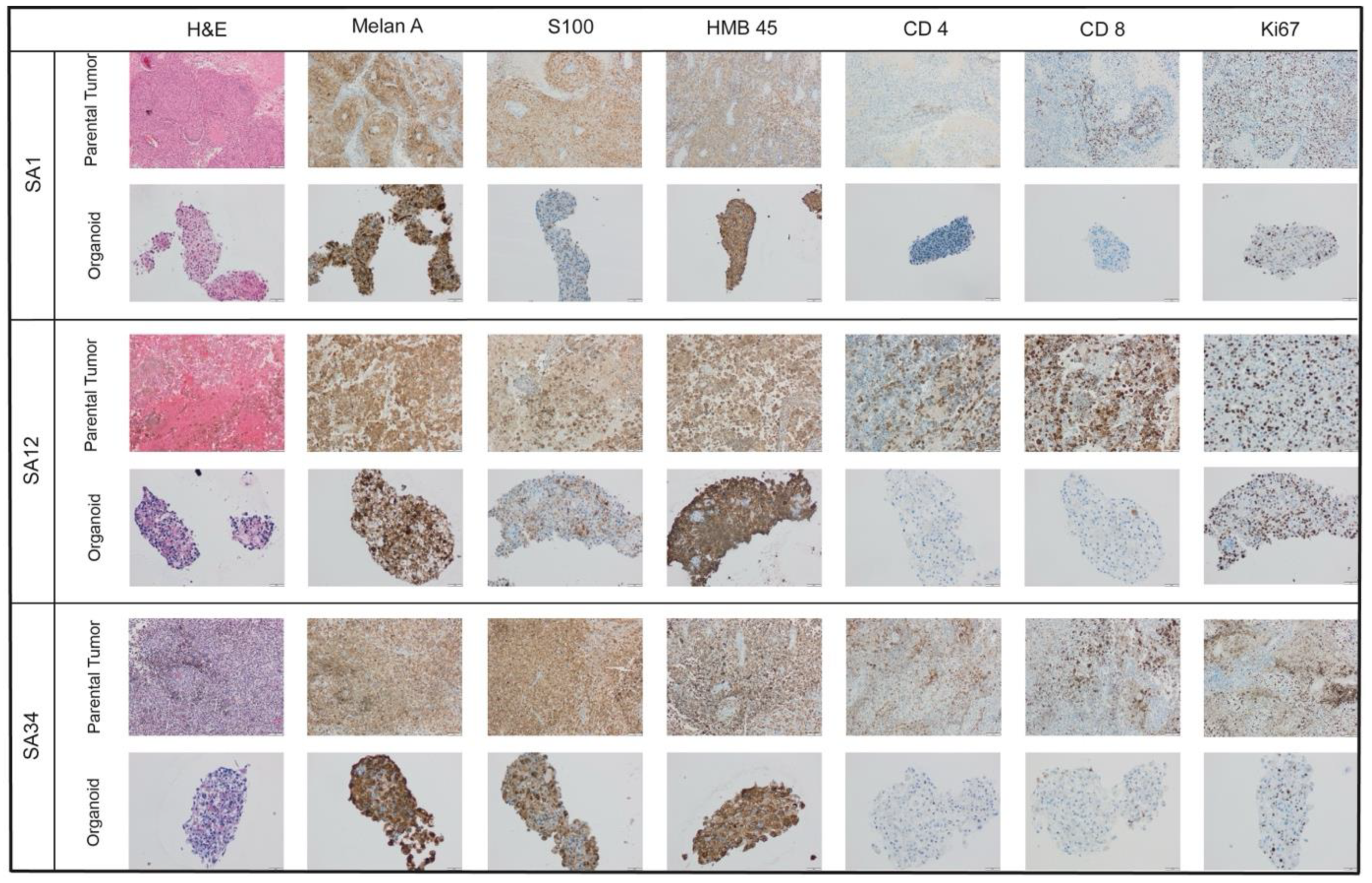
Immunohistochemistry
Mutational Analysis
Next Generation Sequencing (NGS)
Pyrosequencing of BRAF codon 600
Treatment with BRAF and MEK Inhibitors
Measurement of Intracellular ATP
Results
Establishment and Cultivation of MBM-PDOs
MBM-PDOs Preserve Key Histological Features of Their Original Tumors
MBM-PDOs Recapitulate the Mutational Profiles of Their Original Tumors
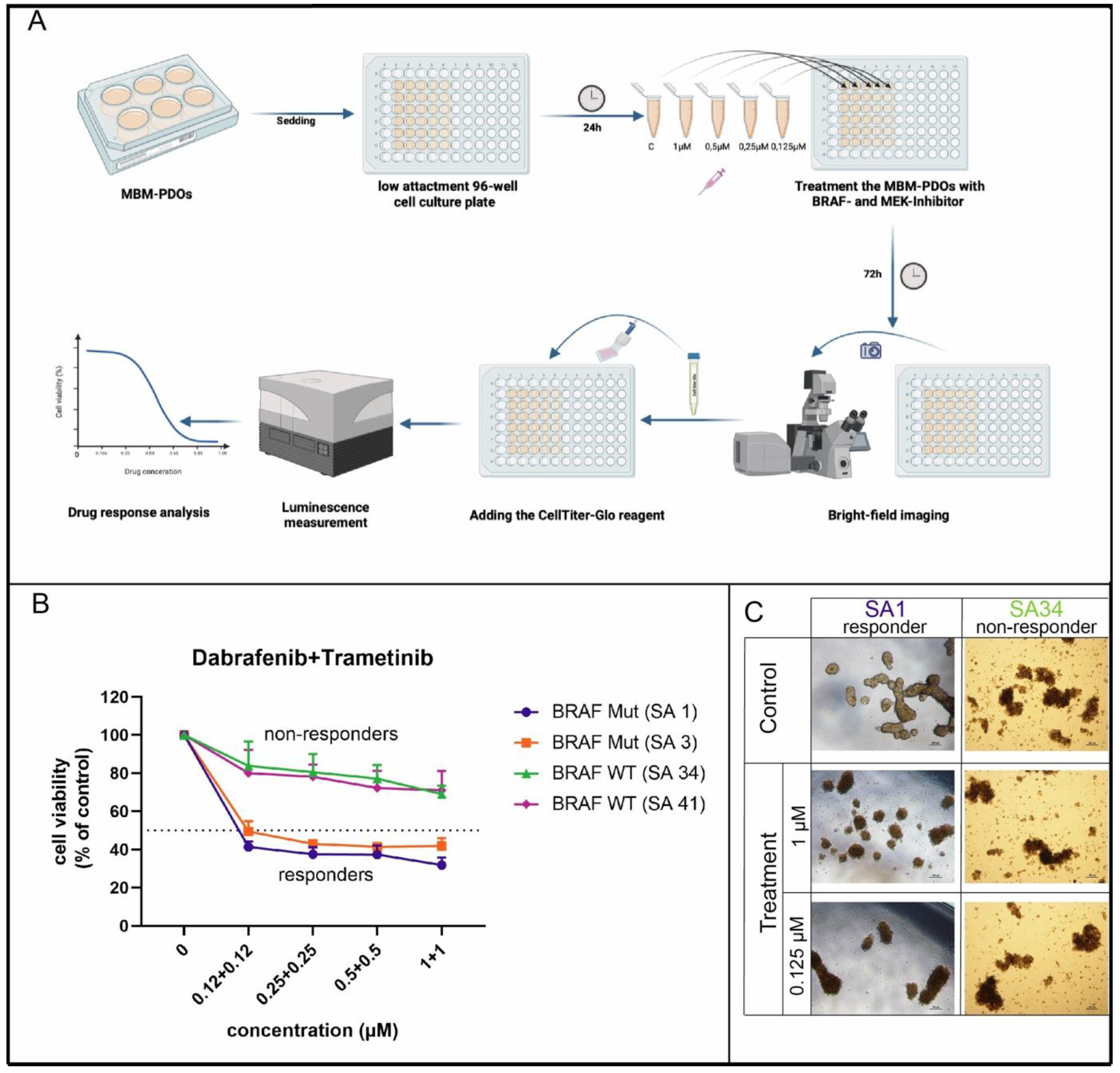
MBM-PDOs with BRAF V600E Mutations Show Therapy Response to BRAF and MEK Inhibitors
Discussion
Supplementary Materials
Funding
Contributors
Acknowledgments
Ethics approval
Competing Interests
Availability of data and materials
Abbreviations
| PDO: patient-derived-organoids |
| MBM: melanoma brain metastases |
| MBM-PDO: patient-derived-organoids from melanoma brain metastases |
| NGS: next-generation sequencing |
References
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. [CrossRef]
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54. [CrossRef]
- Stelzer KJ. Epidemiology and prognosis of brain metastases. Surg Neurol Int. 2013;4(Suppl 4):S192-202. [CrossRef]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-954. [CrossRef]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30-39. [CrossRef]
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248-1260. [CrossRef]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535-1546. [CrossRef]
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239-1251. [CrossRef]
- Robert C, Carlino MS, McNeil C, et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J Clin Oncol. 2023;41(24):3998-4003. [CrossRef]
- Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10(1):3991. [CrossRef]
- Karkampouna S, La Manna F, Benjak A, et al. Patient-derived xenografts and organoids model therapy response in prostate cancer. Nat Commun. 2021;12(1):1117. [CrossRef]
- Wensink GE, Elias SG, Mullenders J, et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis Oncol. 2021;5(1):30. [CrossRef]
- Freitas de Morais E, Da Siquara Rocha LdO, Souza Santos JL de, et al. Use of Three-Dimensional Cell Culture Models in Drug Assays for Anti-Cancer Agents in Oral Cancer: Protocol for a Scoping Review. J Pers Med. 2023;13(11). [CrossRef]
- Sahgal P, Patil DT, Bala P, et al. Replicative stress in gastroesophageal cancer is associated with chromosomal instability and sensitivity to DNA damage response inhibitors. iScience. 2023;26(11):108169. [CrossRef]
- Vazquez-Armendariz AI, Tata PR. Recent advances in lung organoid development and applications in disease modeling. J Clin Invest. 2023;133(22). [CrossRef]
- Ren X, Huang M, Weng W, et al. Personalized drug screening in patient-derived organoids of biliary tract cancer and its clinical application. Cell Rep Med. 2023;4(11):101277. [CrossRef]
- Ou L, Liu S, Wang H, et al. Patient-derived melanoma organoid models facilitate the assessment of immunotherapies. EBioMedicine. 2023;92:104614. [CrossRef]
- Zhou S, Lu J, Liu S, et al. Role of the tumor microenvironment in malignant melanoma organoids during the development and metastasis of tumors. Front Cell Dev Biol. 2023;11:1166916. [CrossRef]
- Blanco-García L, Ruano Y, Blanco Martínez-Illescas R, et al. pTERT C250T mutation: A potential biomarker of poor prognosis in metastatic melanoma. Heliyon. 2023;9(8):e18953. [CrossRef]
- Gutzmer R, Vordermark D, Hassel JC, et al. Melanoma brain metastases - Interdisciplinary management recommendations 2020. Cancer Treat Rev. 2020;89:102083. [CrossRef]
- Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626-636. [CrossRef]
- Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863-873. [CrossRef]
- Berking C, Livingstone E, Debus D, et al. COMBI-r: A Prospective, Non-Interventional Study of Dabrafenib Plus Trametinib in Unselected Patients with Unresectable or Metastatic BRAF V600-Mutant Melanoma. Cancers (Basel). 2023;15(18). [CrossRef]
- Sun L, Kang X, Ju H, et al. A human mucosal melanoma organoid platform for modeling tumor heterogeneity and exploring immunotherapy combination options. Sci Adv. 2023;9(43):eadg6686. [CrossRef]
| Age | Median | 55 | |
| Sex | Male | 5 (62.5%) | |
| Female | 3 (37.5%) | ||
| Localization | Frontal | 4 (50%) | |
| Parietal | 3 (37.5%) | ||
| Occipital | 1 (12.5%) | ||
| Number of brain metastases | singular | 6 (75%) | |
| multiple | 2 (25%) | ||
| Extracranial metastases | 0 | 5 (62.5%) | |
| Pulmonary | 1 (12.5%) | ||
| Osseous | 2 (25%) | ||
| TNM | T | pTx (First diagnosis) | 2 (25%) |
| pT1 | 0 (0%) | ||
| pT2 | 1 (12.5%) | ||
| pT3 | 4 (50%) | ||
| pT4 | 1 (12.5%) | ||
| N | pN0 | 5 (62.5%) | |
| pN1 | 2 (25%) | ||
| pN2 | 1 (12.5%) | ||
| M | pM1 | 8 (100%) | |
| Mutation | BRAF V600E | 5 (62.5%) | |
| BRAf wildtype | 3 (37.5%) | ||
| Therapy | pre neurosurgical resection | Stereotactic Radiotherapie | 3 (37.5%) |
| Interferon therapy | 2 (25%) | ||
| Combination immunotherapy | 3 (37.5%) | ||
| post neurosurgical resection | BRAF and MEK inhibitor (tafinlar mekinist) | 2 (25%) | |
| Combination immunotherapy (Nivolumab,Ipilimumab) | 3 (37.5%) | ||
| Case | Primary tumor | Organoid |
| SA1 | BRAF V600 E | BRAF V600 E |
| SA3 | BRAF V600 E | BRAF V600 E |
| SA12 | BRAF V600 E | BRAF V600 E |
| SA17 | BRAF V600 E | BRAF V600 E |
| SA20 | BRAF V600 E | BRAF V600 E |
| SA34 | BRAF V600 WT NRAS p.Q61K |
BRAF V600 WT NRAS p.Q61K TERT c.146C>T |
| SA41 | BRAF WT KIT p.L576P TERT c.146C>T TERT c.125_124delinsTT POLE c.1360-1>A |
BRAF V600 WT KIT p.L576P. TERT c.146C>T TERT c.125_124delinsTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





