2. Materials and Methods
2.1. Study Design, Ethics and Protocol
This case-control study was based on a retrospective approach, i.e., data collection and outcome assessment took place after the initial records had been obtained for diagnostic purposes and orthodontic treatment planning. It was conducted according to the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [
39]. Ethical approval of the research protocol was granted by the Research and Ethics Committee of the School of Dentistry of the National and Kapodistrian University of Athens to access patients’ records (application No. 592/26.05.2023). Informed consent was obtained from the parents / legal guardians of all participants involved in the study. The study followed the approved protocol without deviation from its original design. The imaging examinations (OPT, LC radiographs and CBCT scans) were conducted according to the ALARA principle, with an indication justifying radiation exposure.
2.2. Study Setting
The present case-control study investigated the prevalence, type, and location of IFs in patients with non-syndromic CL/P and healthy controls recruited from the graduate clinic of the Department of Orthodontics, School of Dentistry, National and Kapodistrian University of Athens, Greece, during October 2023. The overall timeframe for conducting the research was set from October 2023 through March 2024.
2.3. Study Population
The number of cleft cases available in the university’s orthodontic clinic during the study determined the sample size. The study sample included scans and radiographs of a cleft population and controls, all receiving comprehensive orthodontic treatment in the orthodontic clinic until October 2023. Available complete records of patients diagnosed with CL/P were retrieved for 40 cases. The types of non-syndromic orofacial clefts that were investigated in this research study consisted of the unilateral cleft palate at the upper right side (CP—UR), unilateral cleft palate at the upper left side (CP—UL), unilateral cleft lip and palate at the upper right side (CLP—UR), unilateral cleft lip and palate at the upper left side (CLP—UL), bilateral cleft lip and palate (CLP—B) and isolated cleft palate (CP).
2.3.1. Inclusion Criteria
The inclusion criteria were the following: non-syndromic CL/P patients with complete records, including dental casts, photos, OPT, LC radiographs and/or CBCT scans taken during the orthodontic diagnostic evaluation. No age, gender or ethnic limitations were applied.
2.3.2. Exclusion Criteria
Patients with a history of permanent teeth extractions before the initial orthodontic screening were excluded, as well as patients undergone previous orthodontic treatment, pre-surgical orthopedics, gingivoperiosteoplasty or primary bone grafting.
2.3.3. Matching Criteria
The control group comprised of healthy individuals, matched to cases by date of birth and gender. During the matching process, we accepted up to 6 months of age difference (older or younger) of controls compared with cleft cases. Two controls per one cleft case (case: control ratio = 1:2) were selected from the same clinic according to the stated matching criteria. Subjects with craniofacial anomalies, any maxillofacial pathology or trauma were excluded from the study. Consequently, the control group included 80 healthy individuals.
2.4. Study Outcomes
The primary outcome was the comparison of the IF prevalence between patients with and without non-syndromic CL/P. Secondary outcomes included investigating potential correlations of IFs with patients’ gender and cleft type, third molar aberrations, TMJ disorders, and the implications on the mandible.
2.5. Data Collection and Radiographic Evaluation
The OPT, LC radiographs and CBCT scans were taken by expert radiology staff in the Department of Radiology of the School of Dentistry, National and Kapodistrian University of Athens, as part of the orthodontic diagnostic process and treatment planning and not for this study. During the radiographic examination of all participants, the same equipment [Planmeca PM2002CC (Planmeca Oy, Helsinki, Finland)] and standardized method (67 kV, 5.0 mA, 13.9 s) were implemented. All radiographs had been taken according to the ALARA (as low as reasonably achievable) principle with an indication justifying radiation exposure.
Patient characteristics (individual patient file number, age, gender, exam date) and all the IFs found during the radiographic evaluation were recorded on spreadsheets originally designed for this study in Microsoft© Excel© 365 software (version 16.82 for Mac; Microsoft Corp., Redmond, WA, USA). Chronologic age was calculated by subtracting the date of birth from the date of imaging examination, namely either OPT or CBCT, and it was presented as a decimal age.
The data were collected after thoroughly examining the patient’s records, including intraoral photographs, charts, OPT and LC radiographs, in addition to CBCT scans, if available. The findings were based only on a review of the OPT, LC radiographs and CBCTs and were not influenced by what is reported as a clinical observation/finding in the patient’s medical chart. All images were assessed independently by four observers [two oral and maxillofacial radiologists (N.C. and K.B.), an orthodontist (I.S.) and a dental surgeon with three years of experience (I.P.)] under standardized conditions in the same examination setting. In case of disagreement between the observers, consensus was reached by discussion. Unclear findings were further clarified with the assistance of an experienced oral and maxillofacial radiologist (A.M.).
Ten anatomical areas were screened, and the images were reviewed for anomalies in the (i) maxilla, (ii) mandible, (iii) paranasal sinuses, (iv) middle and inner ear cavity, (v) nasal cavity, (vi) orbit, (vii) skull, (viii) cervical spine, (ix) TMJ and (x) soft tissue. The types of IFs investigated in the present study are depicted in Supplementar
Table S1. The following types of dental anomalies/aberrations in the dental development of the maxilla and mandible were screened:
variations in the number of teeth: teeth agenesis (anodontia, hypodontia, or oligodontia) and supernumerary tooth (supplemental tooth, mesiodens, paramolar, or distomolar);
variations in the morphology of teeth: developmental enamel defects (hypoplasia and/or hypocalcification), microdontia, peg-shaped teeth, taurodontism, root dilaceration, dens invaginatus (dens in dente), dens evaginatus, dentine dysplasia, teeth fusion, tooth gemination, curved maxillary central incisors, macrodontia, compound odontoma, dentinogenesis imperfecta, amelogenesis imperfecta, and
variations in tooth position include tooth transposition, malposition (including ectopic teeth), rotation, and impaction (including third molars).
Other abnormalities, such as the presence of mandibular tori, were also reported.
IFs that were studied in paranasal sinuses included retention cysts, obstructed sinuses, inflammatory opacification of the mucosal lining (which may suggest localized sinusitis), mucosal thickening of all the sinuses (which is indicative of pansinusitis), mucous retention pseudocysts, antral polyps, concha bullosa, oroantral fistula, and antrolith.
The middle and inner ears were assessed for inflammatory or abnormal congenital conditions, middle ear opacification and severely underdeveloped vestibule, suggesting a high likelihood of conductive hearing loss. The nasal cavity was screened for deviated septum and nasal polyps, while the orbit was assessed for an enlarged orbit, suggestive of buphthalmia.
Degenerative changes in vertebrae, along with skull malformations suspected to be caused by a congenital deformity, such as craniosynostosis, or an acquired condition, such as plagiocephaly, were examined. Temporomandibular joint abnormalities, including osteoarthrosis, condylar hyperplasia or hypoplasia, condylar degenerative changes, coronoid hyperplasia, subcondylar cyst, flat condylar margin, bifid condyle and osteophyte were recorded. If necessary, further volumetric data analyses were performed to investigate abnormalities in TMJ structures.
Furthermore, the current study screened for soft-tissue calcifications, including sialolith, tonsillolith, calcified pineal gland and calcified stylohyoid ligament. In addition, carotid artery calcification, vertebral artery calcification and thyroid cartilage calcification were documented. Regarding the cleft types, frequencies of the IFs were calculated for each cleft subgroup. Finally, the absolute number of IFs per patient’s anatomical area was recorded.
2.6. Statistical Analysis
All eligible patients served as cases for this case-control study. After obtaining the available records of cases included in the study, controls were identified based on the pre-specified matching criteria. The study sample size consisted of 40 cases and 80 controls. Thus, the statistical power of the study was estimated at 99.43%.
The datasets created in Microsoft
© Excel
© were merged and then submitted to descriptive statistical analysis. All analyses were conducted using the statistical programming language R (R version 4.3.1 [
40]) in R-Studio
® software (version 2023.12.1+402, R Foundation, Vienna, Austria). The present study demonstrated descriptive statistics as frequencies for categorical variables, while mean and standard deviation were provided for continuous ones. Descriptive statistics were provided for all patients and cases and controls separately. The Pearson Chi-square test was used to compare the frequencies of IFs, tooth agenesis, microdontia and third molar aberrations between cases and controls. In cases where the criteria for applying this test were not met, Fisher’s exact test was used. Comparisons of IF frequencies between cases and controls were also provided by anatomical area. A Pearson Chi-square test was applied to compare the presence of IFs by gender in case and control groups separately. A Student’s t-test for unmatched data was used to separately evaluate differences in the number of IFs in the maxilla and mandible in overall patients and cases and controls. A Goodness-of-fit Chi-square test was applied to assess differences in frequencies of IFs by cleft type in the case group, assuming that the proportion of IFs in each cleft type was the same, i.e., equal to 16.7%. For the case group, frequencies of tooth agenesis by tooth were also presented, while observations with missing values were excluded from the corresponding analysis. Logistic regression models were applied to assess further any mutually adjusted potential effects of gender and cleft type on IFs and to examine potential confounding.
The significance level was predetermined and set at α = 0.05. The intra-examiner and inter-examiner reliability was not determined in the present study since the discrepancies were resolved by consensus and, if necessary, by consultation with an oral and maxillofacial radiologist.
4. Discussion
To our knowledge, no published study has addressed the prevalence of IFs in several anatomical locations of patients with non-syndromic CL/P compared with healthy controls until today. The frequency of IFs in the cleft and non-cleft populations has been documented only in separate studies for each group [
14,
15,
16,
25,
28,
29,
37,
38]. Therefore, these results should be assessed cautiously since a direct comparison of the prevalence of IFs between patients with CL/P and controls has not yet been available. More evidence is needed regarding a comprehensive investigation of the prevalence of IFs in various anatomical regions distant from the dentition, which is often the area of primary interest. The current literature also lacks an accurate reporting of the prevalence of IFs in patients with non-syndromic CL/P compared with healthy controls prior to any significant dental intervention. Limited published data concerning potential IFs during the orthodontic diagnostic evaluation of patients with CL/P and healthy orthodontic patients are available in the literature, and their primary focus has been on dentition [
26,
41]. Based on these premises, we utilized a control group of unaffected individuals to conduct a case-control study for an adequate statistical comparison of the prevalence of IFs in several anatomical areas—not just dental abnormalities like previous studies—between CL/P and control subjects. This is the first comprehensive study regarding IFs in various regions of the oral and maxillofacial area of patients with non-syndromic CL/P compared with unaffected peers. Patients undergone any previous orthodontic/surgical intervention were excluded from the study, since tooth agenesis, as well as structural dental anomalies presented in patients, could be considered iatrogenic.
This study retrospectively assessed OPT, LC radiographs, and CBCT scans to identify IFs in a cleft sample of 40 patients and compare these findings with those of 80 healthy subjects. As described earlier, all IFs were recorded and subdivided into ten anatomical areas. We hypothesized that the prevalence of IFs does not differ between cases of CL/P and controls. However, this hypothesis was not confirmed. After matching for age and gender, highly significant differences in the prevalence of IFs were observed between non-syndromic CL/P patients and unaffected individuals. In addition, we found large differences in the anatomical location of reported IFs, which illustrates the association of IFs’ localization with cleft status. The findings of the present study may provide further insight to the clinicians who treat patients with non-syndromic orofacial clefts in establishing refined comprehensive treatment protocols.
In previous studies [
37,
38,
42], significantly more OPT and LC images of cleft patients or healthy orthodontic samples were assessed than in the present study. Yet, the risk of disregarding findings was probably higher, given that the radiographs were examined by two investigators with minimal involvement of a specialist radiologist. The current study demonstrated markedly more IFs, especially in the control group. One explanation might lie in the absence of a structured assessment method of previously published research; thus, the true diagnoses might have been underestimated.
The present study reported that the prevalence of IFs was significantly higher in the case group than in the control group. These results agree with previous studies [
26,
41]. The 62.5% rate of IFs that was estimated in the control group of our study was lower than that of relative studies conducted by Allareddy et al. (94.3%) [
23], Çaǧlayan & Tozoǧlu (92.8%) [
20] and Price et al. (90.7%) [
24], and higher than the 21.4% presented in other research reports [
15,
37,
38]. Such variability in findings can be attributed to the various imaging techniques implemented in former investigations. Researchers have evaluated IFs in cleft or non-cleft orthodontic populations based on either 2D imaging, namely panoramic and lateral cephalometric radiographs [
10,
27,
28,
29,
30,
38,
41], or 3D techniques with CBCT or CT scans [
15,
20,
21,
22,
23,
24,
25,
37,
42]. In addition, there are different indications for a radiograph or a scan in the existing literature, and other study populations analyzed in various settings could eventually result in fluctuating rates of IFs. Further discrepancies could be observed due to varying age, diagnostic criteria, and total sample size.
No correlation between IFs and gender was found in the case and the control group. After evaluating the prevalence of IFs by cleft type, we concluded that most cleft patients belonged to the CLP-UL subgroup (40%), which is in line with other research analyses [
30,
42,
43].
According to the literature, IFs are more frequently observed in the dental rather than the extra-dental regions. In previous investigations of non-cleft orthodontic samples [
37,
38], most IFs occurred in the dentition, followed by nasal and paranasal sinuses. We confirmed that the most common anatomical location that demonstrated IFs in the case group was the maxilla, followed by the nasal cavity, mandible and paranasal sinuses. In the control group, a higher prevalence of IFs was presented in the maxilla, followed by the mandible, TMJ and paranasal sinuses. In addition, we reported IFs in the nasal cavity and paranasal sinuses common in both case and control groups that could lead to severe aesthetic and functional implications for the patient. Such IFs are the enlarged inferior nasal concha in the nasal cavity, which often requires rhinoplasty and mucosal thickening, retention cysts and obstructed sinus in the paranasal sinuses, which could be associated with higher risk of inflammation. Our study confirmed the theory supported by Camporesi that the impact of orofacial clefts is more localized on the cleft area than general on the dentition [
41].
Regarding the IFs in the dentition, our analysis revealed significantly higher prevalence rates in cleft patients’ maxilla and mandible compared to the non-cleft subjects. It is noteworthy that IFs in the mandible were documented in 63.2% of the cases, in contrast with 41.3% of the controls. Moreover, we observed significantly fewer IFs in the mandible than in the maxilla of patients with CL/P since the former presented a mean number of findings of nearly half the number in the latter. Therefore, we can infer that orofacial clefts may affect anatomical structures outside the cleft’s area, particularly the mandible, yet only to a limited degree
A meta-analysis conducted by Marzouk et al. [
26] included 26 studies with 15,213 participants and assessed the frequency of dental anomalies between non-syndromic cleft patients and individuals without clefts. The results suggested a positive association between orofacial clefts and dental abnormalities, which is accounted for surgical interventions, such as primary lip and secondary palate surgery, that may prompt teeth displacement and rotation or affect the development of anterior and posterior permanent teeth [
26]. Marzouk et al. showed that tooth agenesis was the most commonly reported dental abnormality. Our investigation revealed that 72.5% of the orofacial cleft patients presented with tooth agenesis and were in line with several studies [
23,
26,
27,
28,
29] demonstrating a high rate of tooth agenesis in patients with CL/P. In our sample, the highest prevalence of tooth agenesis among cleft cases (excluding third molars) was reported for maxillary permanent lateral incisors in the cleft area, the second maxillary premolars and the second mandibular premolar outside the cleft area. These findings agree with other reports that concluded tooth agenesis occurs mainly at the cleft side, and the most prevalent missing tooth is the lateral incisor [
27,
28,
29,
42,
43,
44]. A cross-sectional study [
45] estimated the prevalence of tooth agenesis in a large sample of 5,005 non-cleft individuals at 7.1%, which is comparable to the reports of Rakhshan [
46] (0.15-16.2%, excluding the third molars). We also observed a considerably lower rate of tooth agenesis in the non-cleft group than in the cleft group.
The existing body of evidence is ambiguous regarding the association of tooth agenesis with gender. Some studies reported no differences in the distribution of developmental dental abnormalities between male and female patients [
42,
47], while others suggested a gender-related tendency in the agenesis of maxillary lateral incisors and maxillary second premolars [
27]. The lack of variability in the dependent variable in our study rendered the investigation of a potential association between tooth agenesis and gender impossible.
The literature highlights the importance of systematic image screening and thorough interpretation of any potential abnormality in the craniofacial complex since several studies revealed a high prevalence of IFs outside the primary dental focus [
23,
24]. A study by Klenke et al. [
38] reported 66% of the findings having occurred distant from the dentition and suggested that a markedly higher prevalence of IFs located in extra-dental regions is expected as patients’ age increases.
After dentition, paranasal sinuses are the next most common anatomical locations, where IFs were found at 52.6% in a cleft sample [
14]. Our study confirmed the same trend since 54.3% of cleft patients presented findings in paranasal sinuses, such as mucosal thickening, antral polyps, antrolith, sinusitis, pansinusitis and retention cysts. In the control group, we reported a 2.7% rate of IFs in paranasal sinuses, a striking difference from the 14.2-59.7% demonstrated in the literature [
15,
22,
24,
48]. This discrepancy may indicate the different imaging indications implemented in each study sample.
Temporomandibular joint abnormalities were observed at a high rate in the cleft and control groups in our analysis, with a rate of 47.1% and 14.7%, respectively. Santos et al. [
14] did not detect any finding in the TMJ region of cleft patients, while studies on non-cleft populations found the rate of incidental TMJ findings at 4.3% [
15] to 11.1% [
20]. Four CBCT studies on non-cleft samples [
15,
20,
21,
23] detected a variety of abnormal findings (coronoid hyperplasia, condylar hyperplasia, condylar hypoplasia), physiological remodeling (flat condylar margins, subchondral sclerosis) and degenerative alterations (osteophytes, erosions) affecting the TMJ structures.
According to our results, findings in the nasal cavity were present in 75.8% of the cleft patients and 1.49% of the controls. Nasal septum deviation constituted most of the detected findings in the nasal cavity of our sample. This abnormality is mentioned as the most common finding concerning the nasal cavity in patients with CL/P [
14], with a prevalence of 34% [
25] and is also considered to increase with age [
49]. Prevalence rates in former studies on non-cleft subjects range from 0.4 to 56.7% [
15,
20,
49].
Soft tissue calcifications were incidentally detected in 7.69% of both groups in our study, which was lower compared with former studies. CBCT studies indicated that soft tissue calcifications were present in 20% [
24] of healthy non-cleft samples, with tonsilloliths, sialoliths, thyroid cartilage and carotid artery calcifications as the most prominent findings [
23,
24].
4.1. Clinical Implications
The assessment of IFs on radiographic images or CBCT scans bears significant clinical implications. Most clinicians who are not radiologists or radiology-trained are unfamiliar with interpreting anatomical structures and/or abnormal findings beyond the area of their primary interest, as this was typically not incorporated in their training. Published reports, however, underline the clear benefit to the patient from a thorough evaluation of all available radiographic records and screening of all depicted anatomical areas [
16,
23,
50]. Orthodontists, dentists, surgeons, and radiologists who are part of the cleft care team should be aware of the potential pathology or important IFs in the dentition that require further diagnostic examination or treatment, as well as extra-dental abnormalities that may be relevant, necessitate further diagnostics, or alter the patient’s prognosis. Thereby, adequate education on the interpretation of images taken from the cranio-maxillofacial complex is imperative during the training of clinicians.
Clinicians should take into consideration the high prevalence of dental as well as extra-dental IFs for effective interceptive treatment of potentially severe oral rehabilitation issues. Understanding the association between CL/P and IFs is critical not only for providing successful interdisciplinary treatment, but it is also essential for raising awareness of the potential need for future dental care for individuals with CL/P and managing any extra-dental aberrations. Therefore, a systematic approach, including a meticulous review of all images and examination of each anatomical region during diagnostic assessment, is suggested to properly screen orthodontic radiographs and CBCT scans for abnormalities and eliminate the risk of overlooking IFs of clinical significance. To prevent under- or overestimating potential findings, consultations with oral and maxillofacial radiologists and appropriate referrals to specialists are highly recommended.
4.2. Limitations
No limitation concerning the age of this study’s participants was implemented in our research. Since the prevalence of IFs increases with age [
38], our estimates may slightly overestimate the true prevalence. This could also explain the higher rate of IFs reported in this study, especially in the control group, compared to previous research.
The limitations of this study can be primarily attributed to the relatively small sample size of the cleft group, especially for the subgroups of cleft lip and palate only at the right side (CLP—UR) and isolated cleft palate (CP—B, CP—UR, CP—UL). Nevertheless, gathering a large sample size can be challenging because orofacial clefts are rare. Another concern is the potential bias resulting from the lack of ethnic variability in the study sample, as all subjects were of Caucasian origin. Furthermore, the radiographic evaluations of this study were based on a retrospective analysis, which constitutes another limitation. However, a prospective study could not be possible due to ethical concerns associated with the radiation exposure of the imaging techniques. Thus, researchers are prohibited from exposing any subject to radiation for research purposes and are restricted to radiographs taken only for therapeutical reasons.
Our study was also limited because a FOV as small as possible is usually selected in each case in CBCT scans; hence, anatomical coverage may vary. The intra-rater and inter-rater reliability were not thoroughly assessed because differences between them were resolved in the present investigation by consensus and, whenever necessary, by a consultant radiologist. Finally, histopathology could not be used to validate our findings due to apparent practical and ethical considerations. The definitive diagnosis was determined merely by imaging.
4.3. Generalizability (External Validity)
The generalizability of this study was limited to the examined population in terms of ethnicity. Our database included patients from the Greek population treated in a single setting. The range of patients was sufficient: male and female, from children and adolescents to young adults, were included. Hence, the major exposure categories were well represented. The severity of orofacial cleft in the case group ranged from isolated cleft palate to cleft lip and palate unilaterally or bilaterally.
4.4. Future Research
Further research regarding potential IFs is still required internationally to determine the most effective approaches to minimize the burden of oral clefts and optimize the quality of care delivered to affected individuals. Therefore, subsequent investigations should incorporate diverse ethnic groups and collect larger sample sizes with a multi-center study design, to fully understand the potential association and illustrate an accurate picture of the prevalence of IFs in the various cleft subgroups compared with the general non-cleft population.
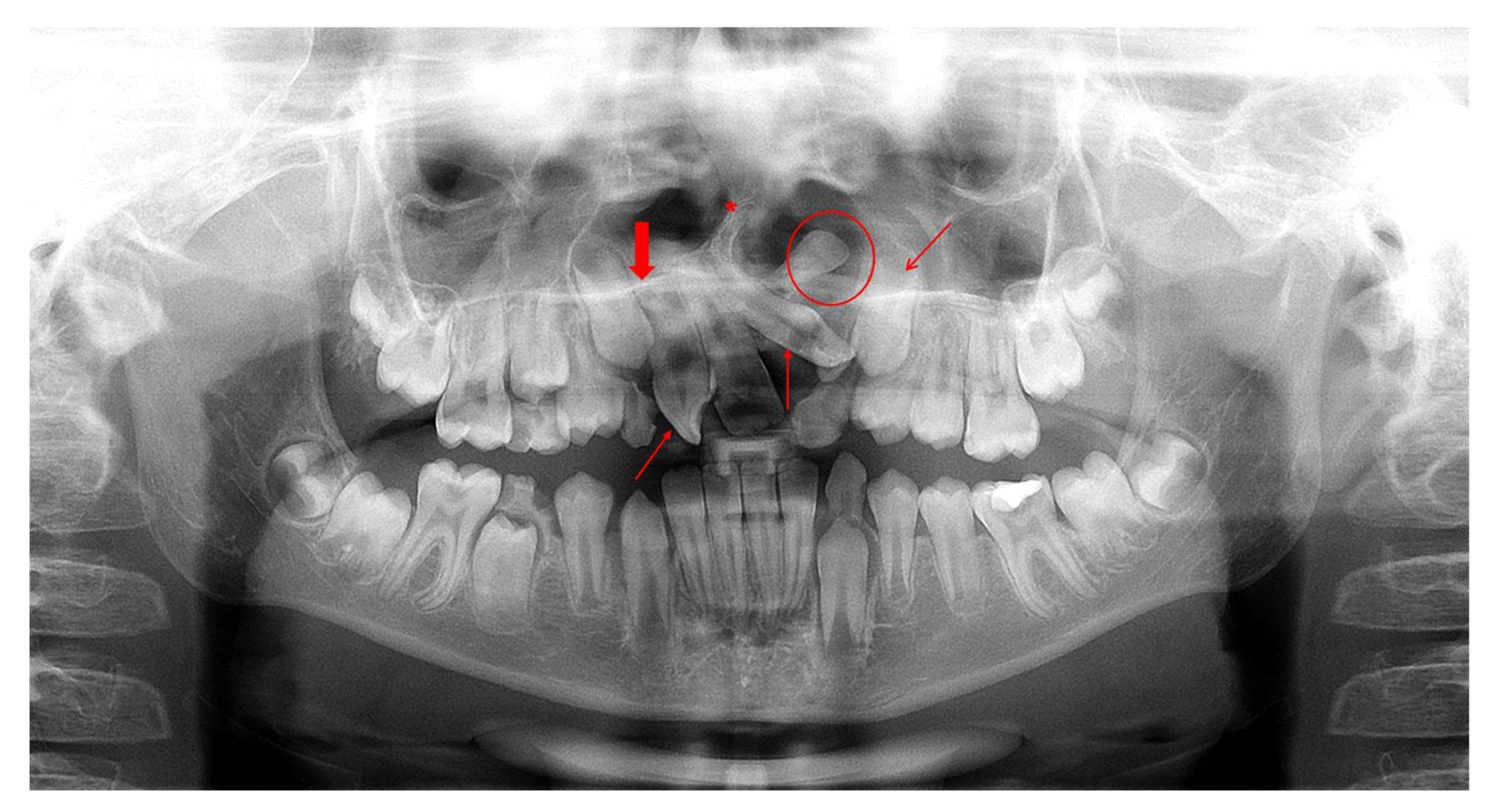
Figure 1.
Orthopantomogram (OPT) of a male patient (10.9 years old) diagnosed with CLP—UL presenting various incidental findings: agenesis of tooth 12 (bold red arrow), malposition of tooth 22 (thin diagonal red arrow), impaction of tooth 23 (thin diagonal red arrow), rotation of tooth 11 (thin vertical red arrows), supernumerary tooth between teeth 22-23 (red circle) and deviated nasal septum to the right (red asterisk).
Figure 1.
Orthopantomogram (OPT) of a male patient (10.9 years old) diagnosed with CLP—UL presenting various incidental findings: agenesis of tooth 12 (bold red arrow), malposition of tooth 22 (thin diagonal red arrow), impaction of tooth 23 (thin diagonal red arrow), rotation of tooth 11 (thin vertical red arrows), supernumerary tooth between teeth 22-23 (red circle) and deviated nasal septum to the right (red asterisk).
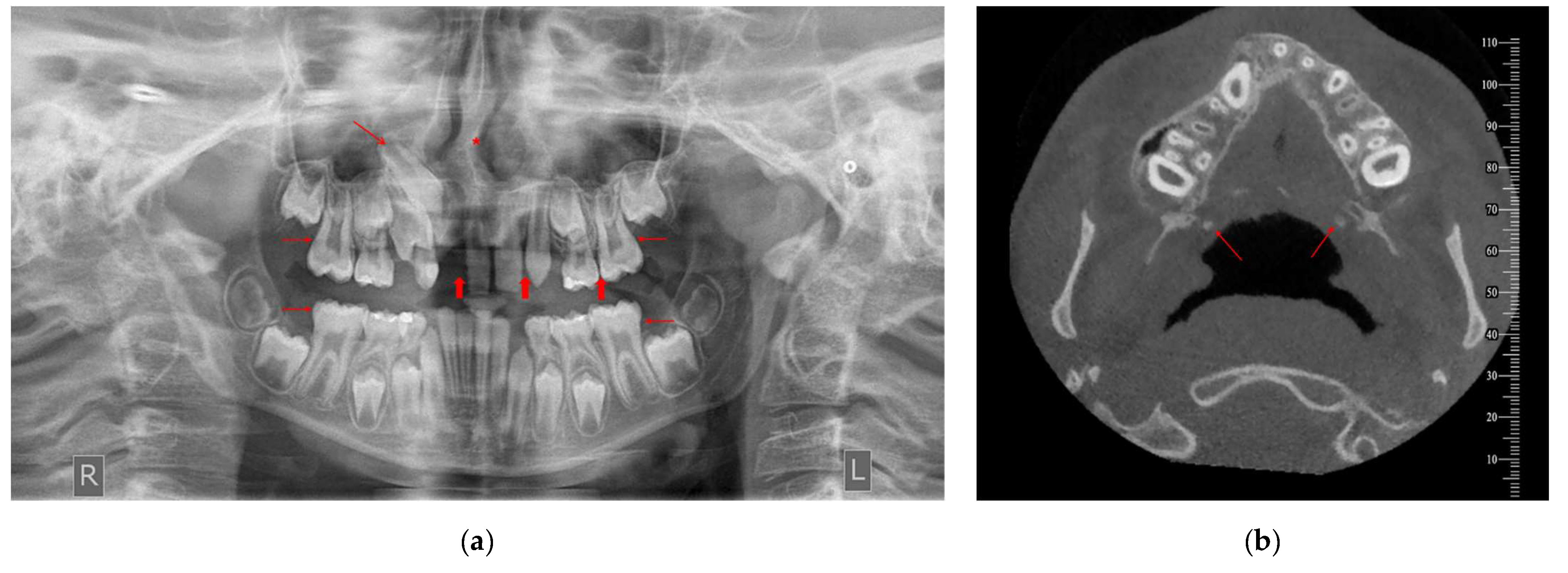
Figure 2.
Imaging records of a male patient (10.7 years old) diagnosed with CP—UR presenting various incidental findings: (a) OPT indicates agenesis of teeth 12, 22 and 25 (bold red arrows), impaction of tooth 13 (thin diagonal red arrow), taurodontism of teeth 16, 26, 36 and 46 (thin horizontal red arrows) and deviated nasal septum to the right (red asterisk). (b) Cone-beam computed tomography (CBCT) image of two incidentally detected tonsilloliths (axial view; red arrows). R: right, L: left.
Figure 2.
Imaging records of a male patient (10.7 years old) diagnosed with CP—UR presenting various incidental findings: (a) OPT indicates agenesis of teeth 12, 22 and 25 (bold red arrows), impaction of tooth 13 (thin diagonal red arrow), taurodontism of teeth 16, 26, 36 and 46 (thin horizontal red arrows) and deviated nasal septum to the right (red asterisk). (b) Cone-beam computed tomography (CBCT) image of two incidentally detected tonsilloliths (axial view; red arrows). R: right, L: left.
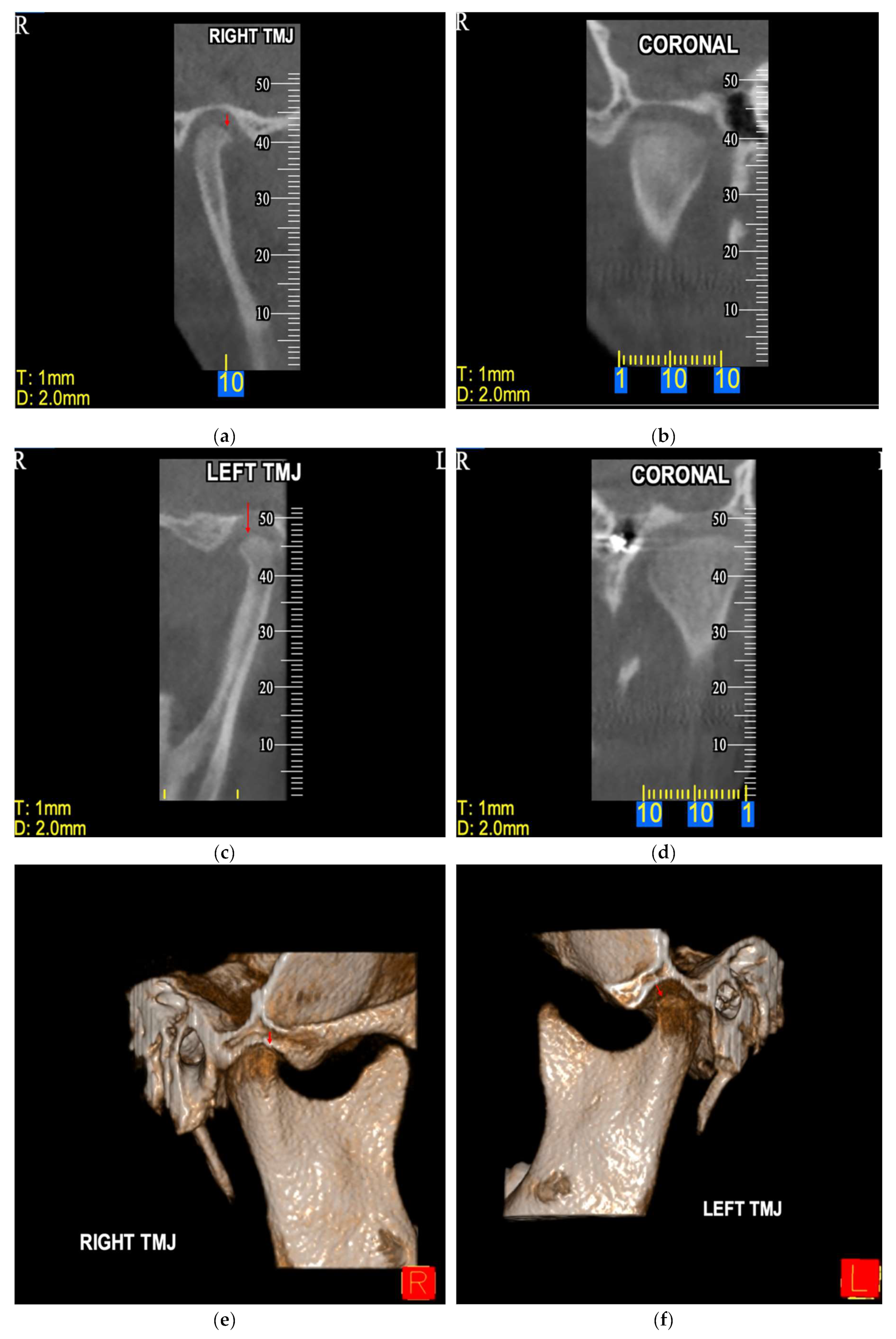
Figure 3.
CBCT image of incidentally detected flat condylar margins bilaterally on TMJ of a male patient (10.7 years old) diagnosed with CP—UR. The findings are imaged in sagittal view [right (a) and left TMJ (c)], coronal view [right (b) and left TMJ (d)] and 3D reconstruction [right (e) and left TMJ (f)]. R: right, L: left.
Figure 3.
CBCT image of incidentally detected flat condylar margins bilaterally on TMJ of a male patient (10.7 years old) diagnosed with CP—UR. The findings are imaged in sagittal view [right (a) and left TMJ (c)], coronal view [right (b) and left TMJ (d)] and 3D reconstruction [right (e) and left TMJ (f)]. R: right, L: left.
Table 1.
Demographic and disease characteristics of the study population.
Table 1.
Demographic and disease characteristics of the study population.
| |
All participants |
Controls |
Cases |
| |
N=120 |
N=80 |
N=40 |
| Gender, n(%) |
|
|
|
| Females |
54 (45.0%) |
36 (45.0%) |
18 (45.0%) |
| Males |
66 (55.0%) |
44 (55.0%) |
22 (55.00%) |
| Age (years), mean (SD1) |
14.6 (5.0) |
14.6 (5.0) |
14.5 (5.0) |
| Cleft type, n(%) |
|
|
|
| CLP—B |
9 (7.5%) |
0 (0.0%) |
9 (22.5%) |
| CLP—UL |
16 (13.3%) |
0 (0.0%) |
16 (40.0%) |
| CLP—UR |
4 (3.3%) |
0 (0.0%) |
4 (10.0%) |
| CP |
5 (4.1%) |
0 (0.0%) |
5 (12.5%) |
| CP—UL |
3 (2.5%) |
0 (0.0%) |
3 (7.5%) |
| CP—UR |
3 (2.5%) |
0 (0.0%) |
3 (7.5%) |
| No |
80 (66.7%) |
80 (100.0%) |
0 (0.0%) |
Table 2.
Descriptive statistics on Incidental Findings (IFs).
Table 2.
Descriptive statistics on Incidental Findings (IFs).
| |
All participants |
Controls |
Cases |
| |
N=120 |
N=80 |
N=40 |
| IFs, n(%) |
|
|
|
| No |
31 (25.8%) |
30 (37.5%) |
1 (2.5%) |
| Yes |
89 (74.2%) |
50 (62.5%) |
39 (97.5%) |
| IFs in Maxilla, n(%) |
|
|
|
| No |
48 (40.0%) |
45 (56.3%) |
3 (7.5%) |
| Yes |
72 (60.0%) |
35 (43.8%) |
37 (92.5%) |
| Number of IFs in Maxilla, n(%) |
|
|
|
| 0 |
48 (40.0%) |
45 (56.3%) |
2 (5.0%) |
| 1 |
19 (15.0%) |
10 (12.5%) |
9 (22.5%) |
| 2 |
21 (17.5%) |
17 (21.3%) |
4 (10.0%) |
| 3 |
15 (12.5%) |
4 (5.0%) |
11 (27.5%) |
| 4 |
10 (8.3%) |
3 (3.8%) |
7 (17.5%) |
| 5 |
3 (2.5%) |
0 (0.0%) |
3 (7.5%) |
| 6 |
4 (3.3%) |
0 (0.0%) |
4 (10.0%) |
| 7 |
1 (0.8%) |
1 (1.3%) |
0 (0.0%) |
| IFs in Mandible, n(%) |
|
|
|
| No |
61 (51.7%) |
47 (58.8%) |
14 (36.8%) |
| Yes |
57 (48.3%) |
33 (41.3%) |
24 (63.2%) |
| Number of IFs in Mandible, n(%) |
|
|
|
| 0 |
61 (51.7%) |
47 (58.8%) |
14 (36.8%) |
| 1 |
16 (13.6%) |
9 (11.3%) |
7 (18.4%) |
| 2 |
32 (27.1%) |
19 (23.8%) |
13 (34.2%) |
| 3 |
1 (0.9%) |
1 (1.3%) |
0 (0.0%) |
| 4 |
5 (4.2%) |
2 (2.5%) |
3 (7.9%) |
| 5 |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
| 6 |
3 (2.5%) |
2 (2.5%) |
1 (2.6%) |
| IFs in Paranasal sinuses, n(%) |
|
|
|
| No |
89 (80.9%) |
73 (97.3%) |
16 (45.7%) |
| Yes |
21 (19.1%) |
2 (2.7%) |
19 (54.3%) |
| IFs in Nasal cavity, n(%) |
|
|
|
| No |
74 (74.0%) |
66 (98.5%) |
8 (24.2%) |
| Yes |
26 (26.0%) |
1 (1.5%) |
25 (75.8%) |
| IFs in Middle and inner ear, n(%) |
|
|
|
| No |
3 (100.0%) |
1 (100.0%) |
2 (100.0%) |
| IFs in Orbit, n(%) |
|
|
|
| No |
71 (100.0%) |
67 (100.0%) |
4 (100.0%) |
| IFs in Skull, n(%) |
|
|
|
| No |
7 (100.0%) |
3 (100.0%) |
4 (100.0%) |
| IFs in Cervical spine, n(%) |
|
|
|
| No |
86 (100.0%) |
66 (100.0%) |
20 (100.0%) |
| IFs in TMJ structures, n(%) |
|
|
|
| No |
73 (79.4%) |
64 (85.3%) |
9 (52.9%) |
| Yes |
19 (20.6%) |
11 (14.7%) |
8 (47.1%) |
| IFs in Soft-tissues, n(%) |
|
|
|
| No |
24 (92.3%) |
|
24 (92.3%) |
| Yes |
2 (7.7%) |
|
2 (7.7%) |
Table 3.
Comparison of the prevalence of IFs between cases and controls.
Table 3.
Comparison of the prevalence of IFs between cases and controls.
| |
No IFs |
IFs |
| Controls |
30 (37.5%) |
50 (62.5%) |
| Cases |
1 (2.5%) |
39 (97.5%) |
|
χ2 = 17.05, df = 1, p < 0.001** |
Table 4.
Comparison of the prevalence of IFs by gender.
Table 4.
Comparison of the prevalence of IFs by gender.
| Cases |
No IFs |
IFs |
| Females |
1 (5.6%) |
17 (94.4%) |
| Males |
0 (0.0%) |
22 (100.0%) |
|
χ2 = 1.25, df = 1, p = 0.263 (ns) |
| Controls |
No IFs |
IFs |
| Females |
15 (41.7%) |
21 (58.3%) |
| Males |
15 (34.1%) |
29 (65.9%) |
|
χ2 = 0.48, df = 1, p = 0.486 (ns) |
Table 5.
Comparison of prevalence of IFs by anatomical area.
Table 5.
Comparison of prevalence of IFs by anatomical area.
| Maxilla |
No IFs |
IFs |
| Cases |
3 (7.5%) |
37(92.5%) |
| Controls |
45 (56.3%) |
35 (43.8%) |
|
χ2 = 26.4, df = 1, p < 0.001 |
| Mandible |
No IFs |
IFs |
| Cases |
14 (36.8%) |
24 (63.2%) |
| Controls |
47 (58.8%) |
33 (41.3%) |
|
χ2 = 4.95, df = 1, p = 0.026 |
| Paranasal sinuses |
No IFs |
IFs |
| Cases |
16 (45.7%) |
19 (54.3%) |
| Controls |
73 (97.3%) |
2 (2.7%) |
|
χ2 = 41.17, df = 1, p < 0.001 |
| Nasal cavity |
No IFs |
IFs |
| Cases |
8 (24.2%) |
25 (75.8%) |
| Controls |
66 (98.5%) |
1 (1.5%) |
|
χ2 = 63.38, df = 1, p < 0.001 |
| TMJ structures |
No IFs |
IFs |
| Cases |
9 (52.9%) |
8 (47.1%) |
| Controls |
64 (85.3%) |
11 (14.7%) |
|
χ2 = 8.87, df = 1, p = 0.003* |
Table 6.
Comparison of number of IFs in maxilla and mandible.
Table 6.
Comparison of number of IFs in maxilla and mandible.
| |
Mean (SD) |
p-Value†
|
| |
Number of IFs in Maxilla |
Number of IFs in Mandible |
|
| All participants |
1.60 (1.74) |
1.03 (1.36) |
0.006* |
| Cases |
2.93 (1.70) |
1.34 (1.42) |
<0.001** |
| Controls |
0.94 (1.33) |
0.88 (1.32) |
0.766 |
Table 7.
Comparison of the prevalence of IFs by cleft type in the case group.
Table 7.
Comparison of the prevalence of IFs by cleft type in the case group.
| |
No IFs |
IFs |
| CLP—B |
0 (0.0%) |
9 (22.5%) |
| CLP—UL |
0 (0.0%) |
16 (40.0%) |
| CLP—UR |
0 (0.0%) |
4 (10.0%) |
| CP |
1 (2.5%) |
4 (10.0%) |
| CP—UL |
0 (0.0%) |
3 (7.5%) |
| CP—UR |
0 (0.0%) |
3 (7.5%) |
|
χ2 = 20.54, df = 5, p < 0.001** |
Table 8.
Descriptive statistics on tooth agenesis and microdontia.
Table 8.
Descriptive statistics on tooth agenesis and microdontia.
| |
All participants |
Controls |
Cases |
| |
N=120 |
N=80 |
N=40 |
| Tooth agenesis, n(%) |
|
|
|
| No |
72 (60.0%) |
61 (76.3%) |
11 (27.5%) |
| Yes |
48 (40.0%) |
19 (23.7%) |
29 (72.5%) |
| Microdontia, n(%) |
|
|
|
| No |
117 (97.5%) |
78 (97.5%) |
39 (97.5%) |
| Yes |
3 (2.5%) |
2 (2.5%) |
1 (2.5%) |
Table 9.
Comparison of prevalence of tooth agenesis and microdontia between cases and controls.
Table 9.
Comparison of prevalence of tooth agenesis and microdontia between cases and controls.
| |
No Tooth agenesis |
Tooth agenesis |
| Cases |
11(27.5%) |
29 (72.5%) |
| Controls |
61(76.3%) |
19 (23.8%) |
|
χ2 = 24.41, df = 1, p < 0.001** |
| |
No Microdontia |
Microdontia |
| Cases |
39 (97.5%) |
1 (2.5%) |
| Controls |
78 (97.5%) |
2 (2.0%) |
| Fisher’s exact test p > 0.999 (ns) |
Table 10.
Frequency of Tooth agenesis by tooth.
Table 10.
Frequency of Tooth agenesis by tooth.
| Tooth |
n (%) |
| 12 |
13 (21.0%) |
| 14 |
2 (3.2%) |
| 15 |
2 (3.2%) |
| 18 |
2 (3.2%) |
| 22 |
15 (24.2%) |
| 25 |
5 (8.1%) |
| 28 |
3 (4.8%) |
| 32 |
1 (1.6%) |
| 35 |
4 (6.5%) |
| 36 |
1 (1.6%) |
| 37 |
1 (1.6%) |
| 38 |
2 (3.2%) |
| 42 |
2 (3.2%) |
| 45 |
4 (6.5%) |
| 46 |
1 (1.6%) |
| 47 |
1 (1.6%) |
| 48 |
3 (4.8%) |
Table 11.
Descriptive statistics on third molar aberrations.
Table 11.
Descriptive statistics on third molar aberrations.
| |
All participants |
Controls |
Cases |
| |
N=120 |
N=80 |
N=40 |
| Third molar aberrations, n(%) |
|
|
|
| No |
100 (83.3%) |
70 (87.5%) |
30 (75.0%) |
| Yes |
20 (16.7%) |
10 (12.5%) |
10 (25.0%) |
| Third molar Impaction, n(%) |
|
|
|
| No |
108 (90.0%) |
75 (93.8%) |
33 (82.5%) |
| Yes |
12 (10.0%) |
5 (6.3%) |
7 (17.5%) |
| Third molar Tooth agenesis, n(%) |
|
|
|
| No |
113 (94.2%) |
75 (93.8%) |
38 (95.0%) |
| Yes |
7 (5.8%) |
5 (6.3%) |
2 (5.0%) |
| Third molar Taurodontism, n(%) |
|
|
|
| No |
119 (99.2%) |
80 (100.0%) |
39 (97.5%) |
| Yes |
1 (0.8%) |
0 (0.0%) |
1 (2.5%) |
Table 12.
Comparison of prevalence of Third molar aberrations.
Table 12.
Comparison of prevalence of Third molar aberrations.
| |
No Third molar aberrations |
Third molar aberrations |
| Cases |
30 (75.0%) |
10 (25.0%) |
| Controls |
70 (87.5%) |
10 (12.5%) |
|
χ2 = 3.00, df = 1, p = 0.083 (ns) |
| |
No Impaction |
Impaction |
| Cases |
33 (82.5%) |
7 (17.5%) |
| Controls |
75 (93.8%) |
5 (6.3%) |
|
χ2 = 3.75, df = 1, p = 0.053 (ns) |
| |
No Tooth agenesis |
Tooth agenesis |
| Cases |
38 (95.0%) |
2 (5.0%) |
| Controls |
75 (93.8%) |
5 (6.3%) |
| Fisher’s exact test p > 0.999 (ns) |
| |
No Taurodontism |
Taurodontism |
| Cases |
39 (97.5%) |
1 (2.5%) |
| Controls |
80 (100.0%) |
0 (0.0%) |
| Fisher’s exact test p = 0.333 (ns) |