Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients and Methods
Patients
Ethical Approval
Baseline Characteristics
TPF Induction Chemotherapy and Standard Treatment
Follow-up Visit and Clinical End-Point Assessment
Statistical Analysis
3. Results
Patients
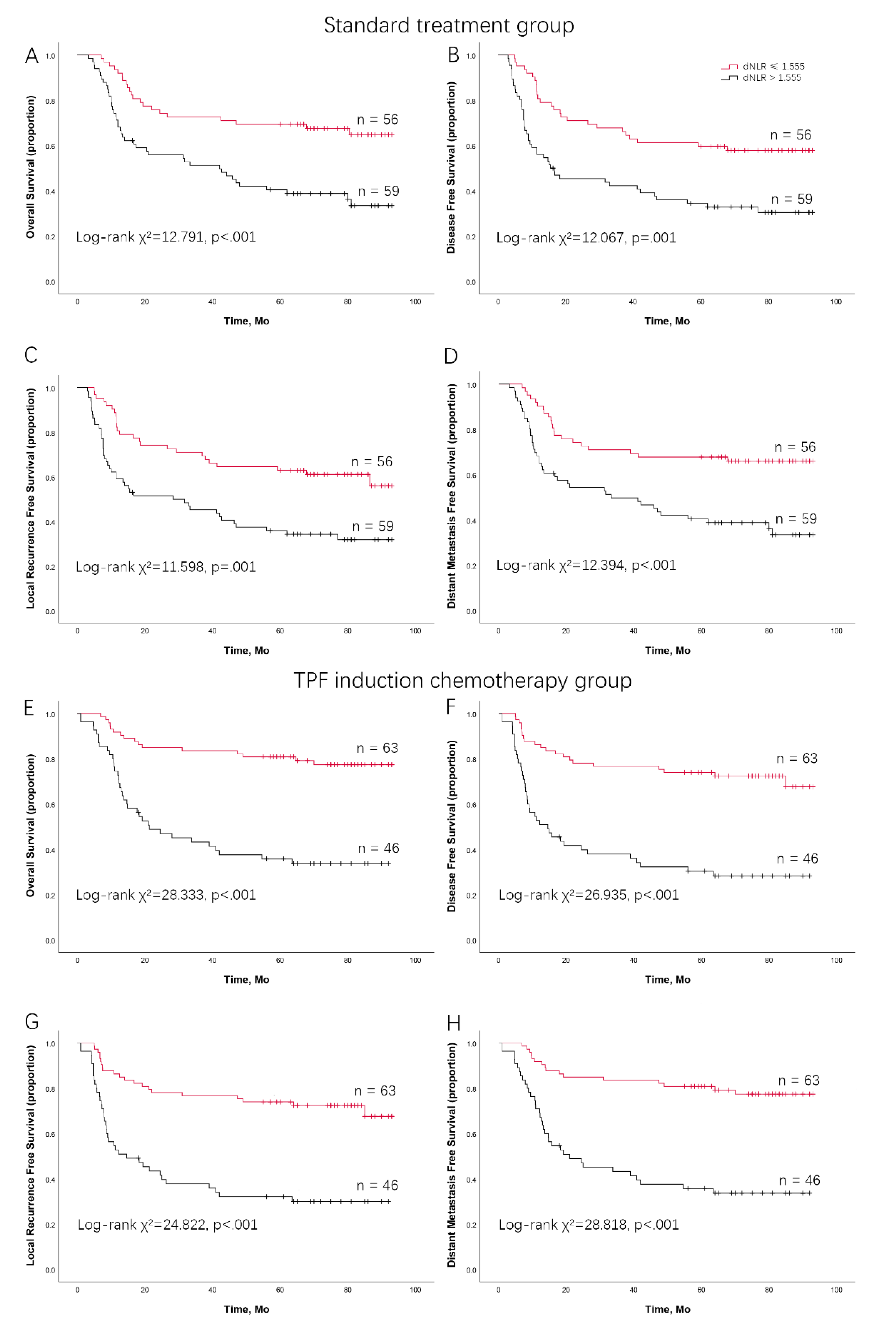
dNLR Predicts Survival Outcomes in LAOSCC Patients Treated by Surgery and Postoperative Radiation
dNLR Predicts Survival Outcomes in LAOSCC Patients Treated by TPF Induction Chemotherapy, Surgery and Postoperative Radiation
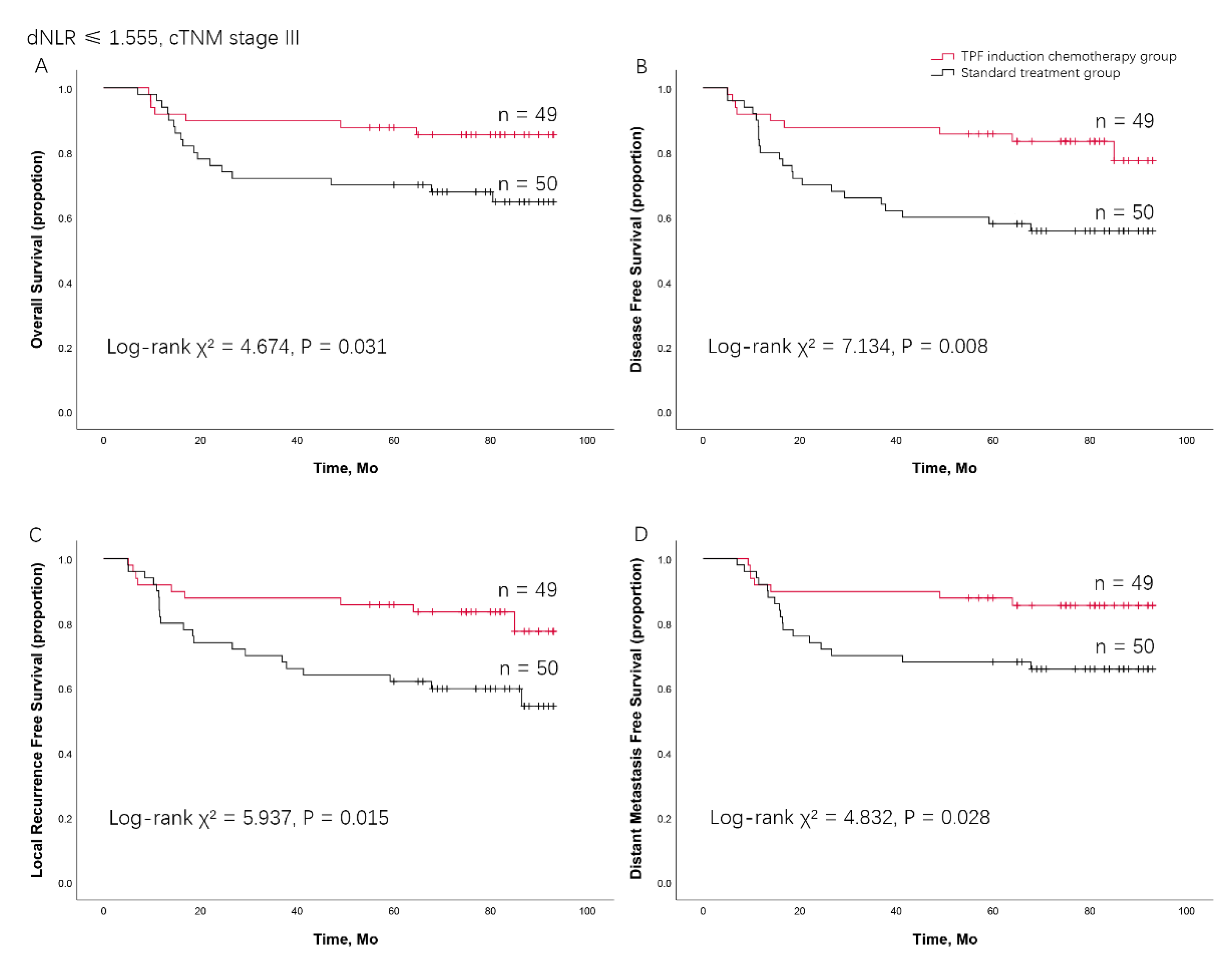
Combining cTNM Stage and dNLR Predicting the Benefit from TPF Induction Chemotherapy for LAOSCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. Jul 20 2019;394(10194):249-260. [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. Apr-May 2009;45(4-5):309-16. [CrossRef]
- Petersen, PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. Dec 2003;31 Suppl 1:3-23. [CrossRef]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Nov 2018;68(6):394-424. [CrossRef]
- Caudell JJ, Gillison ML, Maghami E, et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. Journal of the National Comprehensive Cancer Network: JNCCN. Mar 2022;20(3):224-234. [CrossRef]
- Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. Feb 20 2013;31(6):744-51. [CrossRef]
- Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. Mar 2013;14(3):257-64. [CrossRef]
- Sun Y, Li W, Chen N, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. The Lancet Oncology. 2016;17(11):1509-1520. [CrossRef]
- Ghi MG, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Annals of oncology: official journal of the European Society for Medical Oncology. Sep 1 2017;28(9):2206-2212. [CrossRef]
- Yang CZ, Ma J, Zhu DW, et al. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology. Jun 2014;25(6):1215-22. [CrossRef]
- Zhao TC, Liang SY, Ju WT, et al. Normal BMI predicts the survival benefits of inductive docetaxel, cisplatin, and 5-fluorouracil in patients with locally advanced oral squamous cell carcinoma. Clinical nutrition (Edinburgh, Scotland). Sep 2020;39(9):2751-2758. [CrossRef]
- Alessi JV, Ricciuti B, Alden SL, et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. Journal for immunotherapy of cancer. 2021. [CrossRef]
- Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic Value of the Lung Immune Prognostic Index for Patients Treated for Metastatic Non-Small Cell Lung Cancer. JAMA oncology. Oct 1 2019;5(10):1481-1485. [CrossRef]
- Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. Journal for immunotherapy of cancer. Jul 16 2018;6(1):74. [CrossRef]
- Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Annals of oncology: official journal of the European Society for Medical Oncology. Apr 2016;27(4):732-8. [CrossRef]
- Bauckneht M, Rebuzzi SE, Signori A, et al. The prognostic power of inflammatory indices and clinical factors in metastatic castration-resistant prostate cancer patients treated with radium-223 (BIO-Ra study). European journal of nuclear medicine and molecular imaging. Feb 2022;49(3):1063-1074. [CrossRef]
- van Soest RJ, Templeton AJ, Vera-Badillo FE, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: data from two randomized phase III trials. Annals of oncology: official journal of the European Society for Medical Oncology. Apr 2015;26(4):743-749. [CrossRef]
- Rebuzzi SE, Signori A, Banna GL, et al. Inflammatory indices and clinical factors in metastatic renal cell carcinoma patients treated with nivolumab: the development of a novel prognostic score (Meet-URO 15 study). Therapeutic advances in medical oncology. 2021;13:17588359211019642. [CrossRef]
- Colloca GA, Venturino A, Guarneri D. Reduction of derived neutrophil-to-lymphocyte ratio after four weeks predicts the outcome of patients receiving second-line chemotherapy for metastatic colorectal cancer. Cancer immunology, immunotherapy: CII. Apr 2021;70(4):1115-1125. [CrossRef]
- Grenader T, Nash S, Adams R, et al. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: a post hoc analysis of the MRC COIN study. British journal of cancer. Mar 15 2016;114(6):612-5. [CrossRef]
- Dalpiaz O, Pichler M, Mannweiler S, et al. Validation of the pretreatment derived neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. British journal of cancer. May 13 2014;110(10):2531-6. [CrossRef]
- Jariod-Ferrer Ú M, Arbones-Mainar JM, Gavin-Clavero MA, et al. Are Comorbidities Associated With Overall Survival in Patients With Oral Squamous Cell Carcinoma? Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. Sep 2019;77(9):1906-1914. [CrossRef]
- Kwon HR, Cho J, Park S, et al. Metabolic parameters on baseline (18)F-FDG PET/CT are potential predictive biomarkers for immunotherapy in patients with head and neck squamous cell carcinoma. Frontiers in medicine. 2022;9:896494. [CrossRef]
- Casadei-Gardini A, Rimini M, Kudo M, et al. Real Life Study of Lenvatinib Therapy for Hepatocellular Carcinoma: RELEVANT Study. Liver cancer. Dec 2022;11(6):527-539. [CrossRef]
- Fiore M, Ljevar S, Pasquali S, et al. Preoperative Neutrophil-To-Lymphocyte Ratio And A New Inflammatory Biomarkers Prognostic Index For Primary Retroperitoneal Sarcomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2022. [CrossRef]
- Sui Q, Zhang X, Chen C, et al. Inflammation promotes resistance to immune checkpoint inhibitors in high microsatellite instability colorectal cancer. Nature communications. Nov 28 2022;13(1):7316. [CrossRef]
- Mezquita L, Preeshagul I, Auclin E, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics. European journal of cancer (Oxford, England: 1990). Jul 2021;151:211-220. [CrossRef]
- Abbate V, Barone S, Troise S, et al. The Combination of Inflammatory Biomarkers as Prognostic Indicator in Salivary Gland Malignancy. Cancers. 2022. [CrossRef]
- Li X, Sun W, Ding X, Li W, Chen J. Prognostic model of immune checkpoint inhibitors combined with anti-angiogenic agents in unresectable hepatocellular carcinoma. Frontiers in immunology. 2022;13:1060051. [CrossRef]
- Minici R, Siciliano MA, Ammendola M, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR), Lymphocyte-to-Monocyte Ratio (LMR), Platelet-to-Lymphocyte Ratio (PLR) and Lymphocyte-to-C Reactive Protein Ratio (LCR) in Patients with Hepatocellular Carcinoma (HCC) undergoing Chemoembolizations (TACE) of the Liver: The Unexplored Corner Linking Tumor Microenvironment, Biomarkers and Interventional Radiology. Cancers Dec 30 2022;15(1). 2022. [CrossRef]
- Kachuri L, Jeon S, DeWan AT, et al. Genetic determinants of blood-cell traits influence susceptibility to childhood acute lymphoblastic leukemia. American journal of human genetics. Oct 7 2021;108(10):1823-1835. [CrossRef]
- Sano Y, Kogashiwa Y, Araki R, et al. Correlation of Inflammatory Markers, Survival, and COX2 Expression in Oral Cancer and Implications for Prognosis. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. Apr 2018;158(4):667-676. [CrossRef]
- Zubair F, McMahon J, Kryklyas G, Wicks C. Systemic inflammatory response in predicting outcomes of patients undergoing curative resection for oral squamous cell carcinoma. The British journal of oral & maxillofacial surgery. Jun 2022;60(5):589-595. [CrossRef]
- Ruiz-Ranz M, Lequerica-Fernández P, Rodríguez-Santamarta T, et al. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment. Frontiers in immunology. 2022;13:941351. [CrossRef]
- Košec A, Solter D, Ribić A, Knežević M, Vagić D, Pegan A. Systemic Inflammatory Markers as Predictors of Postoperative Complications and Survival in Patients With Advanced Head and Neck Squamous Cell Carcinoma Undergoing Free-Flap Reconstruction. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. Apr 2022;80(4):744-755. [CrossRef]
- Cho U, Sung YE, Kim MS, Lee YS. Prognostic Role of Systemic Inflammatory Markers in Patients Undergoing Surgical Resection for Oral Squamous Cell Carcinoma. Biomedicines. May 29 2022;10(6). [CrossRef]
- Xiong Y, Zhao N, Zheng Y, Wang J, Wei F, Ren X. Prognostic value of pretreatment inflammatory biomarkers in advanced lung adenocarcinoma patients receiving first-line pemetrexed/platinum doublet. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. Jun 2017;39(6):1010428317701639. [CrossRef]
- Winarto H, Habiburrahman M, Anggraeni TD, et al. The Utility of Pre-Treatment Inflammation Markers as Associative Factors to the Adverse Outcomes of Vulvar Cancer: A Study on Staging, Nodal Involvement, and Metastasis Models. Journal of clinical medicine. 2022. [CrossRef]
- Zhao TC, Liang SY, Ju WT, et al. High-risk lymph node ratio predicts worse prognosis in patients with locally advanced oral cancer. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. Sep 2020;49(8):787-795. [CrossRef]
- Ocaña A, Chacón JI, Calvo L, et al. Derived Neutrophil-to-Lymphocyte Ratio Predicts Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Frontiers in oncology. 2021;11:827625. [CrossRef]
- Bumbasirevic U, Bojanic N, Simic T, et al. Interplay between Comprehensive Inflammation Indices and Redox Biomarkers in Testicular Germ-Cell Tumors. Journal of personalized medicine. 20 May 20 2022;12(5). [CrossRef]
- Song S, Li C, Li S, Gao H, Lan X, Xue Y. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. OncoTargets and therapy. 2017;10:3145-3154. [CrossRef]
- Rajwa P, Życzkowski M, Paradysz A, et al. Novel hematological biomarkers predict survival in renal cell carcinoma patients treated with nephrectomy. Archives of medical science: AMS. 2020;16(5):1062-1071. [CrossRef]
- Liu XC, Dai YL, Huang F, Zhong ZJ, Liu XF. Diagnostic Value of Carcinoembryonic Antigen Combined with Multi-Inflammatory Cell Ratios in Colorectal Cancer. Disease markers. 2022;2022:4889616. [CrossRef]
- Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. Sep 2020;20(9):485-503. [CrossRef]
- Xue R, Zhang Q, Cao Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. Dec 2022;612(7938):141-147. [CrossRef]
- Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. Mar 2022;22(3):173-187. [CrossRef]
- Gungabeesoon J, Gort-Freitas NA, Kiss M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. Mar 30 2023;186(7):1448-1464.e20. [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. Feb 2018;18(2):134-147. [CrossRef]
- Xiao Y, Cong M, Li J, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. Mar 8 2021;39(3):423-437.e7. [CrossRef]
- van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. Apr 2020;20(4):218-232. [CrossRef]

| total | Control group | TPF chemotherapy induction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dNLR≤1.555 | dNLR>1.555 | t/χ²/Fisher exact test | dNLR≤1.555 | dNLR>1.555 | t/χ²/Fisher exact test | ||||||||||
| N | % | N | % | N | % | p | N | % | N | % | p | ||||
| Age(years) | |||||||||||||||
| Average | 55.4 | 54.8 | 56.1 | 56 | 56.2 | ||||||||||
| Range | 26, 75 | 26, 75 | 29, 74 | 32, 73 | 29, 74 | ||||||||||
| <60 | 168 | 66 | 45 | 73 | 40 | 61 | 2.055 | 0.152 | 49 | 67 | 34 | 62 | 0.387 | 0.534 | |
| ≥60 | 88 | 34 | 17 | 27 | 26 | 39 | 24 | 33 | 21 | 38 | |||||
| Gender | |||||||||||||||
| Female | 77 | 30 | 20 | 32 | 20 | 30 | 0.057 | 0.812 | 21 | 29 | 16 | 29 | 0.002 | 0.968 | |
| Male | 179 | 70 | 42 | 68 | 46 | 70 | 52 | 71 | 39 | 71 | |||||
| Smoking status | |||||||||||||||
| Negative | 126 | 49 | 39 | 63 | 32 | 48 | 2.691 | 0.101 | 36 | 49 | 23 | 42 | 0.710 | 0.400 | |
| Positive | 130 | 51 | 23 | 37 | 34 | 52 | 37 | 51 | 32 | 58 | |||||
| Alcohol Status | |||||||||||||||
| Negative | 98 | 38 | 42 | 68 | 40 | 61 | 0.707 | 0.400 | 45 | 62 | 31 | 56 | 0.363 | 0.547 | |
| Positive | 158 | 62 | 20 | 32 | 26 | 39 | 28 | 38 | 24 | 44 | |||||
| Tumor site | |||||||||||||||
| Tongue | 113 | 44 | 31 | 50 | 29 | 44 | 2.568 | 0.766 | 34 | 47 | 19 | 35 | 4.352 | 0.500 | |
| Buccal | 45 | 18 | 9 | 15 | 11 | 17 | 13 | 18 | 12 | 22 | |||||
| Gingiva | 40 | 16 | 9 | 15 | 10 | 15 | 12 | 16 | 9 | 16 | |||||
| Floor of mouth | 30 | 12 | 6 | 10 | 12 | 18 | 6 | 8 | 6 | 11 | |||||
| Palate | 18 | 7 | 3 | 5 | 3 | 5 | 5 | 7 | 7 | 13 | |||||
| Retromolar triangle | 10 | 4 | 4 | 6 | 1 | 2 | 3 | 4 | 2 | 4 | |||||
| BMI | |||||||||||||||
| Underweight | 20 | 8 | 5 | 8 | 6 | 9 | 1.137 | 0.258 | 5 | 7 | 4 | 8 | 0.501 | 0.617 | |
| Normal | 132 | 52 | 34 | 55 | 39 | 59 | 35 | 49 | 24 | 45 | |||||
| Overweight | 72 | 28 | 16 | 26 | 17 | 26 | 24 | 33 | 15 | 28 | |||||
| Obese | 29 | 11 | 7 | 11 | 4 | 6 | 8 | 11 | 10 | 19 | |||||
| cT stage | |||||||||||||||
| T1 | 9 | 4 | 0 | 0 | 2 | 3 | 1.637 | 0.749 | 4 | 5 | 3 | 5 | 0.911 | 0.937 | |
| T2 | 57 | 22 | 12 | 19 | 13 | 20 | 20 | 27 | 12 | 22 | |||||
| T3 | 149 | 58 | 40 | 65 | 40 | 61 | 38 | 52 | 31 | 56 | |||||
| T4 | 41 | 16 | 10 | 16 | 11 | 17 | 11 | 15 | 9 | 16 | |||||
| cN stage | |||||||||||||||
| N0 | 110 | 43 | 31 | 50 | 30 | 45 | 3.516 | 0.172 | 29 | 40 | 20 | 36 | 0.397 | 0.820 | |
| N1 | 94 | 37 | 23 | 37 | 19 | 29 | 30 | 41 | 22 | 40 | |||||
| N2 | 52 | 20 | 8 | 13 | 17 | 26 | 14 | 19 | 13 | 24 | |||||
| TNM clinical stage | |||||||||||||||
| III | 177 | 69 | 50 | 81 | 43 | 65 | 3.863 | 0.049 | 49 | 67 | 35 | 64 | 0.169 | 0.681 | |
| IVa | 79 | 31 | 12 | 19 | 23 | 35 | 24 | 33 | 20 | 36 | |||||
| OSCC: oral squamous cell carcinoma; TPF: docetaxel, cisplatin, and 5-FU; dNLR: derived neutrophil to lymphocyte ratio; BMI: body mass index. | |||||||||||||||
| Standard treatment group (N = 115) | TPF induction chemotherapy group (N = 109) | |||||
|---|---|---|---|---|---|---|
| Low dNLR | High dNLR | Low dNLR | High dNLR | |||
| (n = 56) | (n = 59) | (n = 63) | (n = 46) | |||
| Overall survival | 69.4% | 40.4% | 77.4% | 35.7% | ||
| Disease-free survival | 57.8% | 30.4% | 74.0% | 28.1% | ||
| Locoregional recurrence-free survival | 62.9% | 35.9% | 74.0% | 32.1% | ||
| Distant metastasis-free survival | 67.7% | 40.4% | 80.8% | 35.7% | ||
| TPF: docetaxel, cisplatin, and 5-FU; dNLR: derived neutrophil to lymphocyte ratio. | ||||||
| Variable | OS | DFS | LRFS | DMFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Sex (vs. Female) | 0.965 | 0.568-1.642 | 0.897 | 0.736 | 0.452-1.199 | 0.219 | 0.744 | 0.453-1.222 | 0.242 | 0.985 | 0.580-1.676 | 0.957 |
| Age (vs. <60 years) | 1.199 | 0.716-2.009 | 0.490 | 1.066 | 0.654-1.738 | 0.796 | 1.075 | 0.654-1.768 | 0.775 | 1.166 | 0.697-1.952 | 0.559 |
| Smoking status (vs. non-smoker) | 1.219 | 0.743-2.000 | 0.433 | 1.021 | 0.639-1.630 | 0.932 | 1.013 | 0.630-1.630 | 0.956 | 1.225 | 0.747-2.009 | 0.421 |
| Alcohol status (vs. non-alcohol abuser.) | 1.385 | 0.807-2.376 | 0.237 | 1.441 | 0.867-2.394 | 0.158 | 1.339 | 0.799-2.245 | 0.286 | 1.432 | 0.834-2.456 | 0.193 |
| BMI at diagnosis | 0.005 | 0.009 | 0.007 | 0.008 | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||||||
| Underweight | 2.145 | 1.065-4.321 | 0.033 | 1.915 | 0.961-3.814 | 0.065 | 2.106 | 1.053-4.210 | 0.035 | 2.067 | 1.027-4.162 | 0.042 |
| Overweight | 0.527 | 0.270-1.029 | 0.060 | 0.581 | 0.319-1.062 | 0.077 | 0.113 | 0.334-1.122 | 0.612 | 0.538 | 0.276-1.051 | 0.070 |
| Obese | 0.398 | 0.123-1.287 | 0.124 | 0.332 | 0.103-1.069 | 0.064 | 0.345 | 0.107-1.114 | 0.075 | 0.399 | 0.123-1.292 | 0.125 |
| cTNM (vs. III) | 1.993 | 1.184-3.354 | 0.009 | 1.792 | 1.088-2.952 | 0.022 | 1.939 | 1.173-3.207 | 0.010 | 1.603 | 1.127-3.190 | 0.016 |
| dNLR | 1.227 | 1.099-1.369 | <0.001 | 1.186 | 1.063-1.324 | 0.002 | 1.200 | 1.073-1.341 | 0.001 | 1.218 | 1.091-1.361 | <0.001 |
| OS: overall survival; DFS: disease-free survival; LRFS: locoregional recurrence-free survival; DMFS: distant metastasis-free survival; HR: hazard ratio; BMI: body mass index; dNLR: derived neutrophil to lymphocyte ratio. | ||||||||||||
| Variable | OS | DFS | LRFS | DMFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Alcohol status (vs. non-alcohol abuser) | 1.289 | 0.761-2.181 | 0.345 | 1.257 | 0.771-2.048 | 0.359 | 1.184 | 0.722-1.944 | 0.503 | 1.305 | 0.770-2.212 | 0.322 |
| BMI at diagnosis | 0.067 | 0.063 | 0.062 | 0.083 | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||||||
| Underweight | 1.557 | 0.708-3.425 | 0.271 | 1.495 | 0.700-3.191 | 0.299 | 1.662 | 0.777-3.554 | 0.190 | 1.515 | 0.689-3.335 | 0.302 |
| Overweight | 0.510 | 0.995-1.268 | 0.061 | 0.574 | 0.313-1.050 | 0.071 | 0.605 | 0.329-1.111 | 0.105 | 0.523 | 0.267-1.024 | 0.059 |
| Obese | 0.477 | 0.146-1.557 | 0.220 | 0.385 | 0.118-1.250 | 0.112 | 0.400 | 0.123-1.301 | 0.128 | 0.475 | 0.146-1.552 | 0.218 |
| cTNM (vs. III) | 2.026 | 1.196-3.434 | 0.009 | 1.765 | 1.068-2.916 | 0.027 | 1.917 | 1.155-3.184 | 0.012 | 1.910 | 1.128-3.235 | 0.016 |
| dNLR | 1.154 | 1.018-1.309 | 0.025 | 1.123 | 0.260-1.000 | 0.050 | 1.134 | 1.002-1.283 | 0.047 | 1.146 | 1.010-1.300 | 0.035 |
| OS: overall survival; DFS: disease-free survival; LRFS: locoregional recurrence-free survival; DMFS: distant metastasis-free survival; HR: hazard ratio; BMI: body mass index; dNLR: derived neutrophil to lymphocyte ratio. | ||||||||||||
| Variable | OS | DFS | LRFS | DMFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Sex (vs. Female) | 1.212 | 0.657-2.237 | 0.539 | 1.219 | 0.687-2.160 | 0.499 | 1.189 | 0.670-2.111 | 0.555 | 1.207 | 0.654-2.228 | 0.548 |
| Age (vs. <60 years) | 1.193 | 0.682-2.087 | 0.535 | 1.186 | 0.704-1.996 | 0.521 | 1.213 | 0.719-2.047 | 0.470 | 1.183 | 0.676-2.068 | 0.556 |
| Smoking status (vs. non-smoker) | 1.366 | 0.785-2.378 | 0.270 | 1.266 | 0.757-2.116 | 0.369 | 1.221 | 0.728-2.048 | 0.448 | 1.374 | 0.789-2.393 | 0.261 |
| Alcohol status (vs. non-alcohol abuser) | 1.144 | 0.661-1.978 | 0.631 | 1.175 | 0.706-1.955 | 0.534 | 1.124 | 0.672-1.880 | 0.656 | 1.135 | 0.656-1.963 | 0.650 |
| BMI at diagnosis | 0.550 | 0.504 | 0.511 | 0.567 | ||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | ||||||||
| Underweight | 1.549 | 0.593-4.051 | 0.372 | 1.256 | 0.488-3.236 | 0.637 | 1.314 | 0.509-3.393 | 0.573 | 1.564 | 0.598-4.088 | 0.362 |
| Overweight | 0.743 | 0.380-1.453 | 0.386 | 0.659 | 0.351-1.240 | 0.196 | 0.676 | 0.358-1.276 | 0.227 | 0.757 | 0.387-1.480 | 0.415 |
| Obese | 1.065 | 0.480-2.361 | 0.877 | 1.019 | 0.485-2.140 | 0.961 | 1.054 | 0.500-2.220 | 0.890 | 1.065 | 0.480-2.362 | 0.876 |
| cTNM (vs. III) | 1.588 | 0.915-2.755 | 0.100 | 1.926 | 1.154-3.215 | 0.012 | 1.991 | 1.188-3.337 | 0.009 | 1.603 | 0.924-2.782 | 0.093 |
| dNLR | 1.154 | 1.035-1.285 | 0.010 | 1.141 | 1.029-1.266 | 0.013 | 1.139 | 1.024-1.268 | 0.016 | 1.152 | 1.034-1.282 | 0.010 |
| TPF: docetaxel, cisplatin, and 5-FU; OS: overall survival; DFS: disease-free survival; LRFS: locoregional recurrence-free survival; DMFS: distant metastasis-free survival; HR: hazard ratio; BMI: body mass index; dNLR: derived neutrophil to lymphocyte ratio. | ||||||||||||
| Variable | OS | DFS | LRFS | DMFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Alcohol status (vs. non-alcohol abuser) | 1.121 | 0.648, 1.939 | 0.684 | 1.155 | 0.692, 1.927 | 0.582 | 1.100 | 0.656, 1.845 | 0.717 | 1.111 | 0.642, 1.923 | 0.706 |
| cTNM (vs. III) | 1.558 | 0.896, 2.709 | 0.116 | 1.857 | 1.107, 3.112 | 0.019 | 1.931 | 1.148, 3.248 | 0.013 | 1.570 | 0.903, 2.731 | 0.110 |
| dNLR | 1.147 | 1.031, 1.276 | 0.011 | 1.128 | 1.019, 1.250 | 0.021 | 1.125 | 1.014, 1.249 | 0.027 | 1.145 | 1.030, 1.272 | 0.012 |
| TPF: docetaxel, cisplatin, and 5-FU; OS: overall survival; DFS: disease-free survival; LRFS: locoregional recurrence-free survival; DMFS: distant metastasis-free survival; HR: hazard ratio; BMI: body mass index; dNLR: derived neutrophil to lymphocyte ratio. | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





