1. Introduction
Probiotics and prebiotics are known for their beneficial effects on gut health and overall well-being. Scientific evidence supports their role in improving digestion and enhancing immune function. Probiotics are live beneficial bacteria, while prebiotics are non-digestible food components that promote the growth of these bacteria. Together, they form a symbiotic relationship and enhance each other's effects. These benefits are supported by scientific evidence and come from natural sources and fortified foods [
1,
2].
Fermented dairy products, rich in live beneficial bacteria, are traditional probiotic sources and are pivotal in human diets [
3]. During fermentation, bacteria convert lactose into lactic acid, imparting distinct flavor and extending shelf life [
4]. The food industry offers diverse probiotic products like yogurt, kefir, and acidified milk, containing live biocultures that impact gut microbiota [
5,
6].
Microwave treatment is a modern extraction method that efficiently extracts bioactive compounds from plant materials. This method breaks down cell walls, releasing phytochemicals and improving extraction yields and processing times compared to traditional methods. Studies show that microwave treatment enhances the bioavailability and biological activities of plant compounds [
7,
8].
Hawthorn extract, derived from a medicinal herb, contains bioactive compounds like tannins and flavonoids, known for their anti-inflammatory and antioxidant properties. There is growing interest in utilizing hawthorn extract in food and beverage formulations due to its potential health benefits [
9,
10,
11].
Developing a symbiotic fermented dairy product involves combining probiotics, prebiotics, and bioactive compounds. Microwave-treated hawthorn extract is incorporated to enhance nutritional value, sensory characteristics, and potential health benefits.
Despite the potential advantages of incorporating probiotics and bioactive compounds into fermented products, challenges exist in maintaining probiotic viability during production and storage. Factors such as pH, composition, and storage conditions significantly impact probiotic survival [
12]. Probiotic bacteria, especially bifidobacteria, are sensitive to oxygen, which inhibits their growth in fermented milk products [
13]. Strategies like adjusting redox potential and adding antioxidants are employed to enhance viability.
Research has shown the potential of polyphenols (antioxidants) from plant extracts to influence probiotic growth and viability. Activation methods, such as pre-adaptation of bifidobacteria, are crucial for maintaining probiotic activity in dairy products. Various activation techniques are used in the production of fermented dairy products to ensure high functional properties. Thus, using prebiotic-antioxidants to activate bifidobacteria is promising for developing functional foods containing synbiotics.
This study aims to investigate the impact of incorporating bifidobacteria activated with hawthorn extract on the development and characteristics of symbiotic fermented milk products. To achieve these goals, microwave extracts obtained from native hawthorn were used in specific concentrations (10
-5 and 10
-10 g/cm³). These concentrations were selected based on research results on melanins from aqueous microwave extracts, which showed a significant bifidogenic effect at these levels [
14]. Building on this foundation, the study hypothesizes that plant extracts, especially those rich in bioactive compounds, can enhance the viability of probiotics and preserve them during the development of fermented dairy product technology. The study will compare the composition of symbiotic fermented milk products enriched with
B. bifidum activated with different concentrations of hawthorn extract to determine their effect on product characteristics. The research focuses on elucidating the influence of bifidobacteria activated with hawthorn extract on the organoleptic characteristics and main physicochemical properties of the developed symbiotic fermented milk product.
2. Materials and Methods
2.1. Materials
Shredded Turkestan hawthorn (Crataegus turkestanica) was purchased from a pharmacy chain (Zerde-Fito Pharmaceutical Company LLP, Shymkent, Kazakhstan). The bifidobacteria source was the medicinal product “Bifidumbacterin” (ZAO Partner, Moscow, Russia), and the L. acidophilus probiotic culture was sourced from microMilk PR A (Micromilk, Cremosano (CR), Italy). B. bifidum and L. acidophilus were enumerated on TPY and MRS media (Condalab, Madrid, Spain).
2.2. Microwave-Treated Hawthorn Extracts Obtaining Method
Hawthorn extract was obtained using a microwave treatment method. 20 g of hawthorn powder were mixed with 200 ml of distilled water in a flask, heated in the microwave's defrost mode for 4 minutes until reaching 70°C, and then placed in a desiccator at 70°C for 3 hours. After adding 2 ml of 25% hydrochloric acid solution to the extract and leaving it for 12 hours, the precipitate was filtered and dried in atmospheric air for 12–14 hours [
15].
2.3. Water-Soluble Antioxidants Content Determination
Water-soluble antioxidants in symbiotic fermented milk products were determined using the "TsvetYauza-01-AA" device, based on the amperometric method. Extraction was done with a 70% ethyl alcohol solution, and a gallic acid solution was used for calibration [
16].
2.4. Enumeration of B. bifidum and L. acidophilus in Symbiotic Fermented Milk Products
The number of bacteria (
L. acidophilus and
B. bifidum) was determined by allowing them to grow in specific nutrient media (on DeMan Rogosa Sharpe (MRS) agar for
L. acidophilus and TPY broth for
B. bifidum) in tubes and Petri dishes at 37±1°C for 24–72 hours. The number of bacterial cells in 1.0 cm³ of the sample was calculated by multiplying the number of colonies by the dilution factor. The average of two parallel cultures was accepted as the final result [
17].
2.5. Microscopic Examination
Microscopic examination of the samples was conducted using a Levenhuk D400LCD (China) electron microscope at 100x magnification after pre-staining with methylene blue dye.
2.6. Amino Acid Determination
The mass fraction of amino acids (Arg, Lys, Tyr, Phe, His, Leu+Ile, Met, Val, Pro, Ser, Ala, and Gly) was determined using the Lumex method M 04-38 (2009) in the “CAPEL 105 M” capillary electrophoresis system with a special capillary cassette for amino acid analysis. Samples underwent acid hydrolysis, conversion into free forms, and separation by capillary electrophoresis. Detection occurred at 254 nm, with tryptophan quantified at 219 nm. Data were processed using specialized software.
2.7. Mineral Content Determination
The mineral content of symbiotic fermented milk products was determined using a Scanning Electron Microscope JSM-6490LV (Japan) with an energy-dispersive microanalysis system INCA Energy 350 and HKL Basic system.
2.8. Analysis of Experimental Data
Statistical data processing utilized MS Excel and Statistica (TIBCO Software Inc, CA, USA) software. Values were expressed as means ± standard deviations from three independent experiments (n=3) at a 90% confidence level (P=0.90).
3. Results and Discussion
3.1. Development of Symbiotic Fermented Milk Product Technology
In developing the technology for symbiotic fermented functional products, pasteurized milk was mixed with specific concentrations of Lactobacillus acidophilus starter and activated Bifidobacterium bifidum 1 with hawthorn extract. The process began with pasteurized milk with 2.5% fat and a density of 1028 g/cm³. The L. acidophilus starter was added at a concentration of 2.5% together with activated B. bifidum 1 with hawthorn extract at concentrations of 10-5 and 10-10 g/cm³, representing 10% of the total mixture. The bifidobacteria were first incubated in hawthorn extract solutions for 30 minutes at 37±1 ºC for activation. Three different products were prepared:
Control: Symbiotic fermented milk product containing only L. acidophilus.
Product 1: Symbiotic fermented milk product based on L. acidophilus and enriched with B. bifidum, activated with hawthorn extract at a concentration of 10-5 g/cm³.
Product 2: Symbiotic fermented milk product based on L. acidophilus and enriched B. bifidum activated with hawthorn extract at a concentration of 10-10 g/cm³.
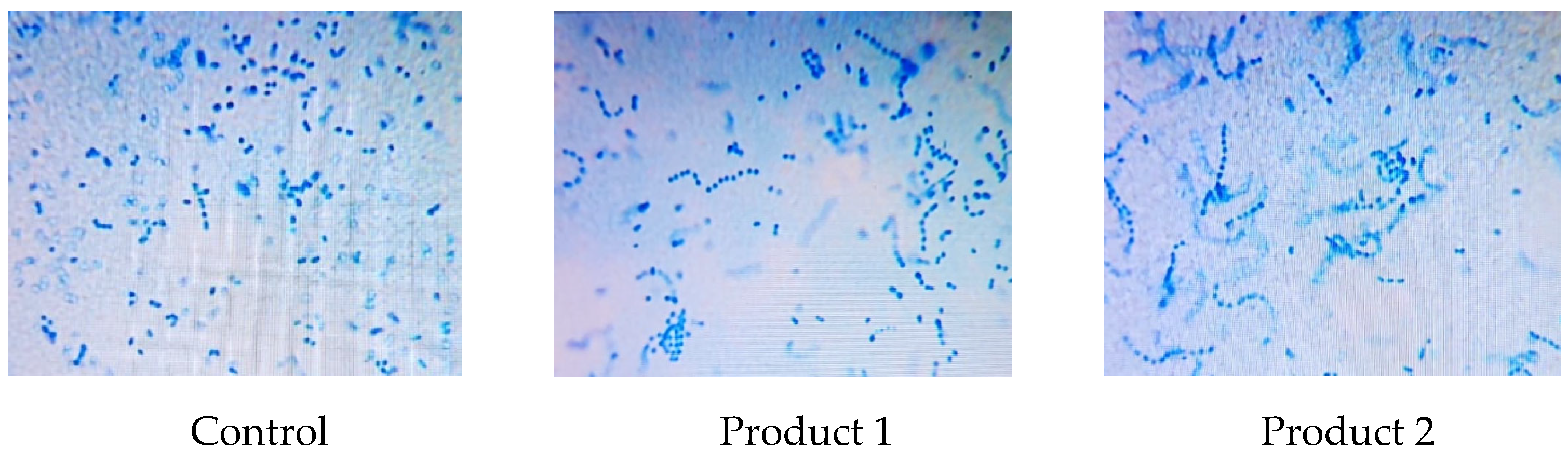
Fermentation lasted 10 hours until an acidity of 75 °T was reached, followed by a maturation period at 6-10 °C for 12 hours. Microscopic examination revealed clear characteristics of the finished symbiotic fermented milk products, as shown in
Figure 1.
It was observed that product 1 and product 2 contained more viable probiotic cells comparison to the control.
3.2. Microbiological Parameters
The microbiological parameters of symbiotic fermented milk products enriched with bifidobacteria activated with hawthorn extract at different concentrations, compared to a control sample, are shown in
Table 1.
According to the results obtained, all products meet or exceed the required concentration of lactic acid microorganisms (GOST 32901-2014) (Federal Agency on Technical Regulating and Metrology, 2014) with product 1 and product 2 having significantly higher concentrations compared to the control. No detectable amounts of E. coli, S. aureus, or other pathogenic microorganisms were found in any sample. The number of yeast and mould cells was below the permitted limit prescribed by the Technical Regulations of the Customs Union “On the safety of milk and dairy products,” thus ensuring the hygienic-sanitary safety of the produced products.
3.3. Organoleptic Characterization
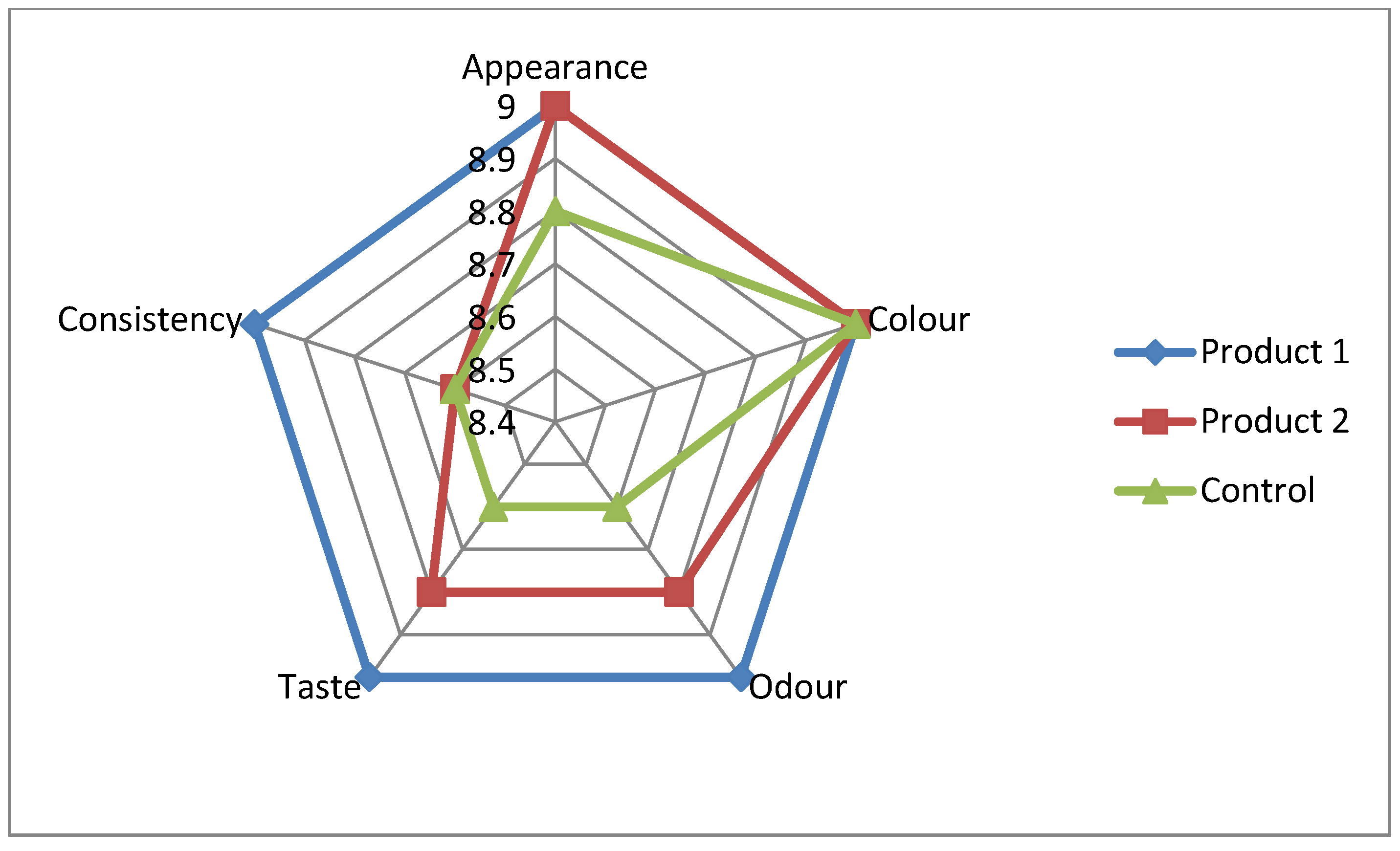
The organoleptic characterization of finished symbiotic fermented milk products provides valuable insights into their sensory properties, including appearance, colour, odour, taste, and consistency. Each parameter is scored on a 9-point scale, with a higher score indicating better sensory quality.
Figure 2 shows the characteristics of the finished symbiotic fermented milk products.
The organoleptic characterization shows that product 1 and product 2 have favorable sensory properties comparable to or even exceeding those of the control. These results show that the enrichment of symbiotic fermented milk products with B. These results demonstrate that enriching symbiotic fermented milk products with B. bifidum activated with hawthorn extract, especially at a concentration of 10-5 g/cm³, improves sensory properties such as odor and taste without affecting other parameters such as appearance, colour, and consistency. This highlights the potential of using bifidobacteria activated with hawthorn extract to develop fermented milk products with improved sensory properties and potentially higher consumer acceptance.
3.4. Main Characteristics of Symbiotic Fermented Milk Products Over 14 Days
Table 2 presents data on the titratable acidity, bacterial counts of
Lactobacillus acidophilus and
Bifidobacterium bifidum, viscosity, and water-holding capacity of control and two symbiotic fermented milk products over a 14-day period.
All products meet the requirements of GOST 54340-2011 and 33491-2015 (Federal Agency on Technical Regulating and Metrology, 2011 and 2015). The data obtained from
Table 2 showed that the increase in titratable acidity was slower over 14 days in products 1 and 2, which were enriched with
B. bifidum activated by hawthorn extract, compared to the control. On day 7, titratable acidity in the control and products 1 and 2 increased by 15.6%, 13.2%, and 14.0%, respectively, compared to the 24-hour values. However, on day 14, titratable acidity in the control increased by 35%, while in products 1 and 2, it increased by 28%. This indicates that the slower increase in acidity in the enriched products suggests better preservation and slower spoilage, potentially maintaining more favorable sensory characteristics.
The viscosity of the control increased by 4% on day 7 and 2% on day 14 compared to the control after 24 hours. In contrast, the viscosity in product 1 and product 2 exceeded the control by 11% and 9.8%, respectively, on day 7, and by 10% and 7% on day 14. Higher viscosities in the enriched products suggest improved texture and mouthfeel, enhancing the sensory experience.
At 7 days, the populations of L. acidophilus in the control and products 1 and 2 increased by 13.1%, 19.4%, and 18.1%, respectively, compared to the levels measured at 24 hours post-production. Similarly, the viable counts of B. bifidum in the control and products 1 and 2 increased by 20.4%, 12.8%, and 19.8%, respectively, compared to the 24-hour measurement.
By the 14th day, a decrease in the population of L. acidophilus was observed in the control and products 1 and 2, with reductions of 14.1%, 13.4%, and 17.6%, respectively, compared to the 7-day counts. A decrease in the viable count of B. bifidum was also noted in the control and products 1 and 2, with reductions of 38.4%, 15.5%, and 15.5%, respectively, compared to the 7-day counts. This reduction may be attributed to nutrient depletion or the accumulation of metabolic by-products.
Over a period of 14 days, the viable count of L. acidophilus in product 1, with B. bifidum activated using hawthorn extract at a concentration of 10-5 g/cm³, increased by 10.6% compared to the control at 24 hours. In product 2, with B. bifidum activated using hawthorn extract at a concentration of 10-10 g/cm³, the viable count of L. acidophilus increased by 7.0% compared to the control at 24 hours. Additionally, over the same period, the viable count of B. bifidum in product 1, with hawthorn extract at a concentration of 10-5 g/cm³, increased by 12.2%, and in product 2, at a concentration of 10-10 g/cm³, increased by 8.7% compared to the control at 24 hours.
The data indicate that the viable counts of L. acidophilus and B. bifidum in symbiotic fermented milk products 1 and 2 with hawthorn extract ranged from 109 - 1010 CFU/cm³ at 7 days. Thus, the addition of hawthorn extract significantly improved the stability and viability of L. acidophilus and B. bifidum in symbiotic fermented milk products. The enriched products demonstrated better control over titratable acidity, higher bacterial counts, and improved viscosity and water-holding capacities over 14 days compared to the control. These enhancements suggest that hawthorn extract serves as an effective prebiotic, supporting the growth and sustainability of probiotic cultures and consequently enhancing the sensory properties of the fermented milk products.
These findings align with previous studies that reported improvements in probiotic viability and sensory qualities in fermented milk products enriched with natural extracts. For example, research by Terpou et al. has shown that the addition of prebiotics can improve the viability of probiotic bacteria in foods [
18]. Similarly, other studies have reported improvements in the number of probiotics and sensory properties of fermented milk products with bifidobacteria and natural plant extracts [
19,
20].
3.5. Amino Acid Composition of Symbiotic Fermented Milk Products
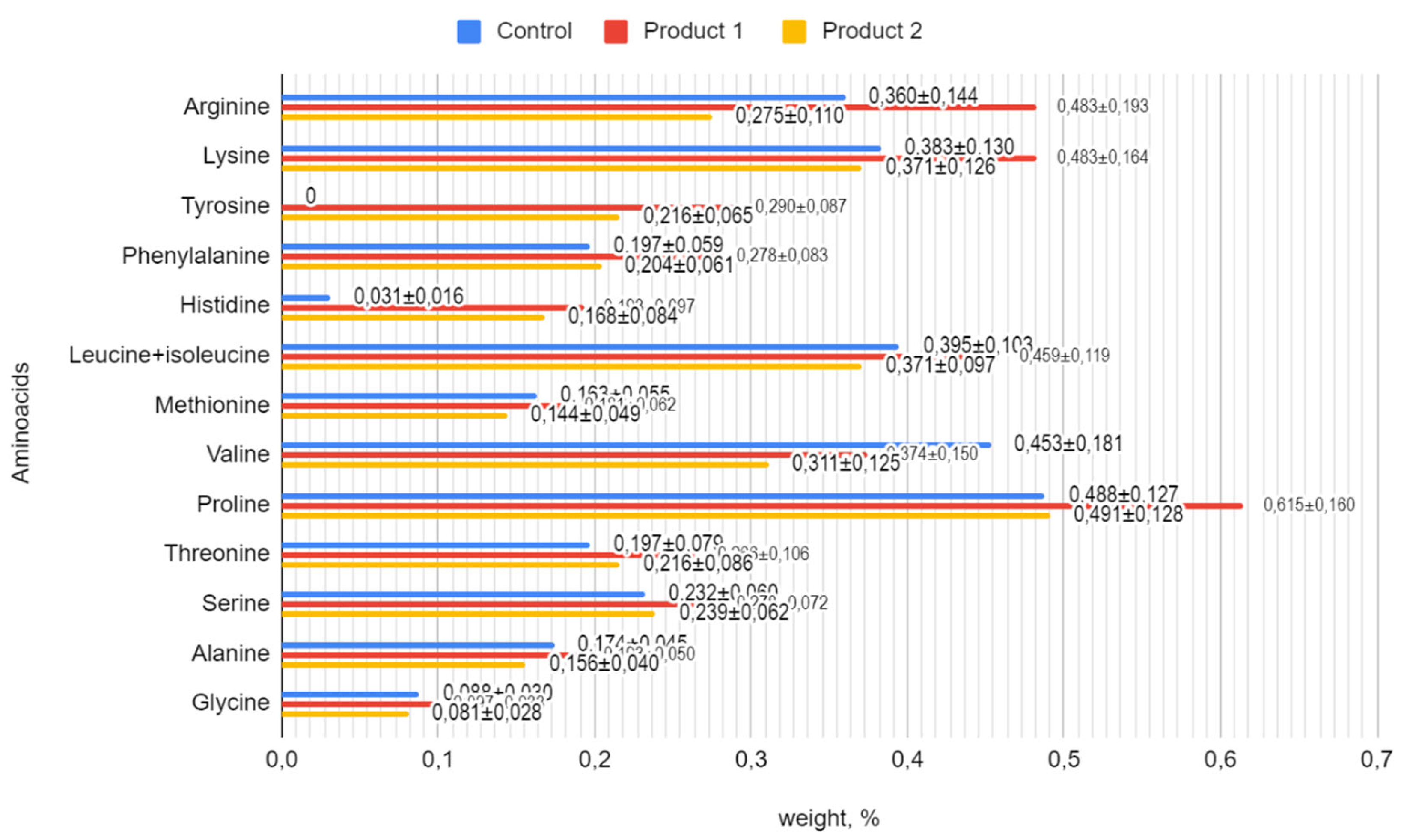
The amino acid composition of the symbiotic fermented milk products was analyzed to assess their nutritional profile. The concentrations of various amino acids in the control and experimental samples (product 1 and product 2) are presented in
Figure 3.
Amino acids are the building blocks of proteins and play essential roles in various physiological functions in the human body. The analysis of the amino acid composition revealed remarkable differences between the samples. Product 1 and product 2 had higher concentrations of certain essential and non-essential amino acids compared to the control, indicating a possible improvement in nutritional value.
The activation of B. bifidum 1 with hawthorn extract at concentrations of 10-5 g/cm³ resulted in an enrichment of the composition of product 1 with the following amino acids: arginine (0.483%), lysine (0.483%), tyrosine (0.290%), phenylalanine (0.278%), histidine (0.193%), leucine+isoleucine (0.459%), methionine (0.181%), proline (0.615%), threonine (0.266%), serine (0.278%), alanine (0.193%), and glycine (0.097%) compared to the control. Activation of the B. bifidum 1 with hawthorn extract at a concentration of 10-10 g/cm³ resulted in an enrichment of the composition of product 2 with the following amino acids: tyrosine (0.216%), histidine (0.168%), and threonine (0.216%) compared to the control.
Arginine is a semi-essential amino acid that is involved in various physiological processes, including immune function and protein synthesis [
21]. Aromatic amino acids such as tyrosine and phenylalanine play a crucial role in protein synthesis and the production of neurotransmitters. Increasing their content could improve the flavor and nutritional value of fermented products.
These changes could be attributed to the fermentation process and the addition of bifidobacteria activated with hawthorn extract. The significant increase in proline content suggests potential benefits in tissue repair and skin health. These results are consistent with previous research highlighting the effects of bifidobacteria and herbal extracts on amino acid composition in fermented dairy products [
22,
23].
3.6. Vitamin and Mineral Composition of Symbiotic Fermented Milk Products
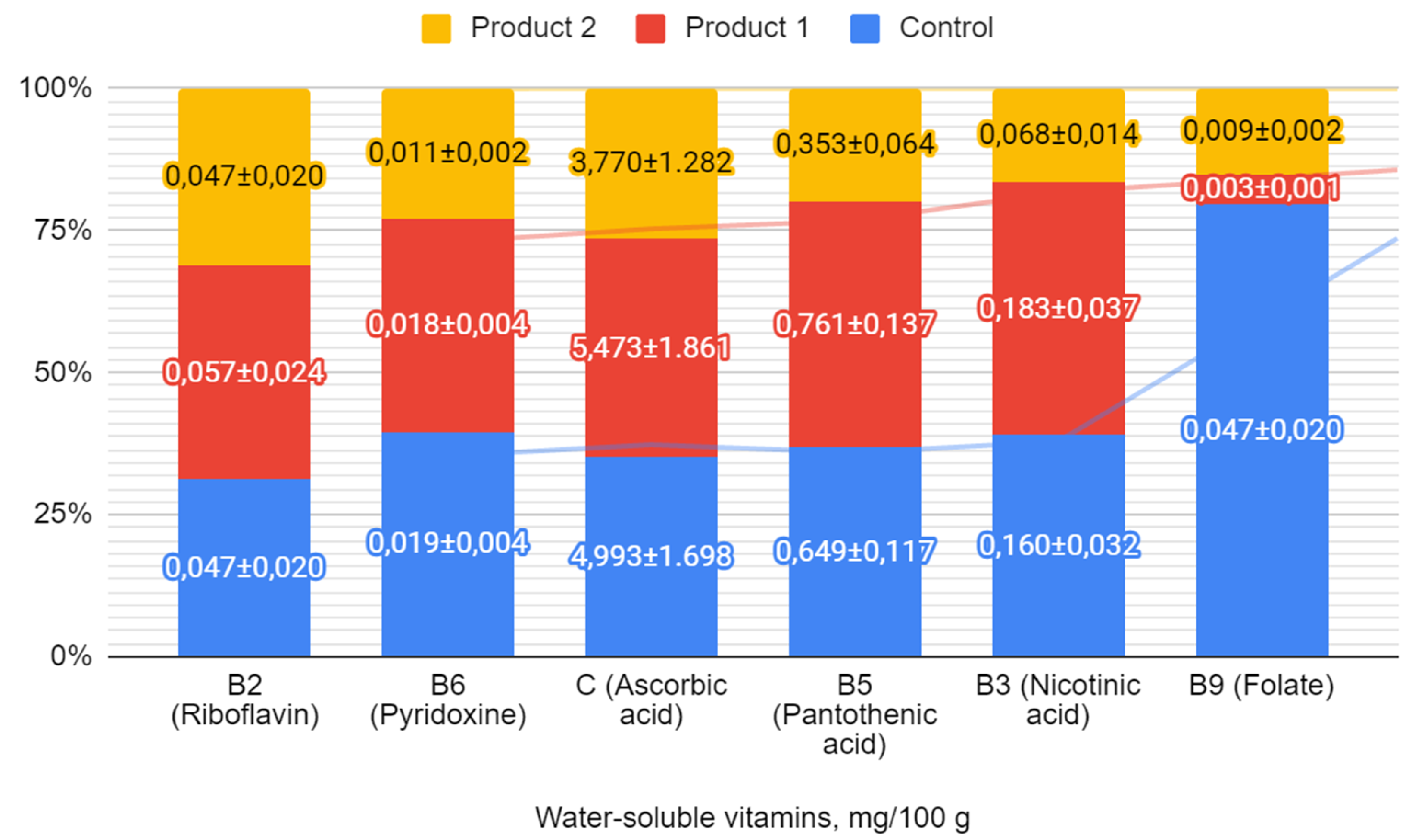
Figure 4 presents the water-soluble vitamin content of the symbiotic fermented milk products enriched with bifidobacteria activated with hawthorn extract, compared to the control.
The analysis presented in
Figure 4 demonstrates that product 1, which is enriched with
B. bifidum activated with hawthorn extract at a concentration of 10
-5 g/cm³, exhibits significantly increased levels of several vitamins compared to the control sample. Specifically, riboflavin (B
2) levels increased by 21.3%, ascorbic acid (C) by 9.6%, nicotinic acid (B
3) by 14.4%, and pantothenic acid (B
5) by 17.2%. These enhancements suggest an improvement in the nutritional value of product 1. In contrast, product 2 showed a decrease in water-soluble vitamin content. This indicates that the effect of bifidobacteria activation by hawthorn extract can vary depending on the concentration used.
These findings are consistent with existing literature, which highlights the role of prebiotics, such as hawthorn extract, in enhancing the activity of probiotics like
B. bifidum. Studies have shown that prebiotics can modulate the metabolic activity of probiotics, leading to increased production of certain vitamins and other beneficial metabolites [
24,
25]. Specifically, the activation of probiotics by plant extracts has been associated with enhanced synthesis of B vitamins, which are crucial for various physiological functions and overall health [
26,
27].
The observed decrease in vitamin content in product 2 underscores the complexity of probiotic activation and the importance of optimizing the concentration of prebiotic extracts. It suggests that while certain concentrations of hawthorn extract can boost vitamin production, others may not be as effective or could potentially inhibit it. This variability is supported by research indicating that the interaction between probiotics and prebiotics is dose-dependent and can be influenced by several factors, including the type of probiotic strain and the specific prebiotic used [
24,
29].
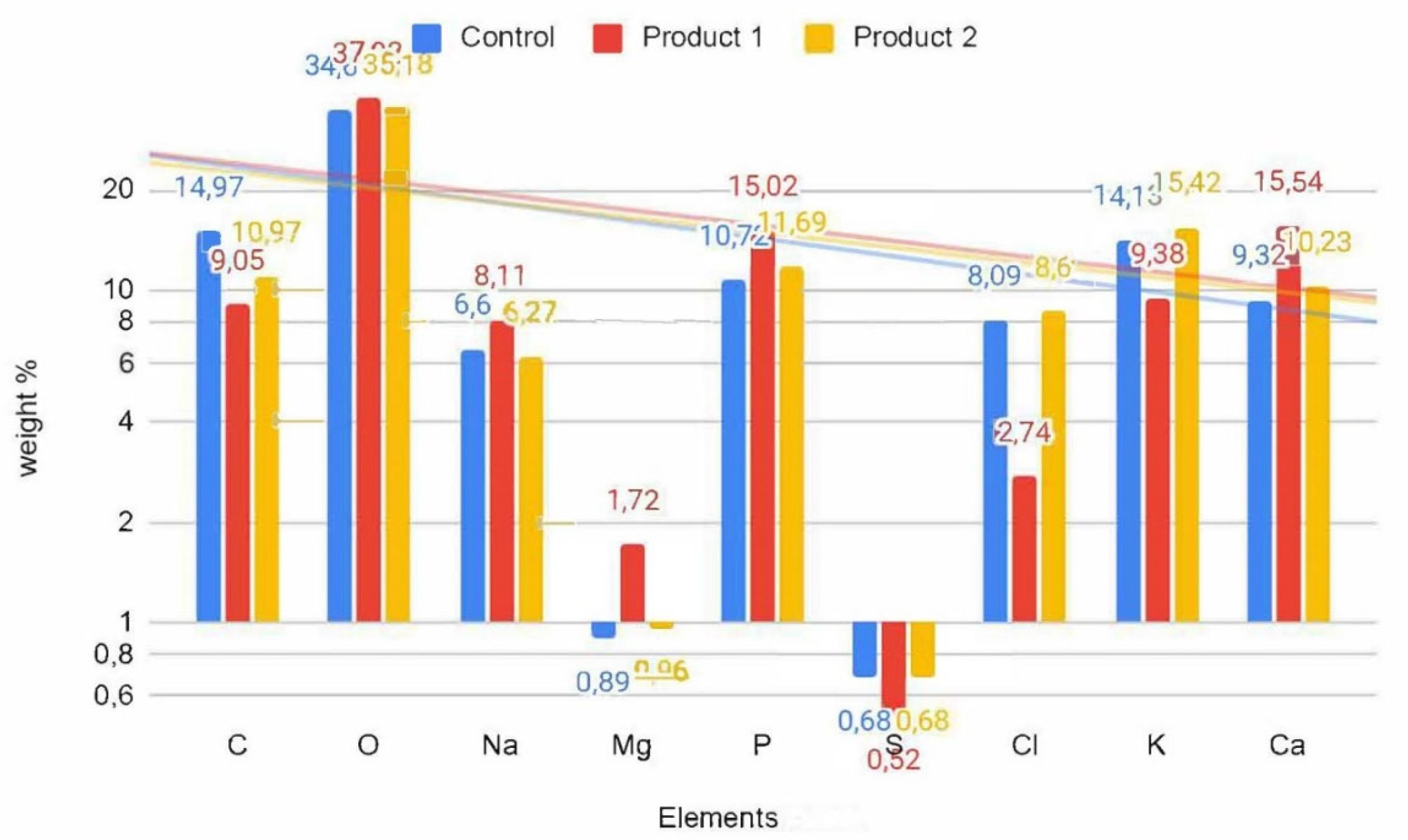
The mineral content is presented in
Figure 5.
The mineral composition also varied (
Figure 5), with product 1 showing an increase in sodium (Na) – 8.11 %, magnesium (Mg) – 1.72 %, phosphorus (P) – 15.02 % and calcium (Ca) – 15.54 %, while product 2 showed an increase in chloride (Cl) – 8.60 %, potassium (K) – 15.42 % and calcium (Ca) – 10.23 %. These results are consistent with research findings that demonstrate the influence of probiotic strains and natural extracts on the mineral content of fermented milk products. For example, recent studies investigated the influence of probiotic strains on the mineral content of fermented milk products and reported changes in sodium, potassium, and calcium levels [
30,
31]. Similarly, research by Yangilar et al. examined the effect of natural plant raw material on mineral composition in fermented dairy product and found alterations in magnesium, phosphorus content [
32].
3.7. Antioxidant Composition of Symbiotic Fermented Milk Products
The antioxidant composition of two different products enriched with
B. bifidum activated with hawthorn extract comparing the control group is presented in
Table 3.
Both product 1 and product 2 showed an increased flavonoid content compared to the control, suggesting improved antioxidant properties. The increase in flavonoid content observed in product 1 and product 2 compared to the control is consistent with the results of studies conducted by Jena et al. and Yang et al. which showed that the fermentation process with bifidobacteria can increase flavonoid content, possibly due to enzymatic activities during fermentation [
33,
34].
The observed changes in water-soluble antioxidants are consistent with research indicating that hawthorn extract may improve antioxidant properties [
35,
36]. These results support the development of fermented milk products with improved antioxidant content by incorporating
B. bifidum 1 activated with hawthorn extract.
3.8. Nutritional Values of Symbiotic Fermented Milk Products
Nutritional values of symbiotic fermented milk products enriched with bifidobacteria activated with hawthorn extract at various concentrations compared to the control are presented in
Table 4.
The protein and fat content remained relatively constant in all products, indicating that enrichment with
B. bifidum activated with hawthorn extract had minimal effect on these components of the fermented milk products. However, the carbohydrate content in product 1 and product 2 decreased significantly compared to the control. This reduction is attributed to the fermentation process in which the bifidobacteria utilize carbohydrates, as shown by the results of Solopova et al [
37].
Accordingly, the energy value of product 1 and product 2 decreased slightly, which correlates with the reduced carbohydrate content. The moisture and ash content remained stable in all samples. Overall, the addition of B. bifidum activated with microwave-treated hawthorn extract had only a minimal impact on the protein and fat content, but led to a significant decrease in the carbohydrate content.
The study's limitations include potential variations in fermentation conditions, hawthorn extract sources, microbial strains, and uncontrolled variables like environmental conditions and milk composition, which may affect the generalizability and reliability of the findings. In general, our results support hypotheses about bifidobacteria metabolism and the impact of bioactive compounds like hawthorn extract on B. bifidum in fermented milk products. The changes in sensory properties, nutritional content, and antioxidant properties align with established theories on their health benefits. Future research should explore the mechanisms of these effects, the synergies between probiotics, prebiotics, and natural extracts, and consumer preferences to enhance market application. This study highlights the potential for hawthorn-activated bifidobacteria to create functional dairy products with improved nutritional and health benefits, offering innovation opportunities in the dairy industry.
5. Conclusions
In this study, a symbiotic fermented milk product based on Lactobacillus acidophilus enriched with Bifidobacterium bifidum activated by microwave-treated hawthorn extract was successfully developed.
It was established that both product 1 and product 2 had a higher content of viable probiotic cells compared to the control. Product 1 showed an increase of 24.1% in L. acidophilus and 14.7% in B. bifidum after 7 days compared to initial levels, which was significantly higher than the control. Product 1 exhibited improved taste and odor, enhancing consumer acceptance.
Both enriched products demonstrated slower increases in titratable acidity and higher viscosities over 14 days, indicating better preservation and texture stability.
Activation of B. bifidum 1 with hawthorn extract at concentrations of 10-5 g/cm³ was found to enrich the composition of product 1 with essential and non-essential amino acids, vitamins, and minerals, enhancing its nutritional value.
Both enriched products showed increased flavonoid content, indicating improved antioxidant properties, which can contribute to health benefits.
These findings underline the potential of using microwave-treated hawthorn extract at concentration 10-5 g/cm³ to activate B. bifidum, resulting in fermented milk products with superior nutritional profiles and enhanced sensory qualities. This approach supports probiotic culture sustainability and aligns with consumer trends toward functional foods with health benefits.
Potential follow-up studies could explore the underlying mechanisms by which hawthorn extract enhances the viability and functionality of probiotics. Studies could also be conducted to understand the functionality of these symbiotic fermented milk products in the human body. In summary, this study demonstrates significant innovation potential in the dairy industry, offering functional dairy products with improved nutritional value and health-promoting properties through the use of bifidobacteria activated by hawthorn extract.
Author Contributions
Conceptualization, A.U. and E.G.; methodology, E.G and Zh.A.; validation, A.U., E.G. and Zh.A.; formal analysis, A.U. and E.G.; investigation, A.U. and E.G.; writing—original draft preparation, A.U.; writing—review and editing, A.U. and E.G.; visualization, A.U. and E.G.; supervision, A.U.; project administration, A.U.; funding acquisition, A.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP13068387.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings are included within.
Acknowledgments
The authors extend their appreciation to the Ministry of Science and Higher Education of the Republic of Kazakhstan for funding this work through the research project No: AP13068387 “Development of technology for the production of innovative functional bio-fermented milk products with probiotics and antioxidants”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ballini, A.; Charitos, I. A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12 (4), 635. [CrossRef]
- Shah, B. R.; Li, B.; Al Sabbah, H.; Xu, W.; Mráz, J. Effects of Prebiotic Dietary Fibers and Probiotics on Human Health: With Special Focus on Recent Advancement in Their Encapsulated Formulations. Trends in Food Science & Technology 2020, 102, pp. 178–192. [CrossRef]
- Dahiya, D.; Nigam, P. S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8 (7), 303. [CrossRef]
- Siddiqui, S. A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H. A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N. A.; Mehdizadeh, M.; Castro-Muñoz, R. An Overview of Fermentation in the Food Industry - Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10 (1), 85. [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front. Microbiol. 2021, 12, 673890. [CrossRef]
- Burton, K. J.; Rosikiewicz, M.; Pimentel, G.; Bütikofer, U.; Ah, U. von; Voirol, M.-J.; Croxatto, A.; Aeby, S.; Drai, J.; McTernan, P. G.; Greub, G.; Pralong, F. P.; Vergères, G.; Vionnet, N. Probiotic Yogurt and Acidified Milk Similarly Reduce Postprandial Inflammation and Both Alter the Gut Microbiota of Healthy, Young Men. British Journal of Nutrition 2017, 117 (9), pp. 1312–1322. [CrossRef]
-
Microwave-Assisted Extraction of Bioactive Compounds (Review) | IntechOpen. 2021. https://www.intechopen.com/chapters/75284.
- Suksaeree, J.; Monton, C. Maximizing Curcuminoid Extraction from Curcuma Aromatica Salisb. Rhizomes via Environmentally Friendly Microwave-Assisted Extraction Technique Using Full Factorial Design. Int J Food Sci 2024, 2024, 4566123. [CrossRef]
- Ngoc, P. C.; Leclercq, L.; Rossi, J.-C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.-S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules 2019, 24 (23), 4420. [CrossRef]
- Kilic, G.; Sengun, I. Y. Bioactive Properties of Kombucha Beverages Produced with Anatolian Hawthorn (Crataegus Orientalis) and Nettle (Urtica Dioica) Leaves. Food Bioscience 2023, 53, 102631. [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11 (18), 2861. [CrossRef]
- De Souza, M.; Drunkler, D. A.; Colla, E. Probiotic Functional Yogurt: Challenges and Opportunities. Fermentation 2023, 10 (1), 6. [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S. V. Technology and Potential Applications of Probiotic Encapsulation in Fermented Milk Products. J Food Sci Technol 2015, 52 (8), pp. 4679–4696. [CrossRef]
- Burmasova, M. A.; Utebaeva, A. A.; Sysoeva, E. V.; Sysoeva, M. A. Melanins of Inonotus Obliquus: Bifidogenic and Antioxidant Properties. Biomolecules 2019, 9 (6), 248. [CrossRef]
- Utebaeva, A.; Gabrilyants, E.; Abish, Z.; Yevlash, V. Evaluation of Quality Characteristics of Fermented Acidophilic Product with B. Bifidum and Prunus Padus Extract. EEJET 2024, 2 (11 (128)), pp. 13–25. [CrossRef]
- Zaitsev, S. Yu.; Voronina, O. A.; Savina, A. A.; Ignatieva, L. P.; Bogolyubova, N. V. Correlations between the Total Antioxidant Activity and Biochemical Parameters of Cow Milk Depending on the Number of Somatic Cells. International Journal of Food Science 2022, 2022, pp. 1–6. [CrossRef]
- GOST 33491-2015. Product fermented-milk, enriched bifidobacteriae bifidum. Specifications. Federal Agency on Technical Regulating and Metrology, 2015.
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11 (7), 1591. [CrossRef]
- Atwaa, E. S. H.; Shahein, M. R.; El-Sattar, E. S. A.; Hijazy, H. H. A.; Albrakati, A.; Elmahallawy, E. K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8 (2), 52. [CrossRef]
- Larasati, B. A.; Panunggal, B.; Afifah, D. N.; Anjani, G.; Rustanti, N. Total Lactic Acid Bacteria, Antioxidant Activity, and Acceptance of Synbiotic Yoghurt with Red Ginger Extract ( Zingiberofficinale Var. Rubrum ). IOP Conf. Ser.: Earth Environ. Sci. 2018, 116, 012037. [CrossRef]
- Wu, G.; Bazer, F. W.; Davis, T. A.; Kim, S. W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S. B.; Spencer, T. E.; Yin, Y. Arginine Metabolism and Nutrition in Growth, Health and Disease. Amino Acids 2009, 37 (1), pp. 153–168. [CrossRef]
- Phang, J. M.; Liu, W.; Hancock, C. N.; Fischer, J. W. Proline Metabolism and Cancer: Emerging Links to Glutamine and Collagen. Current Opinion in Clinical Nutrition and Metabolic Care 2015, 18 (1), 71–77. [CrossRef]
- Beitāne, I.; Ciproviča, I. Nutritional Benefits of Bifidobacterium Lactis in Dairy Products. Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences 2013, 67 (4–5), pp. 378–382. [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9 (9), 1021. [CrossRef]
- Singh, R. P.; Shadan, A.; Ma, Y. Biotechnological Applications of Probiotics: A Multifarious Weapon to Disease and Metabolic Abnormality. Probiotics & Antimicro. Prot. 2022, 14 (6), pp. 1184–1210. [CrossRef]
- Gu, Q.; Li, P. Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health; Rao, V., Rao, L. G., Eds.; InTech, 2016. [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J. B. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera - A promising approach.Croat. J. Food Sci. Technol. 2013, 5 (2), pp. 85-91.
- Cunningham, M.; Vinderola, G.; Charalampopoulos, D.; Lebeer, S.; Sanders, M. E.; Grimaldi, R. Applying Probiotics and Prebiotics in New Delivery Formats – Is the Clinical Evidence Transferable? Trends in Food Science & Technology 2021, 112, pp. 495–506. [CrossRef]
- Fazilah, N. F.; Ariff, A. B.; Khayat, M. E.; Rios-Solis, L.; Halim, M. Influence of Probiotics, Prebiotics, Synbiotics and Bioactive Phytochemicals on the Formulation of Functional Yogurt. Journal of Functional Foods 2018, 48, pp. 387–399. [CrossRef]
- Alhamdan, A. M.; Al Juhaimi, F. Y.; Hassan, B. H.; Ehmed, K. A.; Mohamed Ahmed, I. A. Physicochemical, Microbiological, and Sensorial Quality Attributes of a Fermented Milk Drink (Laban) Fortified with Date Syrup (Dibs) during Cold Storage. Foods 2021, 10 (12), 3157. [CrossRef]
- Varvara, R.-A.; Vodnar, D. C. Probiotic-Driven Advancement: Exploring the Intricacies of Mineral Absorption in the Human Body. Food Chemistry: X 2024, 21, 101067. [CrossRef]
- Yangilar, F.; Cakmakci, S. “Probiotic Shelf-life, Mineral Contents and Others Properties of Probiotic Yogurts Supplemented with Corn Flour. Journal of Agricultural Sciences, 2017, 23, pp. 472–481.
- Jena, R.; Choudhury, P. K. Bifidobacteria in Fermented Dairy Foods: A Health Beneficial Outlook. Probiotics & Antimicro. Prot. 2023. [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12 (17), 3315. [CrossRef]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S.; Li, S. Biological Properties and Potential Application of Hawthorn and Its Major Functional Components: A Review. Journal of Functional Foods 2022, 90, 104988. [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus Monogyna Subsp. Monogyna Jacq.). J Intercult Ethnopharmacol 2014, 3 (2), 51. [CrossRef]
- Solopova, A.; Van Sinderen, D. Determination of Bifidobacterial Carbohydrate Utilization Abilities and Associated Metabolic End Products. In Bifidobacteria; Van Sinderen, D., Ventura, M., Eds.; Methods in Molecular Biology; Springer US: New York, NY, 2021; Vol. 2278, pp. 117–129. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).