Submitted:
19 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
Introduction
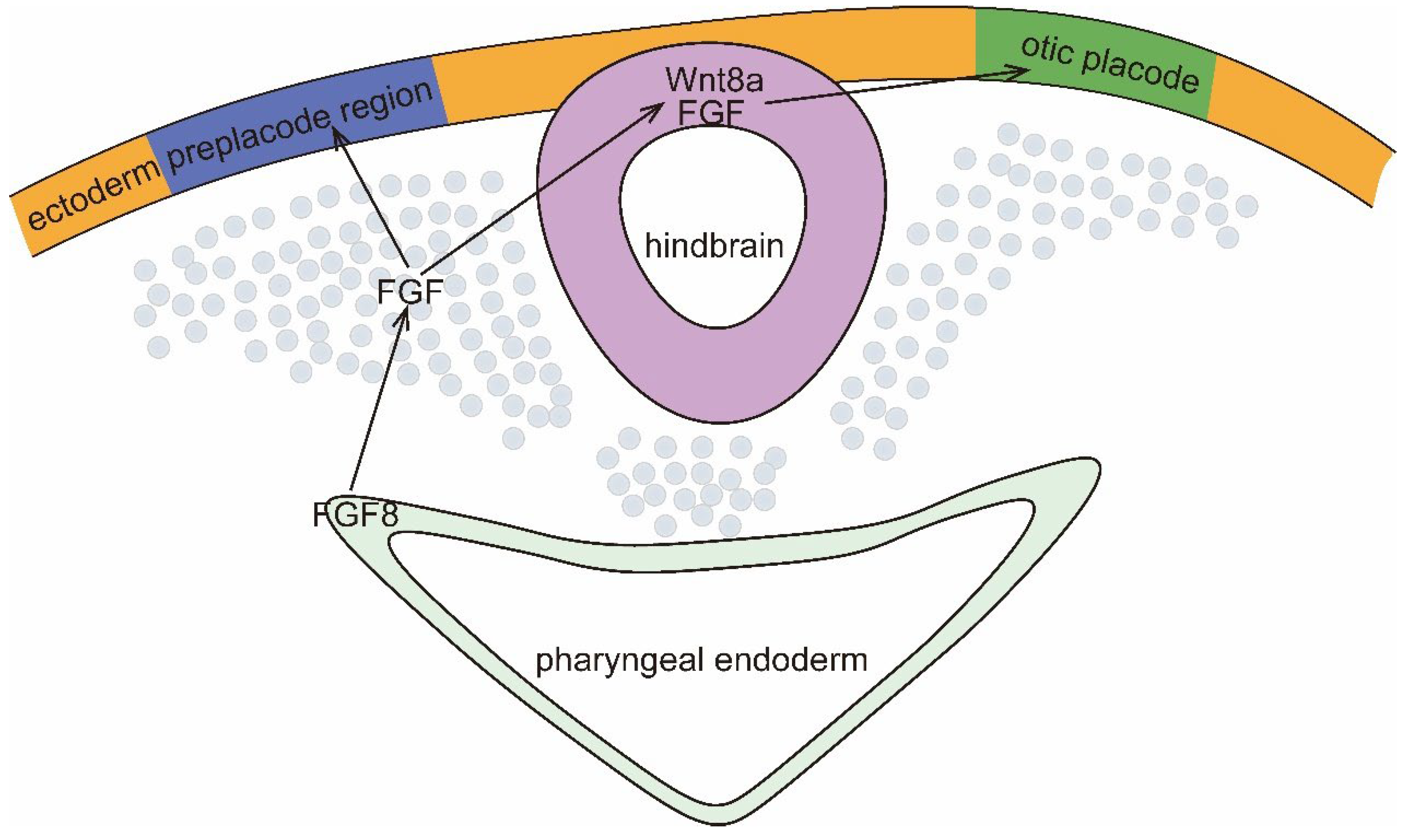
The Development Process of Inner Ear
HCMV Regulates Sox2: A Transcription Factor Required for Inner Ear Growth and Cochlear Nonsensory Formation
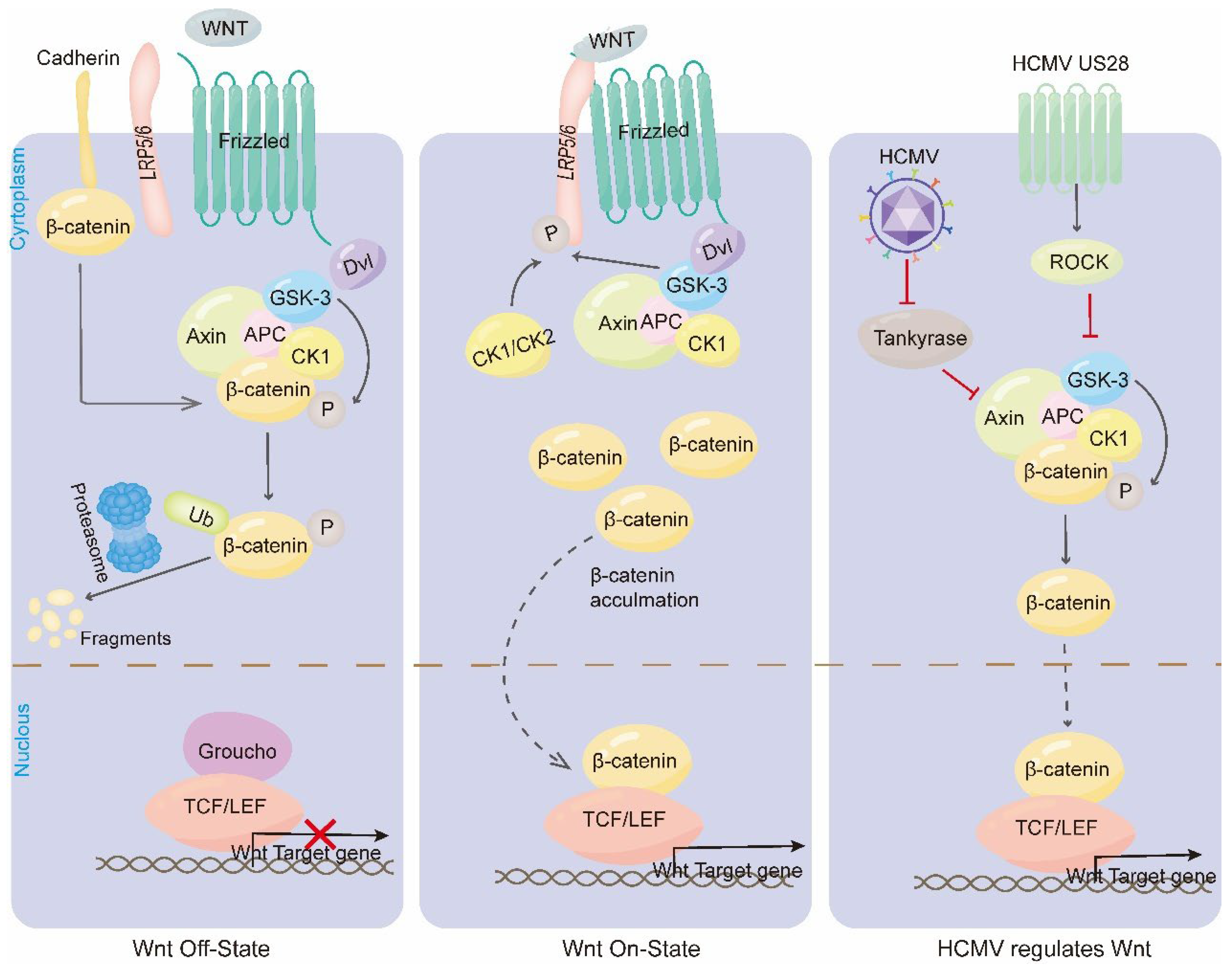
HCMV Regulates the Wnt Signaling Pathway: Affecting Auditory Substrate Specialization, Otic Vesicle Formation and Hair Cell Differentiation
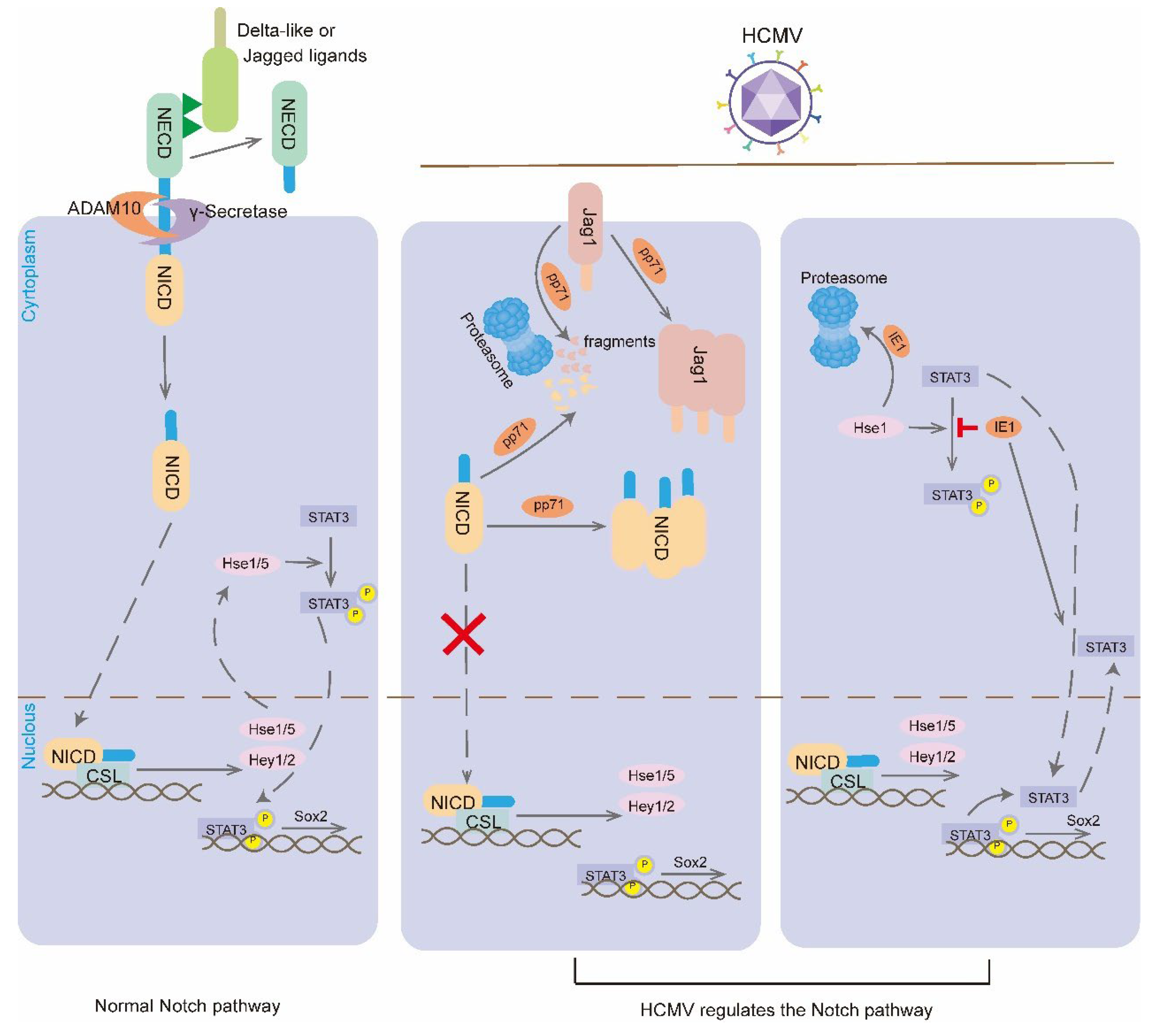
HCMV Regulates the Nonth Signaling Pathway: Affecting Cell Fate and Interfering with Inner Ear Development
Other Signaling Pathways Regulated by HCMV
Viral Infections Affect the Formation of Ossicular Chains
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiertsema, S.P.; Chidlow, G.R.; Kirkham, L.A.; Corscadden, K.J.; Mowe, E.N.; Vijayasekaran, S.; Coates, H.L.; Harnett, G.B.; Richmond, P.C. High detection rates of nucleic acids of a wide range of respiratory viruses in the nasopharynx and the middle ear of children with a history of recurrent acute otitis media. J Med Virol 2011, 83, 2008–17. [Google Scholar] [CrossRef]
- Rujescu, D.; Hartmann, A.M.; Giegling, I.; Konte, B.; Herrling, M.; Himmelein, S.; Strupp, M. Genome-Wide Association Study in Vestibular Neuritis: Involvement of the Host Factor for HSV-1 Replication. Front Neurol 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Pyykko, I.; Zou, J. Do viruses cause inner ear disturbances? ORL J Otorhinolaryngol Relat Spec discussion 40-1. 2008, 70, 32–40. [Google Scholar] [CrossRef]
- Tsubota, M.; Shojaku, H.; Ishimaru, H.; Fujisaka, M.; Watanabe, Y. Mumps virus may damage the vestibular nerve as well as the inner ear. Acta Otolaryngol 2008, 128, 644–7. [Google Scholar] [CrossRef]
- Kikidis, D.; Nikolopoulos, T.P.; Kampessis, G.; Stamatiou, G.; Chrysovergis, A. Sudden sensorineural hearing loss: subclinical viral and toxoplasmosis infections as aetiology and how they alter the clinical course. ORL J Otorhinolaryngol Relat Spec 2011, 73, 110–5. [Google Scholar] [CrossRef]
- Rosenthal, L.S.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; Pass, R.F.; Schmid, S.D.; Stagno, S.; Cannon, M.J. Cytomegalovirus shedding and delayed sensorineural hearing loss: results from longitudinal follow-up of children with congenital infection. Pediatr Infect Dis J 2009, 28, 515–20. [Google Scholar] [CrossRef]
- Bale, J.F. Jr. Human cytomegalovirus infection and disorders of the nervous system. Arch Neurol 1984, 41, 310–20. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007, 17, 253–76. [Google Scholar] [CrossRef]
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol 2006, 35, 226–31. [Google Scholar] [CrossRef] [PubMed]
- Conboy, T.J.; Pass, R.F.; Stagno, S.; Britt, W.J.; Alford, C.A.; McFarland, C.E.; Boll, T.J. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics 1986, 77, 801–6. [Google Scholar] [CrossRef]
- Hawkins, R.D.; Bashiardes, S.; Powder, K.E.; Sajan, S.A.; Bhonagiri, V.; Alvarado, D.M.; Speck, J.; Warchol, M.E.; Lovett, M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS One 2007, 2, e525. [Google Scholar] [CrossRef]
- Stevens, C.B.; Davies, A.L.; Battista, S.; Lewis, J.H.; Fekete, D.M. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol 2003, 261, 149–64. [Google Scholar] [CrossRef]
- Harding, A.T.; Ocwieja, K.; Jeong, M.; Zhang, Y.; Leger, V.; Jhala, N.; Stankovic, K.M.; Gehrke, L. Human otic progenitor cell models of congenital hearing loss reveal potential pathophysiologic mechanisms of Zika virus and cytomegalovirus infections. mBio 2024, 15, e0019924. [Google Scholar] [CrossRef]
- Bradley, T.; Ferrari, G.; Haynes, B.F.; Margolis, D.M.; Browne, E.P. Single-Cell Analysis of Quiescent HIV Infection Reveals Host Transcriptional Profiles that Regulate Proviral Latency. Cell Rep 2018, 25, 107–117.e3. [Google Scholar] [CrossRef]
- Karosi, T.; Kónya, J.; Petkó, M.; Sziklai, I. Histologic otosclerosis is associated with the presence of measles virus in the stapes footplate. Otol Neurotol 2005, 26, 1128–33. [Google Scholar] [CrossRef]
- Ozaki, H.; Nakamura, K.; Funahashi, J.; Ikeda, K.; Yamada, G.; Tokano, H.; Okamura, H.O.; Kitamura, K.; Muto, S.; Kotaki, H.; Sudo, K.; Horai, R.; Iwakura, Y.; Kawakami, K. Six1 controls patterning of the mouse otic vesicle. Development 2004, 131, 551–62. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Pelling, A.L.; Leung, K.K.; Tang, A.S.; Bell, D.M.; Tease, C.; Lovell-Badge, R.; Steel, K.P.; Cheah, K.S. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005, 434, 1031–5. [Google Scholar] [CrossRef]
- Licona, K. d. a. H. W. Congenital LCM virus: Mechanism of brain disease in a rat model of congenital viral infection. 2010. [Google Scholar]
- Raphael, Y.; Altschuler, R.A. Structure and innervation of the cochlea. Brain Res Bull 2003, 60, 397–422. [Google Scholar] [CrossRef]
- Goldberg, J.M. The vestibular end organs: morphological and physiological diversity of afferents. Curr Opin Neurobiol 1991, 1, 229–35. [Google Scholar] [CrossRef]
- Groves, A.K.; Bronner-Fraser, M. Competence, specification and commitment in otic placode induction. Development 2000, 127, 3489–99. [Google Scholar] [CrossRef]
- Ekker, M.; Akimenko, M.A.; Bremiller, R.; Westerfield, M. Regional expression of three homeobox transcripts in the inner ear of zebrafish embryos. Neuron 1992, 9, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chu, H.; Maves, L.; Yan, Y.L.; Morcos, P.A.; Postlethwait, J.H.; Westerfield, M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development 2003, 130, 2213–24. [Google Scholar] [CrossRef] [PubMed]
- Léger, S.; Brand, M. Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev 2002, 119, 91–108. [Google Scholar] [CrossRef]
- Maroon, H.; Walshe, J.; Mahmood, R.; Kiefer, P.; Dickson, C.; Mason, I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development 2002, 129, 2099–108. [Google Scholar] [CrossRef]
- Wright, T.J.; Mansour, S.L. FGF signaling in ear development and innervation. Curr Top Dev Biol 2003, 57, 225–59. [Google Scholar] [PubMed]
- Couly, G.F.; Coltey, P.M.; Le Douarin, N.M. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 1993, 117, 409–29. [Google Scholar] [CrossRef]
- Anniko, M. Cytodifferentiation of cochlear hair cells. Am J Otolaryngol 1983, 4, 375–88. [Google Scholar] [CrossRef]
- Satoh, T.; Fekete, D.M. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development 2005, 132, 1687–97. [Google Scholar] [CrossRef]
- Martin, P.; Swanson, G.J. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev Biol 1993, 159, 549–58. [Google Scholar] [CrossRef]
- Chang, W.; Brigande, J.V.; Fekete, D.M.; Wu, D.K. The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development 2004, 131, 4201–11. [Google Scholar] [CrossRef]
- Adam, J.; Myat, A.; Le Roux, I.; Eddison, M.; Henrique, D.; Ish-Horowicz, D.; Lewis, J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development 1998, 125, 4645–54. [Google Scholar] [CrossRef] [PubMed]
- Hemond, S.G.; Morest, D.K. Ganglion formation from the otic placode and the otic crest in the chick embryo: mitosis, migration, and the basal lamina. Anat Embryol (Berl) 1991, 184, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 2014, 6, 305–11. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; Ko, M.S.; Niwa, H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 2007, 9, 625–35. [Google Scholar] [CrossRef]
- Kopp, J.L.; Ormsbee, B.D.; Desler, M.; Rizzino, A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells 2008, 26, 903–11. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M. SOX after SOX: SOXession regulates neurogenesis. Genes Dev 2011, 25, 2423–8. [Google Scholar] [CrossRef] [PubMed]
- Brazel, C.Y.; Limke, T.L.; Osborne, J.K.; Miura, T.; Cai, J.; Pevny, L.; Rao, M.S. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell 2005, 4, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Foshay, K.M.; Gallicano, G.I. Regulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fate. Stem Cells Dev 2008, 17, 269–78. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Tong, L.M.; Balestra, M.E.; Javier, R.; Andrews-Zwilling, Y.; Li, G.; Walker, D.; Zhang, W.R.; Kreitzer, A.C.; Huang, Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012, 11, 100–9. [Google Scholar] [CrossRef]
- Ragge, N.K.; Lorenz, B.; Schneider, A.; Bushby, K.; de Sanctis, L.; de Sanctis, U.; Salt, A.; Collin, J.R.; Vivian, A.J.; Free, S.L.; Thompson, P.; Williamson, K.A.; Sisodiya, S.M.; van Heyningen, V.; Fitzpatrick, D.R. SOX2 anophthalmia syndrome. Am J Med Genet A discussion 8. 2005, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Jahan, I.; Macova, I.; Chumak, T.; Bohuslavova, R.; Syka, J.; Fritzsch, B.; Pavlinkova, G. Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Sci Rep 2016, 6, 38253. [Google Scholar] [CrossRef]
- Neves, J.; Uchikawa, M.; Bigas, A.; Giraldez, F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One 2012, 7, e30871. [Google Scholar] [CrossRef] [PubMed]
- Kempfle, J.S.; Turban, J.L.; Edge, A.S. Sox2 in the differentiation of cochlear progenitor cells. Sci Rep 2016, 6, 23293. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.H.; Hannemann, H.; Kulkarni, A.S.; Schwartz, P.H.; O'Dowd, J.M.; Fortunato, E.A. Human cytomegalovirus infection causes premature and abnormal differentiation of human neural progenitor cells. J Virol 2010, 84, 3528–41. [Google Scholar] [CrossRef]
- Soroceanu, L.; Matlaf, L.; Khan, S.; Akhavan, A.; Singer, E.; Bezrookove, V.; Decker, S.; Ghanny, S.; Hadaczek, P.; Bengtsson, H.; Ohlfest, J.; Luciani-Torres, M.G.; Harkins, L.; Perry, A.; Guo, H.; Soteropoulos, P.; Cobbs, C.S. Cytomegalovirus Immediate-Early Proteins Promote Stemness Properties in Glioblastoma. Cancer Res 2015, 75, 3065–76. [Google Scholar] [CrossRef]
- Wu, C.C.; Jiang, X.; Wang, X.Z.; Liu, X.J.; Li, X.J.; Yang, B.; Ye, H.Q.; Harwardt, T.; Jiang, M.; Xia, H.M.; Wang, W.; Britt, W.J.; Paulus, C.; Nevels, M.; Luo, M.H. Human Cytomegalovirus Immediate Early 1 Protein Causes Loss of SOX2 from Neural Progenitor Cells by Trapping Unphosphorylated STAT3 in the Nucleus. J Virol 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wang, X.Z.; Qiu, Y.; Zhou, Y.P.; Zhang, Q.Y.; Cheng, S.; Sun, J.Y.; Jiang, X.J.; Rayner, S.; Britt, W.J.; Chen, J.; Hu, F.; Li, F.C.; Luo, M.H.; Cheng, H. SOX2 downregulation of PML increases HCMV gene expression and growth of glioma cells. PLoS Pathog 2023, 19, e1011316. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.; Nusse, R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–30. [Google Scholar] [CrossRef]
- Habas, R.; Dawid, I.B. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol 2005, 4, 2. [Google Scholar] [CrossRef]
- Qian, D.; Jones, C.; Rzadzinska, A.; Mark, S.; Zhang, X.; Steel, K.P.; Dai, X.; Chen, P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 2007, 306, 121–33. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Fekete, D.M. Wnt signaling during cochlear development. Semin Cell Dev Biol 2013, 24, 480–9. [Google Scholar] [CrossRef] [PubMed]
- Jacques, B.E.; Puligilla, C.; Weichert, R.M.; Ferrer-Vaquer, A.; Hadjantonakis, A.K.; Kelley, M.W.; Dabdoub, A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 2012, 139, 4395–404. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A 1996, 93, 8455–9. [Google Scholar] [CrossRef]
- Teo, W.H.; Chen, H.P.; Huang, J.C.; Chan, Y.J. Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells. Int J Oncol 2017, 51, 1415–1426. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Fang, F.; Dong, Y.S.; Zhou, H.; Zhen, H.; Liu, J.; Li, G. [Inhibitory effect of murine cytomegalovirus infection on neural stem cells' differentiation and its mechanisms]. Zhonghua Er Ke Za Zhi 2006, 44, 505–8. [Google Scholar] [PubMed]
- Maussang, D.; Langemeijer, E.; Fitzsimons, C.P.; Stigter-van Walsum, M.; Dijkman, R.; Borg, M.K.; Slinger, E.; Schreiber, A.; Michel, D.; Tensen, C.P.; van Dongen, G.A.; Leurs, R.; Smit, M.J. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res 2009, 69, 2861–9. [Google Scholar] [CrossRef]
- Bongers, G.; Maussang, D.; Muniz, L.R.; Noriega, V.M.; Fraile-Ramos, A.; Barker, N.; Marchesi, F.; Thirunarayanan, N.; Vischer, H.F.; Qin, L.; Mayer, L.; Harpaz, N.; Leurs, R.; Furtado, G.C.; Clevers, H.; Tortorella, D.; Smit, M.J.; Lira, S.A. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest 2010, 120, 3969–78. [Google Scholar] [CrossRef]
- Langemeijer, E.V.; Slinger, E.; de Munnik, S.; Schreiber, A.; Maussang, D.; Vischer, H.; Verkaar, F.; Leurs, R.; Siderius, M.; Smit, M.J. Constitutive β-catenin signaling by the viral chemokine receptor US28. PLoS One 2012, 7, e48935. [Google Scholar] [CrossRef]
- Angelova, M.; Zwezdaryk, K.; Ferris, M.; Shan, B.; Morris, C.A.; Sullivan, D.E. Human cytomegalovirus infection dysregulates the canonical Wnt/β-catenin signaling pathway. PLoS Pathog 2012, 8, e1002959. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Liu, F.; Arav-Boger, R. Human Cytomegalovirus Inhibits the PARsylation Activity of Tankyrase--A Potential Strategy for Suppression of the Wnt Pathway. Viruses 2015, 8. [Google Scholar] [CrossRef]
- van Zuylen, W.J.; Ford, C.E.; Wong, D.D.; Rawlinson, W.D. Human Cytomegalovirus Modulates Expression of Noncanonical Wnt Receptor ROR2 To Alter Trophoblast Migration. J Virol 2016, 90, 1108–15. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: cell fate control and signal integration in development. Science 1999, 284, 770–6. [Google Scholar] [CrossRef] [PubMed]
- Kovall, R.A.; Gebelein, B.; Sprinzak, D.; Kopan, R. The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev Cell 2017, 41, 228–241. [Google Scholar] [CrossRef]
- Seib, E.; Klein, T. The role of ligand endocytosis in notch signalling. Biol Cell 2021, 113, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zang, C.; Taing, L.; Arnett, K.L.; Wong, Y.J.; Pear, W.S.; Blacklow, S.C.; Liu, X.S.; Aster, J.C. NOTCH1-RBPJ complexes drive target gene expression through dynamic interactions with superenhancers. Proc Natl Acad Sci U S A 2014, 111, 705–10. [Google Scholar] [CrossRef]
- Iso, T.; Kedes, L.; Hamamori, Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 2003, 194, 237–55. [Google Scholar] [CrossRef] [PubMed]
- Bray, S. Notch signalling in Drosophila: three ways to use a pathway. Semin Cell Dev Biol 1998, 9, 591–7. [Google Scholar] [CrossRef]
- Brown, R.; Groves, A.K. Hear, Hear for Notch: Control of Cell Fates in the Inner Ear by Notch Signaling. Biomolecules 2020, 10. [Google Scholar] [CrossRef]
- Jayasena, C.S.; Ohyama, T.; Segil, N.; Groves, A.K. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 2008, 135, 2251–61. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.H.; Reh, T.A.; Bermingham-McDonogh, O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A 2010, 107, 15792–7. [Google Scholar] [CrossRef]
- Neves, J.; Parada, C.; Chamizo, M.; Giráldez, F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development 2011, 138, 735–44. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, A.E.; Xu, J.; Gridley, T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet 2006, 2, e4. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jin, Y.; Stanger, B.; Kiernan, A.E. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A 2010, 107, 15798–803. [Google Scholar] [CrossRef] [PubMed]
- Lanford, P.J.; Lan, Y.; Jiang, R.; Lindsell, C.; Weinmaster, G.; Gridley, T.; Kelley, M.W. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet 1999, 21, 289–92. [Google Scholar] [CrossRef]
- Daudet, N.; Lewis, J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 2005, 132, 541–51. [Google Scholar] [CrossRef]
- Tateya, T.; Imayoshi, I.; Tateya, I.; Ito, J.; Kageyama, R. Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev Biol 2011, 352, 329–40. [Google Scholar] [CrossRef] [PubMed]
- Abelló, G.; Khatri, S.; Giráldez, F.; Alsina, B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev 2007, 124, 631–45. [Google Scholar] [CrossRef]
- Jung, J.Y.; Avenarius, M.R.; Adamsky, S.; Alpert, E.; Feinstein, E.; Raphael, Y. siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Mol Ther 2013, 21, 834–41. [Google Scholar] [CrossRef]
- Hartman, B.H.; Hayashi, T.; Nelson, B.R.; Bermingham-McDonogh, O.; Reh, T.A. Dll3 is expressed in developing hair cells in the mammalian cochlea. Dev Dyn 2007, 236, 2875–83. [Google Scholar] [CrossRef]
- Li, X.J.; Liu, X.J.; Yang, B.; Fu, Y.R.; Zhao, F.; Shen, Z.Z.; Miao, L.F.; Rayner, S.; Chavanas, S.; Zhu, H.; Britt, W.J.; Tang, Q.; McVoy, M.A.; Luo, M.H. Human Cytomegalovirus Infection Dysregulates the Localization and Stability of NICD1 and Jag1 in Neural Progenitor Cells. J Virol 2015, 89, 6792–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, M.; Xing, F.; Wang, M.; Wang, B.; Qian, D. Human cytomegalovirus infection promotes the stemness of U251 glioma cells. J Med Virol 2017, 89, 878–886. [Google Scholar] [CrossRef]
- Liu, X.J.; Jiang, X.; Huang, S.N.; Sun, J.Y.; Zhao, F.; Zeng, W.B.; Luo, M.H. Human cytomegalovirus infection dysregulates neural progenitor cell fate by disrupting Hes1 rhythm and down-regulating its expression. Virol Sin 2017, 32, 188–198. [Google Scholar] [CrossRef]
- Liu, X.J.; Yang, B.; Huang, S.N.; Wu, C.C.; Li, X.J.; Cheng, S.; Jiang, X.; Hu, F.; Ming, Y.Z.; Nevels, M.; Britt, W.J.; Rayner, S.; Tang, Q.; Zeng, W.B.; Zhao, F.; Luo, M.H. Human cytomegalovirus IE1 downregulates Hes1 in neural progenitor cells as a potential E3 ubiquitin ligase. PLoS Pathog 2017, 13, e1006542. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.P.; Wing, B.; Coleman, D.; Shenk, T. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J Virol 2001, 75, 12319–30. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.P.; Tang, Q. Identification of cellular proteins that interact with human cytomegalovirus immediate-early protein 1 by protein array assay. Viruses 2013, 6, 89–105. [Google Scholar] [CrossRef]
- Shim, K.; Minowada, G.; Coling, D.E.; Martin, G.R. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell 2005, 8, 553–64. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Cunningham, D.; Bermingham-McDonogh, O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn 2007, 236, 525–33. [Google Scholar] [CrossRef]
- Chen, Z.; Knutson, E.; Kurosky, A.; Albrecht, T. Degradation of p21cip1 in cells productively infected with human cytomegalovirus. J Virol 2001, 75, 3613–25. [Google Scholar] [CrossRef]
- Chen, P.; Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 1999, 126, 1581–90. [Google Scholar] [CrossRef]
- Ankamreddy, H.; Min, H.; Kim, J.Y.; Yang, X.; Cho, E.S.; Kim, U.K.; Bok, J. Region-specific endodermal signals direct neural crest cells to form the three middle ear ossicles. Development 2019, 146. [Google Scholar]
- Miyake, T.; Cameron, A.M.; Hall, B.K. Stage-specific onset of condensation and matrix deposition for Meckel's and other first arch cartilages in inbred C57BL/6 mice. J Craniofac Genet Dev Biol 1996, 16, 32–47. [Google Scholar] [PubMed]
- Amin, S.; Matalova, E.; Simpson, C.; Yoshida, H.; Tucker, A.S. Incudomalleal joint formation: the roles of apoptosis, migration and downregulation. BMC Dev Biol 2007, 7, 134. [Google Scholar] [CrossRef]
- Chapman, S.C. Can you hear me now? Understanding vertebrate middle ear development. Front Biosci (Landmark Ed) 2011, 16, 1675–1692. [Google Scholar] [CrossRef]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci 2003, 4, 806–18. [Google Scholar] [CrossRef]
- Nie, X.; Luukko, K.; Kettunen, P. BMP signalling in craniofacial development. Int J Dev Biol 2006, 50, 511–21. [Google Scholar] [CrossRef]
- Kanzler, B.; Foreman, R.K.; Labosky, P.A.; Mallo, M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development 2000, 127, 1095–104. [Google Scholar] [CrossRef] [PubMed]
- Crossley, P.H.; Martin, G.R. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 1995, 121, 439–51. [Google Scholar] [CrossRef]
- Trumpp, A.; Depew, M.J.; Rubenstein, J.L.; Bishop, J.M.; Martin, G.R. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev 1999, 13, 3136–48. [Google Scholar] [CrossRef]
- Pau, H.; Fuchs, H.; de Angelis, M.H.; Steel, K.P. Hush puppy: a new mouse mutant with pinna, ossicle, and inner ear defects. Laryngoscope 2005, 115, 116–24. [Google Scholar] [CrossRef]
- Teng, C.S.; Yen, H.Y.; Barske, L.; Smith, B.; Llamas, J.; Segil, N.; Go, J.; Sanchez-Lara, P.A.; Maxson, R.E.; Crump, J.G. Requirement for Jagged1-Notch2 signaling in patterning the bones of the mouse and human middle ear. Sci Rep 2017, 7, 2497. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Friedmann, I. [Detection of measles and rubella-specific antigens in the endochondral ossification zone in otosclerosis]. Laryngol Rhinol Otol (Stuttg) 1987, 66, 167–71. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, H.; Arnold, W.; Neubert, W.J.; Höfler, H. Evidence of measles virus RNA in otosclerotic tissue. ORL J Otorhinolaryngol Relat Spec 1994, 56, 130–2. [Google Scholar] [CrossRef]
- McKenna, M.J.; Kristiansen, A.G.; Haines, J. Polymerase chain reaction amplification of a measles virus sequence from human temporal bone sections with active otosclerosis. Am J Otol 1996, 17, 827–30. [Google Scholar]
- Browning, G.G.; Gatehouse, S. Sensorineural hearing loss in stapedial otosclerosis. Ann Otol Rhinol Laryngol 1984, 93, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Friedmann, I. Otosclerosis--an inflammatory disease of the otic capsule of viral aetiology? J Laryngol Otol 1988, 102, 865–71. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; He, R.; Venkatadri, R.; Forman, M.; Arav-Boger, R. Wnt modulating agents inhibit human cytomegalovirus replication. Antimicrob Agents Chemother 2013, 57, 2761–7. [Google Scholar] [CrossRef]
- Mizutari, K.; Fujioka, M.; Hosoya, M.; Bramhall, N.; Okano, H.J.; Okano, H.; Edge, A.S. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 2013, 77, 58–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




