Submitted:
19 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of Cryptosporidium spp.
3. Cryptosporidiosis
Host immune Response to Cryptosporidium Infection
Laboratory Diagnosis of Cryptosporidium spp. Infection
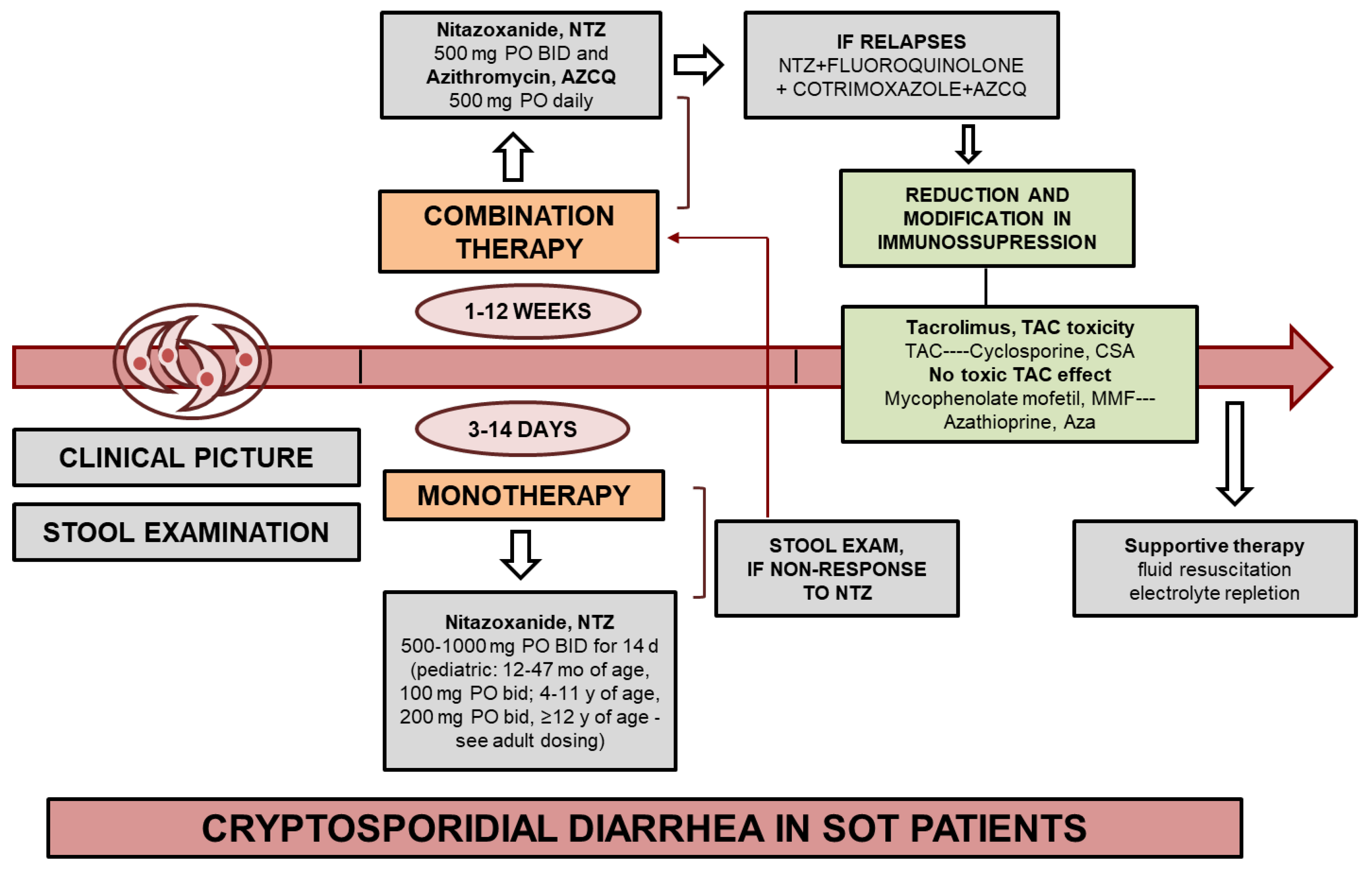
Treatment for Cryptosporidium Infection
Prevention
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J. Enteric protozoa in the developed world: a public health perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L. The burden and etiology of diarrheal illness in developing countries. Pediatr. Clin. North Am. 2017, 64, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Deltombe, C.; Lefebvre, M.; Morio, F.; Boutoille, D.; Imbert, B.M.; Le Pape, P.; Raffi, F.; Hourmant, M. Cryptosporidiosis and microsporidiosis as causes of diarrhea in kidney and/or pancreas transplant recipients. Med. Mal. Infect. 2020, 50, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Sonambekar, A.; Mehta, V.; Desai, D.; Abraham, P.; Almeida, A.; Joshi, A.; Gupta, T.; Sirsat, R.; Kothari, J. Diarrhea in kidney transplant recipients: etiology and outcome. Indian. J. Gastroenterol. 2020, 39, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Calmet, F.H.; Yarur, A.J.; Pukazhendhi, G.; Ahmad, J.; Bhamidimarri, K.R. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann. Gastroenterol. 2015, 28, 366–373. [Google Scholar] [PubMed]
- Plutzer J, Karanis P. Genetic polymorphism in Cryptosporidium species: an update. Vet Parasitol. 2009;165:187–199.
- Helmy, Y.A.; Hafez, H.M. Cryptosporidiosis: from prevention to treatment, a narrative review. Microorganisms. 2022, 10, 2456. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Lim, Y.A.; Mahdy, M.A.; Dixon, B.R.; Surin, J. Epidemiology of cryptosporidiosis in HIV-infected individuals: a global perspective. Open Access Sci. Rep. 2012, 1, 431. [Google Scholar]
- Bouzid, M.; Hunter, P.R.; Chalmers, R.M.; Tyler, K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013, 26, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Hatalova, E.; Guman, T.; Bednarova, V.; Simova, V.T.; Logoida, M.; Halanova, M. Occurrence of Cryptosporidium parvum IIaA17G1R1 in hospitalized hemato-oncological patients in Slovakia. Parasitol. Res. 2022, 121, 471–476. [Google Scholar] [CrossRef]
- Cengiz, Z.T.; Yilmaz, H.; Sahin, I.H.; Kapmaz, M.; Ekici, P. The frequency of Cryptosporidium spp. in immunocompromised patients by modified acid-fast staining, cassette Kit and ELISA methods: comparison of the diagnostic techniques. Jundishapur J. Microbiol 2017, 10, e36479. [Google Scholar] [CrossRef]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Pumipuntu, N.; Piratae, S. Cryptosporidiosis: a zoonotic disease concern. Vet. World. 2018, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenzie, W.R.; Hoxie, N.J.; Proctor, M.E.; Gradus, M.S.; Blair, K.A.; Peterson, D.E.; Kazmierczak, J.J.; Addiss, D.G.; Fox, K.R.; Rose, J.B.; et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994, 331, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.J. Clinical aspects of human cryptosporidiosis. Contrib. Microbiol. 2000, 6, 50–74. [Google Scholar] [PubMed]
- Shirley, D.A.; Moonah, S.N.; Kotloff, K.L. Burden of disease from cryptosporidiosis. Curr. Opin. Infect. Dis. 2012, 25, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Leitch, G.J.; He, Q. Cryptosporidiosis-an overview. J. Biomed. Res. 2012, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R.; Hadfield, S.J.; Wilkinson, D.; Lake, I.R.; Harrison, F.C.; Chalmers, R.M. Correlation between subtypes of Cryptosporidium parvum in humans and risk. Emerg. Infect. Dis. 2007, 13, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Sinclair, M.I.; Forbes, A.B.; Veitch, M.; Kirk, M.; Cunliffe, D.; Willis, J.; Fairley, C.K. Case-control studies of sporadic cryptosporidiosis in Melbourne and Adelaide, Australia. Epidemiol. Infect. 2002, 128, 419–431. [Google Scholar] [CrossRef]
- Chalmers, R.M.; Davies, A.P. Minireview: clinical cryptosporidiosis. Exp. Parasitol. 2010, 124, 138–146. [Google Scholar] [CrossRef]
- Shrateh, O.N.; Jobran, A.; Zaid, M.A.; Saleh, M. Successful management of life-threatening post-COVID-19 cryptosporidiosis in a renal transplant patient: a case report. Pan Afr. Med. J. 2023, 45, 10. [Google Scholar]
- Bahdi, F.; Jain, S.; Agarwal, S.K. Cryptosporidiosis in an immunosuppressed patient with persistent diarrhea. Cleve Clin. J. Med. 2021, 88, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Aulagnon, F.; Scemla, A.; DeWolf, S.; Legendre, C.; Zuber, J. Diarrhea after kidney transplantation: a new look at a frequent symptom. Transplantation. 2014, 98, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Clifford, C.P.; Crook, D.W.; Conlon, C.P.; Fraise, A.P.; Day, D.G.; Peto, T.E. Impact of waterborne outbreak of cryptosporidiosis on AIDS and renal transplant patients. Lancet. 1990, 335, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, A.J.; Gomez, M.A.; Mera, J.; Cagnoni, P.; Michel, M.D. Cryptosporidium infection in renal transplant patients. J. Infect. Dis. 1989, 160, 559. [Google Scholar] [CrossRef] [PubMed]
- Golan Shaposhnik, E.; Abozaid, S.; Grossman, T.; Marva, E.; On, A.; Azrad, M.; Peretz, A. The prevalence of Cryptosporidium among children hospitalized because of gastrointestinal symptoms and the efficiency of diagnostic methods for Cryptosporidium. Am. J. Trop. Med. Hyg. 2019, 101, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Current, W.L.; Garcia, L.S. Cryptosporidiosis. Clin. Microbiol. Rev. 1991, 4, 325–358. [Google Scholar] [CrossRef]
- Sponseller, J.K.; Griffiths, J.K.; Tzipori, S. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clin. Microbiol. Rev. 2014, 27, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Clavel, A.; Arnal, A.C.; Sánchez, E.C.; Cuesta, J.; Letona, S.; Amiguet, J.A.; Castillo, F.J.; Varea, M.; Gómez-Lus, R. Respiratory cryptosporidiosis: case series and review of the literature. Infection. 1996, 24, 341–346. [Google Scholar] [CrossRef]
- Pellicelli, A.M.; Palmieri, F.; Spinazzola, F.; D’Ambrosio, C.; Causo, T.; De Mori, P.; Bordi, E.; D’Amato, C. Pulmonary cryptosporidiosis in patients with acquired immunodeficiency syndrome. Minerva Med. 1998, 89, 173–175. [Google Scholar]
- Greenberg, P.D.; Koch, J.; Cello, J.P. Diagnosis of Cryptosporidium parvum in patients with severe diarrhea and AIDS. Dig. Dis. Sci. 1996, 41, 2286–2290. [Google Scholar] [CrossRef]
- Ditrich, O.; Palkovic, L.; Stĕrba, J.; Prokopic, J.; Loudová, J.; Giboda. , M. The first finding of Cryptosporidium baileyi in man. Parasitol. Res. 1991, 77, 44–47. [Google Scholar] [CrossRef] [PubMed]
- de Souza Ldo, R.; Rodrigues, M.A.; Morceli, J.; Kemp, R.; Mendes, R/P. Cryptosporidiosis of the biliary tract mimicking pancreatic cancer in an AIDS patient. Rev. Soc. Bras. Med. Trop. 2004, 37, 182–185. [Google Scholar] [PubMed]
- Kalantari, N.; Gorgani-Firouzjaee, T.; Ghaffari, S.; Bayani, M.; Ghaffari, T.; Chehrazi, M. Association between Cryptosporidium infection and cancer: a systematic review and meta-analysis. Parasitol. Int. 2020, 74, 101979. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, D.; Goel, A.; Kaul, A.; Sharma, R.K.; Gupta, A.; Ruhela, V.; Gupta, A.; Vardhan, H.; Prasad, N. Cryptosporidium infection after renal transplantation in an endemic area. Transpl. Infect. Dis. 2015, 17, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bonatti, H.; Barroso, L.F. 2nd; Sawyer, R.G.; Kotton, C.N.; Sifri, C.D. Cryptosporidium enteritis in solid organ transplant recipients: multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transpl. Infect. Dis. 2012, 14, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Rosser, J.I.; Blackburn, B.G. Pathogenic intestinal parasites in transplant recipients emerging transplant infections. In: Emerging Transplant Infections, Springer 2020, pp. 1397–1450.
- Cheng, F.; Li, Q.; Cui, Z.; Wang, Z.; Zeng, F.; Zhang, Y. Tacrolimus concentration is effectively predicted using combined clinical and genetic factors in the perioperative period of kidney transplantation and associated with acute rejection. J. Immunol. Res. 2022, 2022, 3129389. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Razakandrainibe, R.; Valot, S.; Vannier, M.; Sautour, M.; Basmaciyan, L.; Gargala, G.; Viller, V.; Lemeteil, D.; Ballet, J.J.; French National Network on Surveillance of Human Cryptosporidiosis; Dalle, F. ; Favennec, L. Epidemiology of cryptosporidiosis in France from 2017 to 2019. Microorganisms. 2020, 8, 1358. [Google Scholar] [CrossRef]
- Brunet, J.; Lemoine, J.P.; Pesson, B.; Valot, S.; Sautour, M.; Dalle, F.; Muller, C.; Borni-Duval, C.; Caillard, S.; Moulin, B.; Pfaff, A.W.; Razakandrainibe, R.; Abou-Bacar, A.; Favennec, L.; Candolfi, E. Ruling out nosocomial transmission of Cryptosporidium in a renal transplantation unit: case report. BMC Infect. Dis. 2016, 16. [Google Scholar] [CrossRef]
- Ok, U.Z.; Cirit, M.; Uner, A.; Ok, E.; Akcicek, F.; Basci, A.; Ozcel, M.A. Cryptosporidiosis and blastocystosis in renal transplant recipients. Nephron. 1997, 75, 171–174. [Google Scholar] [CrossRef]
- Zheng, S.; Ko, K.K.; Chan, K.S.; Venkatachalam, I. Case report: diagnosis of cryptosporidiosis in renal transplantation in a low-prevalence setting. Am. J. Trop. Med. Hyg. 2019, 100, 78–80. [Google Scholar] [CrossRef]
- Burdese, M.; Veglio, V.; Consiglio, V.; Soragna, G.; Mezza, E.; Bergamo, D.; Tattoli, F.; Rossetti, M.; Jeantet, A.; Segoloni, G.P.; Piccoli, G.B. A dance teacher with kidney-pancreas transplant and diarrhoea: what is the cause? Nephrol. Dial. Transplant. 2005, 20, 1759–1761. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, E.; McDougal, A.N.; White, A.C. Jr. resolution of cryptosporidiosis in transplant recipients: review of the literature and presentation of a renal transplant patient treated with nitazoxanide, azithromycin, and rifaximin. Open Forum Infect. Dis. 2021, 9, ofab610. [Google Scholar] [CrossRef] [PubMed]
- Abdo, A.; Klassen, J.; Urbanski, S.; Raber, E.; Swain, M. G. Reversible sclerosing cholangitis secondary to cryptosporidiosis in a renal transplant patient. J. Hepatol. 2003, 38, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Rocamora, N.; Merino, E.; Paya, A. Cryptosporidiosis. A rare infection in renal transplantation. Nefrologia. 2006, 26, 753–754. [Google Scholar] [PubMed]
- Tran, M.Q.; Gohh, R.Y.; Morrissey, P.E.; Dworkin, L.D.; Gautam, A.; Monaco, A.P.; Yango, A.F. Jr. Cryptosporidium infection in renal transplant patients. Clin. Nephrol. 2005, 63, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Ungar, B.L. Cryptosporidium spp. and cryptosporidiosis. Microbiol. Rev. 1986, 50, 458–483. [Google Scholar] [CrossRef] [PubMed]
- Conlon, C.P.; Simone, M.C. Cryptosporidium and cryptosporidiosis. In: Oxford Textbook of Medicine, Firth J, Conlon C, Cox T (eds). Oxford, 2020.
- Mercado, R.; Buck, G.A.; Manque, P.A.; Ozaki, L.S. Cryptosporidium hominis infection of the human respiratory tract. Emerg. Infect. Dis. 2007, 13, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: molecular advances. Parasitology. 2014, 141, 1511–1532. [Google Scholar] [CrossRef] [PubMed]
- Hlavsa, M.C.; Watson, J.C.; Beach, M.J. Cryptosporidiosis surveillance--United States 1999-2002. MMWR Surveill Summ. 2005, 54, 1–8. [Google Scholar]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 2014, 141, 1667–185. [Google Scholar] [CrossRef]
- Bruce, B.B.; Blass, M.A.; Blumberg, H.M.; Lennox, J.L.; del Rio, C.; Horsburgh, C.R. Jr. Risk of Cryptosporidium parvum transmission between hospital roommates. Clin. Infect. Dis. 2000, 31, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, T.K.; Fayer, R.; Knight, R.; Mhangami-Ruwende, B.; Trout, J.M.; Da Silva, A.J.; Pieniazek, N.J. Mechanical transport and transmission of Cryptosporidium parvum oocysts by wild filth flies. Am. J. Trop. Med. Hyg. 2000, 63, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Khalil, S.; Mirdha, B. Molecular appraisal of intestinal parasitic infection in transplant recipients. Indian J. Med. Res. 2016, 144, 258–263. [Google Scholar] [PubMed]
- Reina, F.T.; Ribeiro, C.A.; Araujo, R.S.; Matte, M.H.; Castanho, R.E.; Tanaka, I.I.; Viggiani, A.M.; Martins, L.P. Intestinal and pulmonary infection by Cryptosporidium parvum in two patients with HIV/AIDS. Rev. Inst. Med. Trop. Sao Paulo. 2016, 58, 21. [Google Scholar] [CrossRef]
- Gentile, G.; Baldassarri, L.; Caprioli, A.; Donelli, G.; Venditti, M.; Avvisati, G.; Martino, P. Colonic vascular invasion as a possible route of extraintestinal cryptosporidiosis. Am. J. Med. 1987, 82, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.; Jakobi, V.; Tessema, T.S. Host immune response to Cryptosporidium parvum infection. Exp. Parasitol. 2010, 126, 304–309. [Google Scholar] [CrossRef]
- Siński, E. Resistance to intestinal opportunistic infections using Cryptosporidium sp. as an example]. Wiad Parazytol. 2000;46:29-40.
- Kaushik, K.; Khurana, S.; Wanchu, A.; Malla, N. Serum immunoglobulin G, M and A response to Cryptosporidium parvum in Cryptosporidium-HIV co-infected patients. BMC Infect. Dis. 2009, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Barrier, M.; Lacroix-Lamandé, S.; Mancassola, R.; Auray, G.; Bernardet, N.; Chaussé, A.M.; Uematsu, S.; Akira, S.; Laurent, F. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 2006, 193, 1400–1407. [Google Scholar] [CrossRef]
- Chen, X.M.; O’Hara, S.P.; Nelson, J.B.; Splinter, P.L.; Small, A.J.; Tietz, P.S.; Limper, A.H.; LaRusso, N.F. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-κB. J. Immunol. 2005, 175, 7447–7456. [Google Scholar] [CrossRef]
- Lantier, L.; Drouet, F.; Guesdon, W.; Mancassola, R.; Metton, C.; Lo-Man, R.; Werts, C.; Laurent, F.; Lacroix-Lamandé, S. Poly(I:C)-induced protection of neonatal mice against intestinal Cryptosporidium parvum infection requires an additional TLR5 signal provided by the gut flora. J. Infect. Dis. 2014, 209, 457–467. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.P.; Bogert, P.S.; Trussoni, C.E.; Chen, X.; LaRusso, N.F. TLR4 promotes Cryptosporidium parvum clearance in a mouse model of biliary cryptosporidiosis. J. Parasitol. 2011, 97, 813–821. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A.; Paparini, A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016, 38, 535–547. [Google Scholar] [CrossRef]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zaalouk, T.K.; Bajaj-Elliott, M.; George, J.T.; McDonald, V. Differential regulation of beta-defensin gene expression during Cryptosporidium parvum infection. Infect. Immun. 2004, 72, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Elwin, K.; Hadfield, S.J.; Robinson, G.; Chalmers, R.M. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol. Infect. 2012, 140, 673–683. [Google Scholar] [CrossRef]
- Ludington, J.G.; Ward, H.D. Systemic and mucosal immune responses to Cryptosporidium-vaccine development. Curr. Trop. Med. Rep. 2015, 2, 171–180. [Google Scholar] [CrossRef]
- Jobin, C.; Sartor, R.B. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am. J. Physiol. Cell Physiol. 2000, 278, C451–C462. [Google Scholar] [CrossRef]
- Riggs, M.W. Recent advances in cryptosporidiosis: the immune response. Microbes Infect. 2002, 4, 1067–1080. [Google Scholar] [CrossRef]
- Ghafari, R.; Rafiei, A.; Tavalla, M.; Moradi Choghakabodi, P.; Nashibi, R.; Rafiei, R. Prevalence of Cryptosporidium species isolated from HIV/AIDS patients in southwest of Iran. Comp. Immunol. Microbiol. Infect. Dis. 2018, 56, 39–44. [Google Scholar] [CrossRef]
- Borowski, H.; Clode, P.L.; Thompson, R.C. Active invasion and/or encapsulation? A reappraisal of host-cell parasitism by Cryptosporidium. Trends Parasitol. 2008, 24, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Dann, S.M.; Okhuysen, P.C.; Lewis, D.E.; Chappell, C.L.; Adler, D.G.; White, A.C. Jr. High levels of CXCL10 are produced by intestinal epithelial cells in AIDS patients with active cryptosporidiosis but not after reconstitution of immunity. Infect. Immun. 2007, 75, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R. Early immune and host cell responses to Cryptosporidium infection. Front Parasitol. 2023, 2, 1113950. [Google Scholar] [CrossRef] [PubMed]
- Borad, A.; Ward, H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010, 5, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, M.; Rashid, R.; Mubarak, M. Gastrointestinal complications in renal transplant recipients detected by endoscopic biopsies in a developing country. Indian J. Gastroenterol. 2015, 34, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M.; Dieterich, D.T. The histopathology of consecutive colonoscopy biopsies from 82 symptomatic patients with acquired immunode¢ciency syndrome. Arch. Pathol. Lab. Med. 2001, 125, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chadwick, V.; Tie, A.; Harp, J. Cryptosporidium parvum in intestinal mucosal biopsies from patients with inflammatory bowel disease. Am. J. Gastroenterol. 2001, 96, 3463–3464. [Google Scholar] [CrossRef]
- Delis, S.G.; Tector, J.; Kato, T.; Mittal, N.; Weppler, D.; Levi, D.; Ruiz, P.; Nishida, S.; Nery, J.R.; Tzakis, A.G. Diagnosis and treatment of Cryptosporidium infection in intestinal transplant recipients. Transplant. Proc. 2002, 34, 951–952. [Google Scholar] [CrossRef]
- Khurana, S.; Chaudhary, P. Laboratory diagnosis of cryptosporidiosis. Trop. Parasitol. 2018, 8, 2–7. [Google Scholar] [CrossRef]
- Chieffi, P.P.; Sens, Y.A.; Paschoalotti, M.A.; Miorin, L.A.; Silva, H.G.; Jabur, P. Infection by Cryptosporidium parvum in renal patients submitted to renal transplant or hemodialysis. Rev. Soc. Bras. Med. Trop. 1998, 31, 333–337. [Google Scholar] [CrossRef]
- Minz, M.; Udgiri, N.K.; Heer, M.K.; Kashyap, R.; Malla, N. Cryptosporidiasis in live related renal transplant recipients: a single center experience. Transplantation. 2004, 77, 1916–1917. [Google Scholar] [CrossRef] [PubMed]
- Udgiri, N.; Minz, M.; Kashyap, R.; Heer, M.; Gupta, C.S.; Mohandas, K.; Minz, R.W.; Malla, N. Intestinal cryptosporidiasis in living related renal transplant recipients. Transplant Proc. 2004, 36, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Hazrati Tappeh, K.H.; Gharavi, M.J.; Makhdoumi, K.; Rahbar, M.; Taghizadeh, A. Prevalence of Cryptosporidium spp. infection in renal transplant and hemodialysis patients. Iran J. Public Health. 2006, 35, 54–57. [Google Scholar]
- Arslan, H.; Inci, E.K.; Azap, O.K.; Karakayali, H.; Torgay, A.; Haberal, M. Etiologic agents of diarrhea in solid organ recipients. Transpl. Infect. Dis. 2007, 9, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Abbas, Z.; Hassan, S.M.; Luck, N.H.; Aziz, T.; Mubarak, M. Prevalence of cryptosporidiosis in renal transplant recipients presenting with acute diarrhea at a single center in Pakistan. J. Nephropathol. 2014, 3, 127–131. [Google Scholar] [PubMed]
- Mohamed, N.S.; Siddig, E.E.; Mohamed, M.A.; Alzein, B.A.; Osman, H.H.S.; Tanyous, E.E.; Elamin, B.K.; Edris, A.M.M. Enteroparasitosis infections among renal transplant recipients in Khartoum state, Sudan 2012-2013. BMC Res. Notes. 2018, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.; Ranjan, P.; Dey, A.; Ghoshal, U.C. Intestinal cryptosporidiosis in renal transplant recipients: prevalence, species detection and comparative evaluation of SSU rRNA and Cryptosporidium oocyst wall protein genes. Indian J. Med. Microbiol. 2018, 36, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Lanternier, F.; Amazzough, K.; Favennec, L.; Mamzer-Bruneel, M.F.; Abdoul, H.; Tourret, J.; Decramer, S.; Zuber, J.; Scemla, A.; Legendre, C.; Lortholary, O.; Bougnoux, M.E. ; ANOFEL Cryptosporidium National Network and Transplant Cryptosporidium Study Group. Cryptosporidium spp. infection in solid organ transplantation: The Nationwide “TRANSCRYPTO” Study. Transplantation. 2017, 101, 826–830. [Google Scholar] [PubMed]
- Caccio, S.M.; Widmer, G. Cryptosporidium: parasite and disease; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- O’Leary, J.K.; Sleator, R.D.; Lucey, B. Cryptosporidium spp. diagnosis and research in the 21st century. Food Waterborne Parasitol. 2021, 24, e00131. [Google Scholar] [CrossRef]
- Van den Bossche, D.; Cnops, L.; Verschueren, J.; Van Esbroeck, M. Comparison of four rapid diagnostic tests, ELISA, microscopy and PCR for the detection of Giardia lamblia, Cryptosporidium spp. and Entamoeba histolytica in feces. J. Microbiol. Methods. 2015, 110, 78–84. [Google Scholar] [CrossRef]
- Love, M.S.; Choy, R.K.M. Emerging treatment options for cryptosporidiosis. Curr. Opin. Infect. Dis. 2021, 34, 455–462. [Google Scholar] [CrossRef]
- Florescu, D.F.; Sandkovsky, U. Cryptosporidium infection in solid organ transplantation. World J. Transplant. 2016, 6, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Eckmann, L. Drug development against the major diarrhea-causing parasites of the small intestine, Cryptosporidium and Giardia. Front Microbiol. 2015, 6, 1208. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.F.; Ayoub, A.; Ayers, M.S. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 2001, 184, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, J.F.; Kabil, S.M.; el-Gohary, Y.; Younis, A.M. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin. Gastroenterol. Hepatol. 2006, 4, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Faraci, M.; Cappelli, B.; Morreale, G.; Lanino, E.; Moroni, C.; Bandettini, R.; Terranova, M.P.; Di Martino, D.; Coccia, C.; Castagnola, E. Nitazoxanide or CD3+/CD4+ lymphocytes for recovery from severe Cryptosporidium infection after allogeneic bone marrow transplant? Pediatr. Transplant. 2007, 11, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Krause, I.; Amir, J.; Cleper, R.; Dagan, A.; Behor, J.; Samra, Z.; Davidovits, M. Cryptosporidiosis in children following solid organ transplantation. Pediatr. Infect. Dis. J. 2012, 31, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Grenouillet, F.; Larosa, F.; Dalle, F.; Saas, P.; Millon, L.; Deconinck, E.; Rohrlich, P.S. Diagnosis and treatment of digestive cryptosporidiosis in allogeneic haematopoietic stem cell transplant recipients: a prospective single centre study. Bone Marrow Transplant. 2011, 46, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Mawhorter, S.D. Parasitic infections in solid organ transplantation. Am. J. Transplant. 2013, 13, 280–303. [Google Scholar] [CrossRef]
- Tie, X.; Zhang, Z.; Zhou, R.; Li, Y.; Xu, J.; Yin, W. A case of septic shock due to delayed diagnosis of Cryptosporidium infection after liver transplantation. BMC Infect. Dis. 2023, 23, 260. [Google Scholar] [CrossRef]
- Baishanbo, A.; Gargala, G.; Duclos, C.; François, A.; Rossignol, J.F.; Ballet, J.J.; Favennec, L. Efficacy of nitazoxanide and paromomycin in biliary tract cryptosporidiosis in an immunosuppressed gerbil model. J. Antimicrob. Chemother. 2006, 57, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Okhuysen, P.C.; Chappell, C.L.; Crabb, J.; Valdez, L.M.; Douglass, E.T.; DuPont. H.L. Prophylactic effect of bovine anti-Cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin. Infect. Dis. 1998, 26, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, F.; Valot, S.; Dalle, F.; Sterin, A.; L’Ollivier, C. Disseminated Cryptosporidium infection in an infant with CD40L deficiency. IDCases. 2021, 24, e01115. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.H.B.; Recuenco, F.C.; Mohd Zin, N.K.; Watanabe, N.; Fukuda, Y.; Bando, H.; Watanabe, K.; Bochimoto, H.; Xuan, X.; Kato, K. Identification of potent anti-Cryptosporidium new drug leads by screening traditional Chinese medicines. PLoS Negl. Trop. Dis. 2022, 16, e0010947. [Google Scholar] [CrossRef] [PubMed]
- Yavuzcan Yildiz, H.; Phan Van, Q.; Parisi, G.; Dam Sao, M. Anti-parasitic activity of garlic (Allium sativum) and onion (Allium cepa) juice against crustacean parasite, Lernantropus kroyeri, found on European sea bass (Dicentrarchus labrax). Ital. J. Anim. Sci. 2019, 18, 833–837. [Google Scholar] [CrossRef]
- Farid, A.; Yousry, M.; Safwat, G. Garlic (Allium sativum Linnaeus) improved inflammation and reduced cryptosporidiosis burden in immunocompromised mice. J. Ethnopharmacol. 2022, 292, 115174. [Google Scholar] [CrossRef] [PubMed]
- Elbahaie, E.S.; El Gamal, R.L.; Fathy, G.M.; Al-Ghandour, A.M.F.; El-Akabawy, N.; Abd El Hameed, B.H.; Yahia, S.H. The controverted therapeutic efficacy of Allium sativum and Artemisia herba-alba extracts on Cryptosporidium-infected mice. J. Infect. Dev. Ctries. 2023, 17, 732–743. [Google Scholar] [CrossRef]
- Hamdy, D.A.; Ismail, M.A.M.; El-Askary, H.M.; Abdel-Tawab, H.; Ahmed, M.M.; Fouad, F.M.; Mohamed, F. Newly fabricated zinc oxide nanoparticles loaded materials for therapeutic nano delivery in experimental cryptosporidiosis. Sci. Rep. 2023, 13, 19650. [Google Scholar] [CrossRef]
| Patient age/gender | kidney transplantation | Cryptosporidium infection | Treatment of vryptosporidiosis | Reference | ||||
| Time after | Immunosuppressive treatment | Symptoms | Environmental risk factor | Species (intensity) | Drugs | Treatment result | ||
| 60 | 8 years /1st graft |
TAC (4 mg/day) + MMF) (1 g x 2/day) + PRED(7.5 mg/day) | watery diarrhea, nausea, vomiting, weight loss (6 kg) | admitted to own a dog |
C. felis (5–10 oocysts/slide) |
NTZ (500 mg x 2/day for 14 days) | negative 2 weeks after treatment, with no recurrence of diarrhea observed 4 months | [40] |
| 64 | 2 years /1st graft | TAC (7 mg x 2/day), MMF (750 mg x 2/day), and PRED (10 mg/day) | watery diarrhea, abdominal pain, weight loss (13 Kg) | had travelled to Mali | C. hominis (>100 oocysts/slide) | 1. reduction of TAC | diarrhea regressed after 8 days, 3 months after therapy stools still tested positive | |
| 2. NTZ (500 mg x 2/day for 14 days) | One month stools were tested negative | |||||||
| 34 | 8 years /2st graft |
TAC (6 mg x 2/day), MMF (750 mg x 2/day), and PRED (25 mg/day). | watery diarrhea, abdominal pain weight loss (10 Kg) | had travelled to Kosovo | C. parvum (1–5 oocysts/slide) | 1. reduction of TAC | diarrhea was regressing 1 month after treatment | |
| 2. NTZ (500 mg x 2/day for 14 days) | months after the second treatment course, his stools were tested negative | |||||||
| 38 | 2 years /1st graft | ND | diarrhea | ND | ND | Spiramycin 2 g daily for 10 days | Symptoms resolved second days therapy and reduction oocyst | [41] |
| 42 | 1 year /1st graft |
ND | Abdominal pain, distention | ND | ND | Spiramycin 2 g daily for 10 days | After treatment no symptoms and oocysts | |
| 41/M | 1 year /1st graft |
ND | Weakness, fever of 39°C, yellowish diarrhea occurring 4-5 times daily without blood | ND | ND | NTZ 500 mg twice daily for 3 days. | successful recovery of Cryptosporidium spp. infection | [21] |
| 37 | 1st graft | ND | acute diarrhea, up to 10 times daily, abdominal discomfort, coryzal symptoms | ND | ND | paromomycin 1 g twice daily + AZM 500 mg daily | 1 week after treatment was still positive, after 4 weeks clinical and parasitological resolution |
[42] |
| 42/W | 1 year | TAC (present levels 8–10 ng/ml), MMF (1250 mg/day) and steroids (PRED, 5 mg/day) | abdominal pain, diarrhoea (5 days) | travelled to Cuba | ND | rifaximin (600 mg thrice daily), reduced MMF to 1 g/day | patient’s symptoms resolved within 1 week | [43] |
| 24/M | 1 month/ 2st graft |
TAC + MMF | chronic watery diarrhea, nausea | ND | ND | NTZ monotherapy | ineffective | [44] |
| NTZ (1 g twice daily) + AZM (600 mg daily) + rifaximin (550 mg twice daily) + intravenous fluids + diphenoxylate-atropine; AZM was stopped; TAC was 17 ng/mL, and it was discontinued and later restarted at a lower dose | diarrhea within 10 days without recurrence | |||||||
| 78/W | 9 years /1st graft |
Steroids 3 months post-transplant, TAC + MMF | watery diarrhoea without any pathological substance that had a 7 day evolution, without fever, vomiting or abdominal pain /sclerosing cholangitis | paromomycin + AZM of 14 days, subsequently NTZ for 6 days, doses of TAC and MMF were reduced | diarrhoea disappeared, kidney function recovered to basaline levels, 17 months later the patient was still asymptomatic | [45] | ||
| 60 | 3 months | MMF/CsA/Cs, MMF switched to AZA | Severe diarrhoea | Spiramycin 10 days | Resolved | [46] | ||
| 59 | 1 month | Sir/FK/Cs | Severe diarrhoea | Paromomycin 4 weeks | Resolved | [47] | ||
| 68/M | 56 months /1st graft |
Anti-IL2r CNI + MMF | Diarrhea, vomiting, dehydration, weight loss (8 kg), acute kidney injury, acidosis | Contact with animals and children | C. parvum | Reduced MMF or stopped until diarrhea resolved, 500 mg of NTZ twice a day for four weeks | for the three patients gastrointestinal disorders resolved in the first two weeks; MMF was newly initiated as soon as diarrhea resolved; no relapse was observed | [3] |
| 42/F | 25 months /1st graft |
Anti-IL2r CNI + MMF | Fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (4 kg) | Previous antibiotic therapy with amoxicillinclavulanic acid | Cryptosporidium spp. | |||
| 77/M | 14 days /1st graft |
Anti-IL2r CNI + MMF | Severe diarrhea, dehydration, weight loss (3 kg), acute kidney injury | Contact with untreated water | C. parvum | |||
| 53/M | 2 days /1st graft |
Anti-IL2r CNI + MMF | Diarrhea, vomiting, dehydration, weight los (4 kg) | Contact with cat | C. felis | |||
| 64/F | 65 months /1st graft |
Depleting therapy CNI + MMF | Fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (2 kg) | None | C. parvum | |||
| 37/F | 57 months /3st graft |
Desensitization, depleting therapy CNI + MMF | Fever, abdominal pain, diarrhea, vomiting, dehydration, weight loss (3 kg) | Work as a nurse, contact with recreative water, treated with phenoxymethylpenicillin | C. parvum | |||
| Country | Number of participation | diagnostic method | patients with diarrhea | citation | |
|---|---|---|---|---|---|
| all (n) | infected (n/%) | ||||
| Argentina | 26 | 11/42.3% | MAF after M-FECT | 0 | [25] |
| Türkiye | 69 | 13/18.8% | MAF | 8 | [41] |
| Brazil | 23 | 8/34.8% | ZN after FECT | - | [84] |
| India | 60 | 12/20% | MAF | 2 | [85] |
| India | 60 | 12/20% | MAF | 16.6% | [86] |
| Iran | 87 | 10/11.5% | MAF | - | [87] |
| Türkiye | 43 transplant (40 renal and 3 liver) | 7/21.2% | ZN | 40 | [88] |
| Pakistan | 644 | 343/53% | MZN | 343 | [89] |
| Sudan | 300 | 5/1.7% | NZN, MAF | ND | [90] |
| India | 358 | 13/8.4% | MAF after FECT | - | [91] |
| 307 | 30/9,8% | PCR | 26 | ||
| France | 88 | 41/46.6% | - | [92] | |
| 73 | 6/8.2% | MZN | 6 | [3] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





