1. Introduction
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disorder of the white matter. Pathophysiology is thought to be immune mediated as in most cases the condition follows an infection or triggering incident. Clinical manifestations include encephalopathy and rapid neurological decline over the course of days to weeks [
1]. More recent literature has demonstrated that there may be a link between autoimmune conditions and ADEM [
2]. ADEM is rare disease that is primarily present in children with a prevalence of about one in 100,000 children being effected. Additionally up to 85% of cases of ADEM have identifiable infection at time of diagnosis or were recently vaccinated [
3]. This case is unique as a middle aged female is diagnosed with ADEM with no known triggering event such as infection or vaccination. Here we present a case of ADEM in a middle-aged woman with systemic lupus erythematosus that recovered well after treatment with corticosteroids and Rituximab.
2. Case Presentation
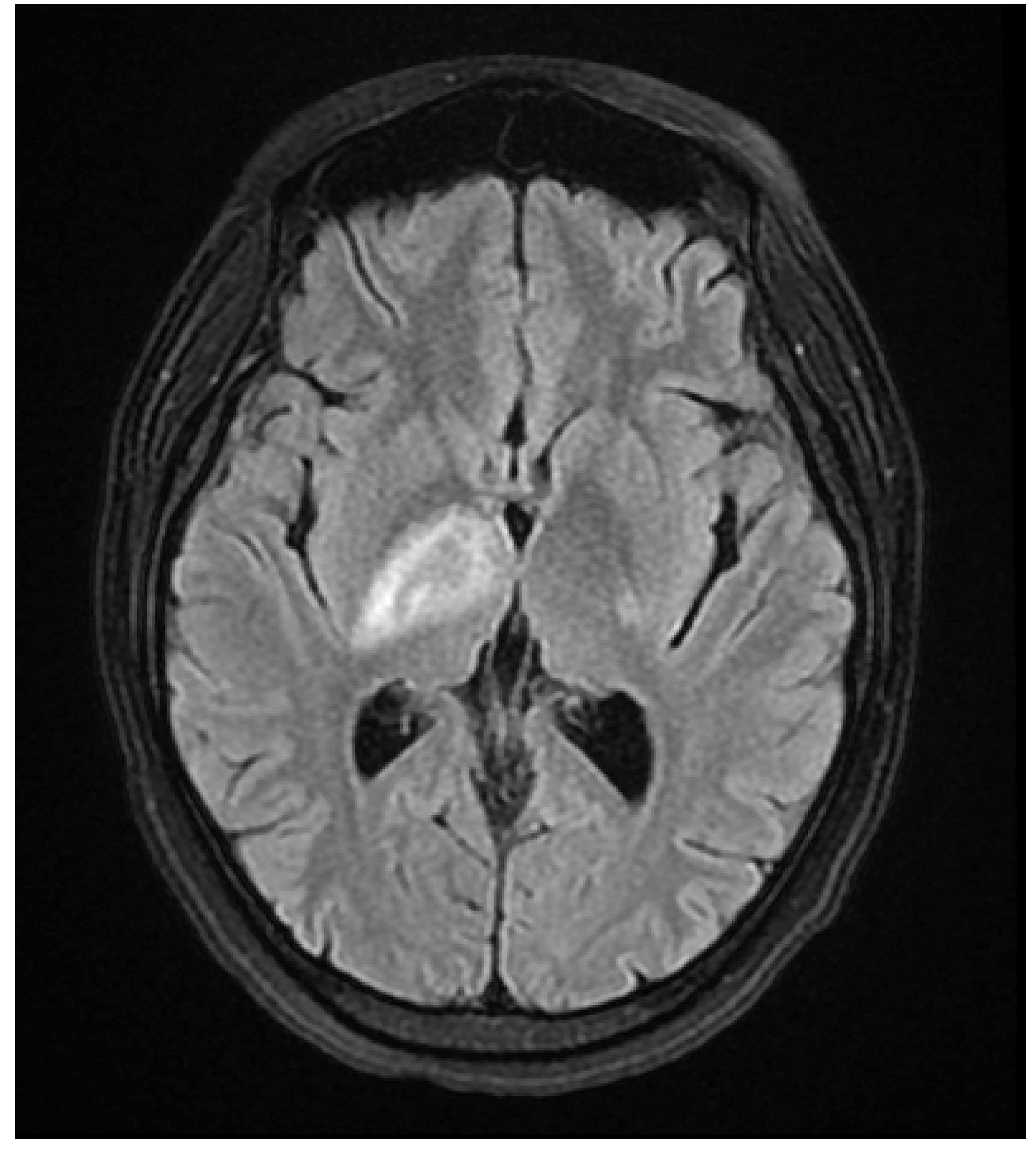
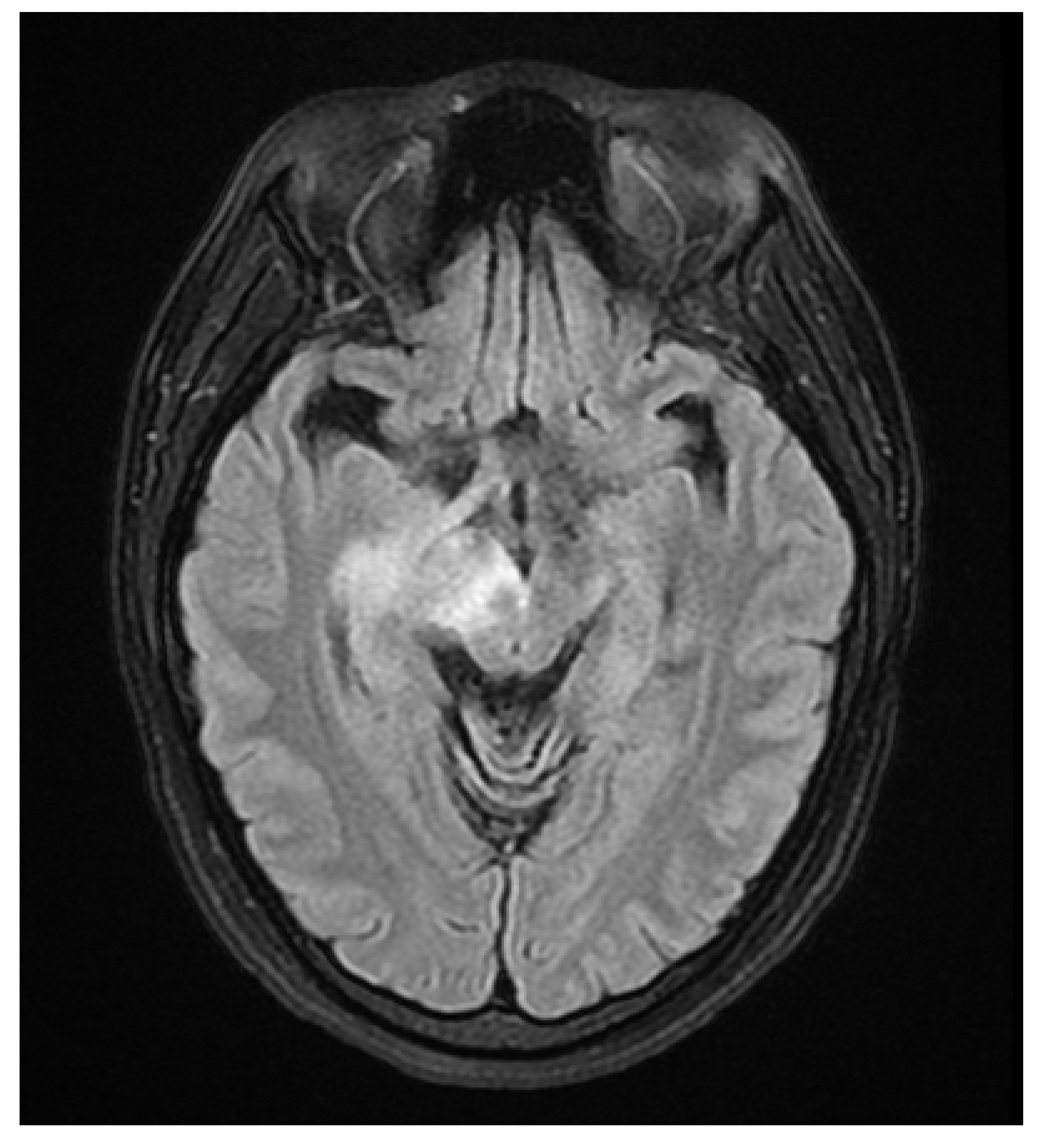
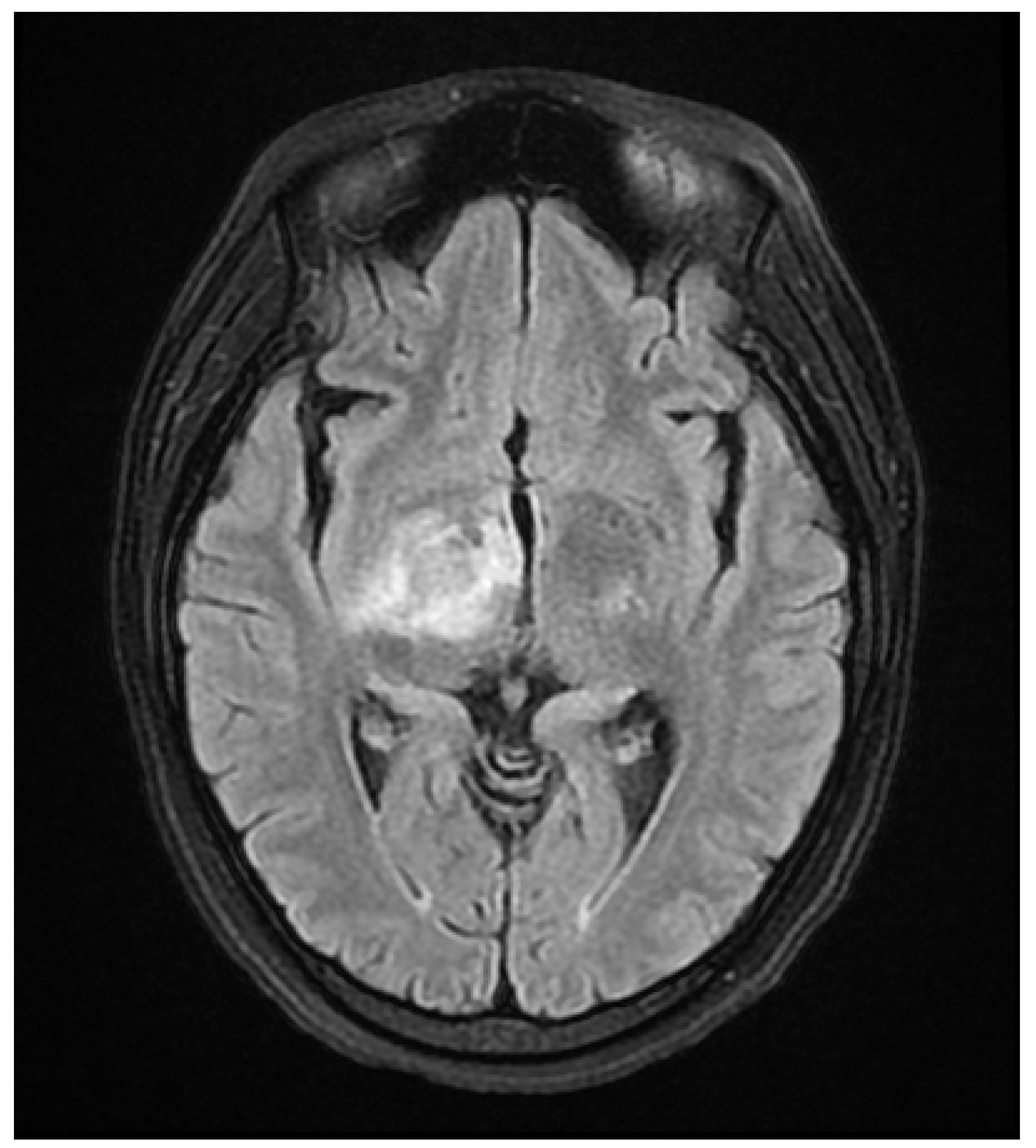
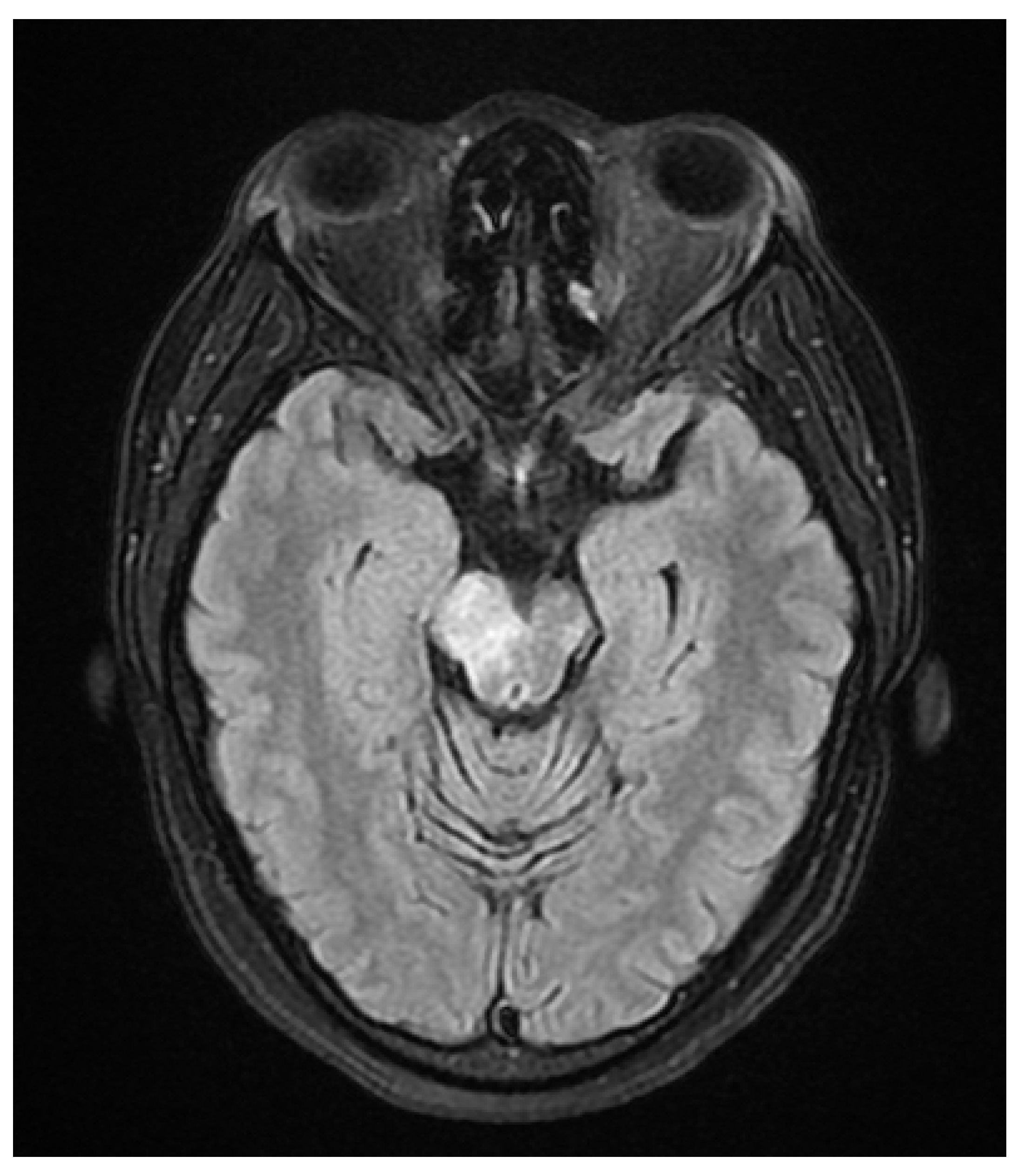
A 37 year old female presented to our hospital due to new onset severe headaches and right sided weakness with facial droop. The patient did not have any significant past medical history before this point. Patient was unable to speak properly and was found to have decreased responsiveness at bedside. Initial CT scan of the head without contrast revealed ill-defined edema involving the left periatrial white matter, involving the central gray matter and external limited internal capsule. MRI head with T2 Flair revealed diffuse edema and inflammation throughout the brainstem, pons, midbrain, and basal ganglia (
Figure 1,
Figure 2,
Figure 3 and
Figure 4). EEG showed diffuse slowing suggestive of moderate diffuse cerebral dysfunction without evidence of seizures or epileptiform activity. Lumbar puncture demonstrated a normal opening pressure, with CSF containing 2 red blood cells, 9 white blood cells of which 94% were lymphocytes, glucose of 58, and protein of 42. CSF bacterial cultures were negative, along with no evidence of active HSV or EBV infection. RPR was also negative. Pulse dose steroids were initiated in the ICU. Patient was slow to improve and was transferred to the neurological intensive care unit for further management. After 17 days in the ICU patient was discharged to a rehabilitation facility. Patient slowly improved with physical therapy but did continue to have some refractory lower extremity weakness. At the time of discharge, our patient was discharged on 20mg prednisone daily. Outpatient brain biopsy of the right frontal cortex revealed moderate gliosis but no abnormal lymphocytic infiltration. After thorough review of imaging findings and clinical presentation a diagnosis of acute disseminated encephalomyelitis was made by Neurology.
This patient was referred to rheumatology due to the inflammatory findings seen on MRI head with T2 FLAIR. Initial Laboratory testing revealed C-reactive protein 7.3 mg/dL (normal < 8 mg/dL), ESR of 17 mm/h (normal 20 mm/h), RNP negative, p-ANCA negative, c-ANCA negative, myeloperoxidase antibody negative, proteinase-3 antibody negative, SSA negative, SSB negative, RNP negative, C3 144 mg/dL (normal < 193 mg/dL), C4 50 mg/dL (normal <57 mg/dL), ANA 1:80, and anti-dsDNA antibody of 22 IU/mL (normal < 10 IU/mL). On exam the patient was found to have joint inflammation of the metacarpophalangeal joints. A diagnosis of SLE was made according to EULAR/ACR criteria. Patient was started on disease modifying antirheumatic drugs including methotrexate and hydroxychloroquine which she did not tolerate due to bleeding and anemia. Prednisone 20mg daily was continued in an attempt to reduce flares of ADEM. Unfortunately, flares of ADEM continued which presented as lower extremity weakness, headaches, and facial droop in the months following diagnosis of ADEM. Subsequently, the patient was started on Rituximab 1,0000 mg every 4 weeks in an attempt to manage flares and improve symptoms. At 6 month follow up the patient’s weakness and headaches greatly improved. However, some residual left lower extremity weakness persisted which necessitated the use of a walker. Bloodwork revealed an anti-dsDNA antibody of 16 IU/mL at 6 month follow up visit. Follow up MRI head with FLAIR revealed marked decrease in T2 Flair signal within the basal ganglia and midbrain with complete resolution of enhancement in the brainstem. During this time neurology also recommended that the patient start IVIG at 1g/kg every 3 weeks. Gradual improvement in symptoms was seen over the next several months on a regiment of Prednisone 15mg daily, Rituximab 1,000mg every four weeks, and IVIG 1g/kg every 3 weeks. During her one year follow up post diagnosis, the patient had minimal residual weakness in her lower extremities and was able to ambulate without a walker. At this visit her anti- dsDNA antibody continued to drop and was measured to be 12 IU/mL (
Table 1). The patient has continued this regiment of prednisone, Rituximab, and IVIG in an effort to avoid flairs of ADEM. She has endorsed a significant improvement in symptoms and quality of life. We appreciate the patient giving consent for us to publish her case.
3. Discussion
ADEM is thought to be an autoimmune disease in which it is theorized that autoimmune constituents attack myelin components such as myelin basic protein, proteolipid protein, and myelin oligodendrocyte protein leading to demyelination and diffuse white matter changes [
4]. Demyelination is theorized to be precipitated by T cell activation through a cascade of inflammation involving Inflammatory cytokines including tumor necrosis factor α (TNFα), interleukin 2 (IL2) and interferon γ (INFγ) [
5]. Due to the active role of inflammatory cytokines in the pathogenesis of ADEM, any disease contributing to systemic formation of inflammatory cytokines can potentially be an etiologic factor for the initiation of ADEM.
In SLE the number of T-cells, T helper type 1 cytokines and other inflammatory cytokines such as TNFα increase substantially. The role of TNFα is especially important which exerts multiple stimulatory effects on T cells [
6]. This inflammatory cascade leads to a altering the permeability of the brain blood barrier, which in turn allows the presentation of autoantigens to the activated immune system. This may lead to the autoimmune disease ADEM [
7]. Migratory immune cells attack the basic myelin protein and the final result is the demyelination seen in ADEM.
Under normal circumstances double stranded DNA is not accessible to the immune system because it is contained in the nucleus and mitochondria. However, nuclear materials can be released from apoptotic cells in the setting of infection, inflammation, and UV radiation. The released DNA can be recognized by anti-DNA antibodies and compose immune complexes. The immune complexes act as an antigen to stimulate B cells to produce anti-dsDNA antibodies.
A wealth of proinflammatory factors, including monocyte chemotactic protein 1, TNF-α, IL-1β, IL-6, and IL-8 are overexpressed with stimulation with anti-dsDNA antibodies. Accumulation of inflammatory cytokines is sufficient for accelerating the recruitment of immune cells and the induction of inflammatory processes. The anti-dsDNA antibodies can gain access to the brain tissue after the destruction of the blood-brain barrier, leading to inflammation and neuronal cell death [
8]. Anti-dsDNA level is correlated with systemic lupus erythematosus activity [
9]. In our patient lower anti-dsDNA was correlated with improvement of symptoms.
There has been growing evidence of autoimmune disease associated with ADEM in patients with systemic lupus erythematosus, vasculitis, and rheumatoid arthritis [
10]. We suggest the screening of all patients with ADEM for rheumatologic conditions. Immediate treatment should be initiated if autoimmune disease is diagnosed. Further clinical and experimental research will have to be performed to understand the underlying link between ADEM and systemic lupus erythematosus.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
References
- Elhassanien, Ahmed Farag. “Acute demyelinating encephalomyelitis (ADEM): Clinical characteristics and outcome.” Pediatrics & Therapeutics, vol. 03, no. 01, 2013. [CrossRef]
- Safadi, S., et al. “Acute disseminated encephalomyelitis (ADEM) and systemic lupus erythematosus (SLE) in children: What relationship?” Global Pediatrics, vol. 8, June 2024, p. 100169. [CrossRef]
- Anilkumar, Arayamparambil, et al. “Acute Disseminated Encephalomyelitis.” Statpearls, 26 Jan. 2024.
- Filippi M, Rocca MA. “Acute Disseminated Encephalomyelitis.” White Matter Diseases. 2020 Feb 12:109–25.
- Garg, Ravindra Kumar, et al. “Pathophysiology of acute disseminated encephalomyelitis.” Multiple Sclerosis, 2016, pp. 201–248.
- Postal, Mariana, and Simone Appenzeller. “The role of tumor necrosis factor-alpha (TNF-α) in the pathogenesis of systemic lupus erythematosus.” Cytokine, vol. 56, no. 3, Dec. 2011, pp. 537–543. [CrossRef]
- Safadi, S., et al. “Acute disseminated encephalomyelitis (ADEM) and systemic lupus erythematosus (SLE) in children: What relationship?” Global Pediatrics, vol. 8, June 2024, p. 100169. [CrossRef]
- Wang, Xiaoyu, and Yumin Xia. “Anti-double stranded DNA antibodies: Origin, pathogenicity, and targeted therapies.” Frontiers in Immunology, vol. 10, 17 July 2019. [CrossRef]
- Venner, Allison A., et al. “Comparison of three anti-dsdna assays: Performance and correlation with systemic lupus erythematosus disease activity.” Clinical Biochemistry, vol. 46, no. 4–5, Mar. 2013, pp. 317–320. [CrossRef]
- Sabayan, B., and Abdolali Zolghadrasli. “Vasculitis and rheumatologic diseases may play role in the pathogenesis of acute disseminated encephalomyelitis (ADEM).” Medical Hypotheses, vol. 69, no. 2, Jan. 2007, pp. 322–324. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).