Submitted:
16 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
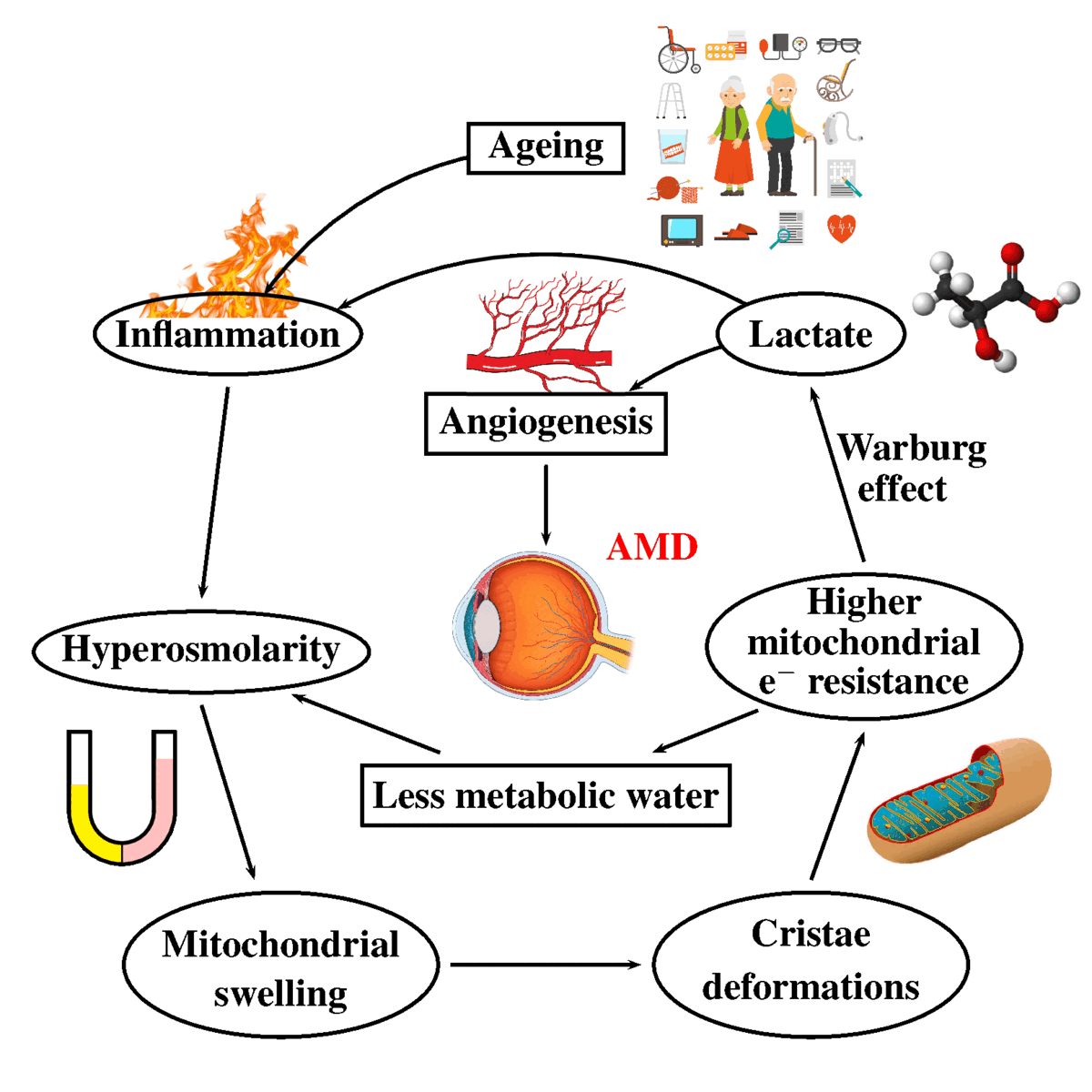
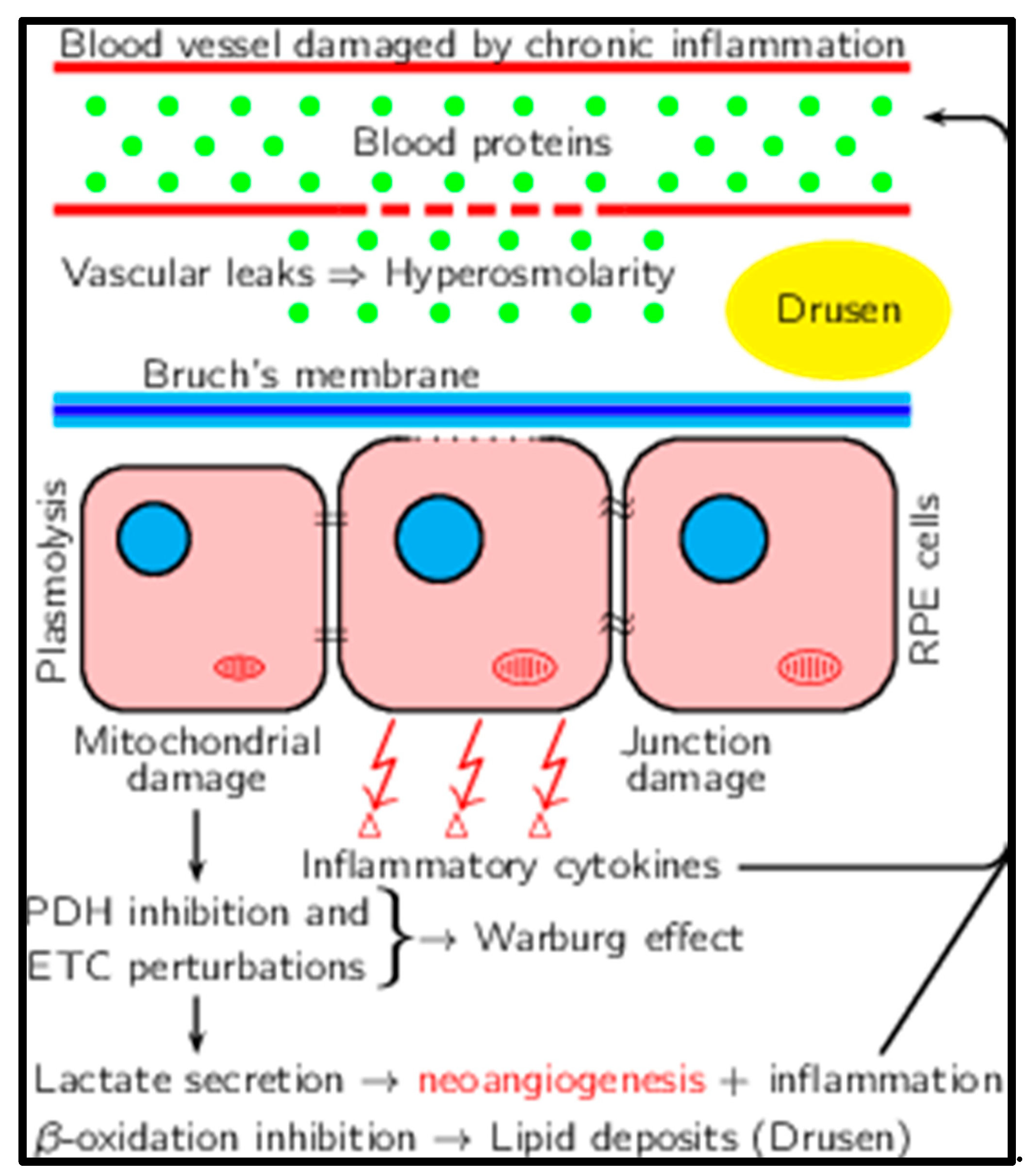
Keywords:
1. Introduction
2. Overview
3. Epidemiology
4. Risk Factors
5. Inflammation as the Driving Force in Cancer, Alzheimer's, and Aging
6. Inflammation and Increased Osmolarity
7. Osmosis in the Posterior Segment
8. Aging of the Posterior Segment: Vascular Changes
9. Increased Osmolarity and AMD
10. Metabolic Shift Because of Increased Pressure
11. Shift to Alternative Energy Sources
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer Disease. Nature Reviews Neurology 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Adami, H.-O.; Hunter, D.; Trichopoulos, D. Textbook of Cancer Epidemiology; Oxford University Press, 2008; ISBN 0-19-971863-6.
- Nogueira, M.L.; Moreira, J.d.V.; Baronzio, G.F.; Dubois, B.; Steyaert, J.-M.; Schwartz, L. Mechanical Stress as the Common Denominator between Chronic Inflammation, Cancer, and Alzheimer’s Disease. Front. Oncol. 2015, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Craft, S. The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia: Two Roads Converged. Archives of neurology 2009, 66, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Aerobic Glycolysis Hypothesis Through WNT/Beta-Catenin Pathway in Exudative Age-Related Macular Degeneration. J. Mol. Neurosci. 2017, 62, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Léveillard, T.; Philp, N.J.; Sennlaub, F. Is Retinal Metabolic Dysfunction at the Center of the Pathogenesis of Age-Related Macular Degeneration? International journal of molecular sciences 2019, 20, 762. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, M.; Wertz, X.; Pooya, M.; Chaumet-Riffaud, P.; Guais, A.; Schwartz, L. Hyperosmolarity causes inflammation through the methylation of protein phosphatase 2A. Inflamm. Res. 2008, 57, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Peres, S.; Jolicoeur, M.; da Veiga Moreira, J. Cancer and Alzheimer’s disease: intracellular pH scales the metabolic disorders. Biogerontology 2020, 21, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.; Korb, C.; Erke, M.G. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: a review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Buitendijk, G.H.; Rochtchina, E.; Myers, C.; van Duijn, C.M.; Lee, K.E.; Klein, B.E.; Meuer, S.M.; de Jong, P.T.; Holliday, E.G.; Tan, A.G. Prediction of Age-Related Macular Degeneration in the General Population: The Three Continent AMD Consortium. Ophthalmology 2013, 120, 2644–2655. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Brodzka, S.; Baszyński, J.; Rektor, K.; Hołderna-Bona, K.; Stanek, E.; Kurhaluk, N.; Tkaczenko, H.; Malukiewicz, G.; Woźniak, A.; Kamiński, P. The Role of Glutathione in Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2024, 25, 4158. [Google Scholar] [CrossRef] [PubMed]
- Hrishikesh, V.; Pranaykumar, S. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14. [Google Scholar]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration: AREDS Report No. 17. Archives of ophthalmology (Chicago, Ill.: 1960) 2005, 123, 1484–1498. [Google Scholar] [PubMed]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.-Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A Simplified Severity Scale for Age-Related Macular Degeneration: AREDS Report No. 18. Archives of ophthalmology (Chicago, Ill.: 1960) 2005, 123, 1570–1574. [Google Scholar] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. The Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Joachim, N.; Mitchell, P.; Burlutsky, G.; Wang, J.J. Validating the AREDS Simplified Severity Scale of Age-Related Macular Degeneration with 5- and 10-Year Incident Data in a Population-Based Sample. Ophthalmology 2016, 123, 1874–1878. [Google Scholar] [CrossRef]
- Garcia-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- Friedman, D.S.; O'Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; De Jong, P.T.V.M.; Nemesure, B.; Mitchell, P.; Kempen, J.; Congdon, N. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef]
- von der Emde, L.; Pfau, M.; Holz, F.G.; Fleckenstein, M.; Kortuem, K.; Keane, P.A.; Rubin, D.L.; Schmitz-Valckenberg, S. AI-based structure-function correlation in age-related macular degeneration. Eye 2021, 35, 2110–2118. [Google Scholar] [CrossRef]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef] [PubMed]
- Telander, D.G. Inflammation and Age-Related Macular Degeneration (AMD). Semin. Ophthalmol. 2011, 26, 192–197. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.; Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Schlanitz, F.G.; Baumann, B.; Kundi, M.; Sacu, S.; Baratsits, M.; Scheschy, U.; Shahlaee, A.; Mittermüller, T.J.; Montuoro, A.; Roberts, P.; et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.A.; Or, C.; Young, M.; Chui, L.; Fallah, N.; Kirker, A.W.; Albiani, D.A.; Merkur, A.B.; Forooghian, F. Optical Coherence Tomography–Based Measurement of Drusen Load Predicts Development of Advanced Age-Related Macular Degeneration. Arch. Ophthalmol. 2014, 158, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Folgar, F.A.; Yuan, E.L.; Sevilla, M.B.; Chiu, S.J.; Farsiu, S.; Chew, E.Y.; Toth, C.A. Drusen Volume and Retinal Pigment Epithelium Abnormal Thinning Volume Predict 2-Year Progression of Age-Related Macular Degeneration. Ophthalmology 2016, 123, 39–50. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, S.-G.; Bae, J.H.; Yu, H.G.; Song, S.J. Risk Factors for Progression of Early Age-Related Macular Degeneration in Koreans. Ophthalmic Epidemiology 2016, 23, 80–87. [Google Scholar] [CrossRef]
- Joachim, N.D.; Mitchell, P.; Kifley, A.; Wang, J.J. Incidence, Progression, and Associated Risk Factors of Medium Drusen in Age-Related Macular Degeneration: Findings from the 15-Year Follow-up of an Australian Cohort. JAMA ophthalmology 2015, 133, 698–705. [Google Scholar] [CrossRef]
- Hallak, J.A.; de Sisternes, L.; Osborne, A.; Yaspan, B.; Rubin, D.L.; Leng, T. Imaging, Genetic, and Demographic Factors Associated with Conversion to Neovascular Age-Related Macular Degeneration: Secondary Analysis of a Randomized Clinical Trial. JAMA ophthalmology 2019, 137, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement Activation and Inflammatory Processes in Drusen Formation and Age Related Macular Degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Arch. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; Schultz, D.W.; Weleber, R.G.; Schain, M.B.; Edwards, A.O.; Matise, T.C.; Acott, T.S.; Ott, J.; Klein, M.L. Age-Related Macular Degeneration—a Genome Scan in Extended Families. Am. J. Hum. Genet. 2003, 73, 540–550. [Google Scholar] [CrossRef] [PubMed]
- E Hughes, A.; Orr, N.; Esfandiary, H.; Diaz-Torres, M.; Goodship, T.; Chakravarthy, U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006, 38, 1173–1177. [Google Scholar] [CrossRef]
- Cipriani, V.; Leung, H.-T.; Plagnol, V.; Bunce, C.; Khan, J.C.; Shahid, H.; Moore, A.T.; Harding, S.P.; Bishop, P.N.; Hayward, C.; et al. Genome-wide association study of age-related macular degeneration identifies associated variants in the TNXB–FKBPL–NOTCH4 region of chromosome 6p21.3. Hum. Mol. Genet. 2012, 21, 4138–4150. [Google Scholar] [CrossRef]
- Smailhodzic, D.; Muether, P.S.; Chen, J.; Kwestro, A.; Zhang, A.Y.; Omar, A.; Van de Ven, J.P.; Keunen, J.E.; Kirchhof, B.; Hoyng, C.B.; et al. Cumulative Effect of Risk Alleles in CFH, ARMS2, and VEGFA on the Response to Ranibizumab Treatment in Age-related Macular Degeneration. Ophthalmology 2012, 119, 2304–2311. [Google Scholar] [CrossRef]
- Medina, F.M.C.; da Motta, A.A.L.; Takahashi, W.Y.; Carricondo, P.C.; Motta, M.M.d.S.; Melo, M.B.; Vasconcellos, J.P.C. Pharmacogenetic Effect of Complement Factor H Gene Polymorphism in Response to the Initial Intravitreal Injection of Bevacizumab for Wet Age-Related Macular Degeneration. Ophthalmic Res. 2015, 54, 169–174. [Google Scholar] [CrossRef]
- McCarty, C.A.; Mukesh, B.N.; Fu, C.L.; Mitchell, P.; Wang, J.J.; Taylor, H.R. Risk Factors for Age-Related Maculopathy: The Visual Impairment Project. Archives of ophthalmology 2001, 119, 1455–1462. [Google Scholar] [CrossRef]
- Joachim, N.; Mitchell, P.; Kifley, A.; Rochtchina, E.; Hong, T.; Wang, J.J. Incidence and Progression of Geographic Atrophy: Observations from a Population-Based Cohort. Ophthalmology 2013, 120, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Joachim, N.; Mitchell, P.; Rochtchina, E.; Tan, A.G.; Wang, J.J. Incidence and Progression of Reticular Drusen in Age-Related Macular Degeneration: Findings from an Older Australian Cohort. Ophthalmology 2014, 121, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, F.; Fisher, D.E.; Eiriksdottir, G.; Sigurdsson, S.; Klein, R.; Launer, L.J.; Harris, T.; Gudnason, V.; Cotch, M.F. Five-Year Incidence, Progression, and Risk Factors for Age-Related Macular Degeneration: The Age, Gene/Environment Susceptibility Study. Ophthalmology 2014, 121, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Velilla, S.; García-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Durán, M.D.; Gómez-Ulla, F.; Arévalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and Age-Related Macular Degeneration: Review and Update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Marin, A.I.; Poppelaars, F.; Wagner, B.D.; Palestine, A.G.; Patnaik, J.L.; Holers, V.M.; Frazer-Abel, A.A.; Mathias, M.T.; Manoharan, N.; Fonteh, C.N.; et al. Sex and Age-Related Differences in Complement Factors Among Patients With Intermediate Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2022, 11, 22–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Mitchell, P.; Wang, J. Gender, Oestrogen, Hormone Replacement and Age-related Macular Degeneration: Results from the Blue Mountains Eye Study. Australian and New Zealand journal of ophthalmology 1997, 25, 13–15. [Google Scholar] [CrossRef]
- Clemons, T.E.; Milton, R.C.; Klein, R.; Seddon, J.M.; Ferris 3rd, F.L. Risk Factors for the Incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS Report No. 19. Ophthalmology 2005, 112, 533–539. [Google Scholar]
- Tikellis, G.; Robman, L.D.; Dimitrov, P.; Nicolas, C.; A McCarty, C.; Guymer, R.H. Characteristics of progression of early Age-related macular degeneration: the Cardiovascular Health and Age-related maculopathy Study. Eye 2006, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cougnard-Grégoire, A.; Delyfer, M.-N.; Korobelnik, J.-F.; Rougier, M.-B.; Malet, F.; Le Goff, M.; Dartigues, J.-F.; Colin, J.; Barberger-Gateau, P.; Delcourt, C. Long-Term Blood Pressure and Age-Related Macular Degeneration: The ALIENOR Study. Investig. Opthalmology Vis. Sci. 2013, 54, 1905–1912. [Google Scholar] [CrossRef]
- Parekh, N.; Voland, R.P.; Moeller, S.M.; Blodi, B.A.; Ritenbaugh, C.; Chappell, R.J.; Wallace, R.B.; Mares, J.A. ; CAREDS Research Study Group Association between Dietary Fat Intake and Age-Related Macular Degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): An Ancillary Study of the Women’s Health Initiative. Archives of ophthalmology 2009, 127, 1483–1493. [Google Scholar] [CrossRef]
- Garber, J.E.; Offit, K. Hereditary Cancer Predisposition Syndromes. J. Clin. Oncol. 2005, 23, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Pellegrini, S.; Siciliano, G.; Murri, L. Causative and susceptibility genes for Alzheimer’s disease: a review. Brain Res. Bull. 2003, 61, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Bermejo, J.L.; Sundquist, J.; Hemminki, K. Age of onset in familial cancer. Ann. Oncol. 2008, 19, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s Disease. The Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Waring, S.C.; Rosenberg, R.N. Genome-Wide Association Studies in Alzheimer Disease. Arch. Neurol. 2008, 65, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 1999, 399, A23–A31. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Chen, C.A.; Santangelo, S.L.; Seddon, J.M. The Genetics of Age-Related Macular Degeneration: A Review of Progress to Date. Surv. Ophthalmol. 2006, 51, 316–363. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Weber, O. In Memoriam of Rudolf Virchow: A Historical Retrospective Including Aspects of Inflammation, Infection and Neoplasia. In Infection and inflammation: impacts on oncogenesis; Karger Publishers, 2006; Vol. 13, pp. 1–15.
- James, S.; Pogribna, M.; Miller, B.J.; Bolon, B.; Muskhelishvili, L. Characterization of cellular response to silicone implants in rats: implications for foreign-body carcinogenesis. Biomaterials 1997, 18, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yang, W.; Wu, C.; Liu, B.; Lu, H.; Wang, H.; Yan, H. Assessment of the role of intracranial hypertension and stress on hippocampal cell apoptosis and hypothalamic-pituitary dysfunction after TBI. Sci. Rep. 2017, 7, 3805. [Google Scholar] [CrossRef]
- Nogueira, M.L.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Lafitte, O.; Steyaert, J.-M.; Dubois, B.; Schwartz, L. Mechanical Stress Increases Brain Amyloid β, Tau, and α-Synuclein Concentrations in Wild-Type Mice. Alzheimer’s & Dementia 2018, 14, 444–453. [Google Scholar]
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, Ageing and Chronic Disease. Current opinion in immunology 2014, 29, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Mengoli, L.P.; De Martinis, M. Osteoporosis, Inflammation and Ageing. Handbook on Immunosenescence: Basic Understanding and Clinical Applications 2009, 1329–1352.
- Sparrow, J.R. Bisretinoids of RPE Lipofuscin: Trigger for Complement Activation in Age-Related Macular Degeneration. Inflammation and Retinal Disease: Complement Biology and Pathology 2010, 63–74.
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Barron, E.; Hageman, G.S.; Johnson, L.V. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Cotran, R.S.; Majno, G. The Delayed and Prolonged Vascular Leakage in Inflammation: I. Topography of the Leaking Vessels after Thermal Injury. The American Journal of Pathology 1964, 45, 261. [Google Scholar] [CrossRef] [PubMed]
- Roviezzo, F.; Tsigkos, S.; Kotanidou, A.; Bucci, M.; Brancaleone, V.; Cirino, G.; Papapetropoulos, A. Angiopoietin-2 Causes Inflammation in Vivo by Promoting Vascular Leakage. J. Pharmacol. Exp. Ther. 2005, 314, 738–744. [Google Scholar] [CrossRef]
- Schwartz, L.; Israël, M.; Philippe, I. Inflammation and Carcinogenesis: A Change in the Metabolic Process. Cancer Microenvironment and Therapeutic Implications: Tumor Pathophysiology Mechanisms and Therapeutic Strategies 2009, 3–18.
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; del Castillo, J.M.B.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear Osmolarity in the Diagnosis and Management of Dry Eye Disease. Arch. Ophthalmol. 2011, 151, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Gilbard, J.P.; Farris, R.L. OCULAR SURFACE DRYING AND TEAR FILM OSMOLARITY IN THYROID EYE DISEASE. Acta Ophthalmol. 1983, 61, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.; Wellik, S.R.; Galor, A. Glaucoma Therapy and Ocular Surface Disease: Current Literature and Recommendations. Current opinion in ophthalmology 2013, 24, 136–143. [Google Scholar] [CrossRef]
- Jacob, T.; Duncan, G. Osmotic influences on lens membrane characteristics. Exp. Eye Res. 1980, 31, 505–512. [Google Scholar] [CrossRef]
- Joyal, J.-S.; Gantner, M.L.; Smith, L.E. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2017, 64, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Alm, A.; Bill, A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp. Eye Res. 1973, 15, 15–29. [Google Scholar] [CrossRef]
- Moore, D.J.; Clover, G.M. The effect of age on the macromolecular permeability of human Bruch's membrane. . 2001, 42, 2970–5. [Google Scholar] [PubMed]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef] [PubMed]
- Boscher, C.; Erol, O.; Luscan, R. Remodeling of the choroidal vasculature and the role of choriocapillaris perfusion drop in pachychoroid diseases: a global rheological approach. Graefe's Arch. Clin. Exp. Ophthalmol. 2023, 261, 3045–3046. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Yazdi, H.; Vincent, S.J.; Collins, M.J.; Read, S.A.; Alonso-Caneiro, D. Wide-field choroidal thickness in myopes and emmetropes. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Arch. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, S.; Yu, A.S.L. Regulation of Epithelial Cell Functions by the Osmolality and Hydrostatic Pressure Gradients: A Possible Role of the Tight Junction as a Sensor. Int. J. Mol. Sci. 2019, 20, 3513. [Google Scholar] [CrossRef] [PubMed]
- Shirao, Y.; Steinberg, R.H. Mechanisms of effects of small hyperosmotic gradients on the chick RPE. . 1987, 28, 2015–25. [Google Scholar]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. . 1996, 37, 1929–34. [Google Scholar] [CrossRef]
- Hollborn, M.; Vogler, S.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: Involvement of NFAT5. 2015, 21, 360–377.
- Veltmann, M.; Hollborn, M.; Reichenbach, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Osmotic Induction of Angiogenic Growth Factor Expression in Human Retinal Pigment Epithelial Cells. PLOS ONE 2016, 11, e0147312. [Google Scholar] [CrossRef]
- Marmor, M. Retinal Detachment from Hyperosmotic Intravitreal Injection. Investigative Ophthalmology & Visual Science 1979, 18, 1237–1244.
- Turner, J.L.; Bierman, E.L. Effects of glucose and sorbitol on proliferation of cultured human skin fibroblasts and arterial smooth-muscle cells. Diabetes 1978, 27, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Tytarenko, R.G.; Liu, J.; Bora, N.S.; Bora, P.S. Polyethylene Glycol (PEG)-induced Mouse Model of Choroidal Neovascularization. J. Biol. Chem. 2011, 286, 16229–16237. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Logan, C.; Lyzogubov, V.V.; Bora, N.S.; Bora, P.S. Wet and Dry Age-Related Macular Degeneration Induced by Polyethylene Glycol. 2023.
- Christen, R.; Schackmann, R.W.; Shapiro, B.M. Metabolism of sea urchin sperm. Interrelationships between intracellular pH, ATPase activity, and mitochondrial respiration. J. Biol. Chem. 1983, 258, 5392–5399. [Google Scholar] [CrossRef] [PubMed]
- Sinning, A.; Hübner, C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Zebhauser, P.T.; Berthele, A.; Goldhardt, O.; Diehl-Schmid, J.; Priller, J.; Ortner, M.; Grimmer, T. Cerebrospinal Fluid Lactate Levels along the Alzheimer’s Disease Continuum and Associations with Blood-Brain Barrier Integrity, Age, Cognition, and Biomarkers. Alzheimer’s Research & Therapy 2022, 14, 1–8. [Google Scholar]
- Demetrius, L.A.; Simon, D.K. An inverse-Warburg effect and the origin of Alzheimer’s disease. Biogerontology 2012, 13, 583–594. [Google Scholar] [CrossRef]
- Poitry-Yamate, C.; Poitry, S.; Tsacopoulos, M. Lactate released by Muller glial cells is metabolized by photoreceptors from mammalian retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [CrossRef]
- Ng, S.K.; Wood, J.P.; Chidlow, G.; Han, G.; Kittipassorn, T.; Peet, D.J.; Casson, R.J. Cancer-like metabolism of the mammalian retina. Clin. Exp. Ophthalmol. 2015, 43, 367–376. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A. Role of Muller cells in retinal degenerations. Front. Biosci. 2001, 6, e77–92. [Google Scholar] [CrossRef]
- Yokosako, K.; Mimura, T.; Funatsu, H.; Noma, H.; Goto, M.; Kamei, Y.; Kondo, A.; Matsubara, M. Glycolysis in Patients with Age-Related Macular Degeneration. Open Ophthalmol. J. 2014, 8, 39–47. [Google Scholar] [CrossRef]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth,Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Song, J.; Lee, K.; Park, S.W.; Chung, H.; Jung, D.; Na, Y.R.; Quan, H.; Cho, C.S.; Che, J.-H.; Kim, J.H.; et al. Lactic Acid Upregulates VEGF Expression in Macrophages and Facilitates Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Reber, F.; Kasper, M.; Siegner, A.; Kniep, E.; Seigel, G.; Funk, R.H. Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefe's Arch. Clin. Exp. Ophthalmol. 2002, 240, 1022–1032. [Google Scholar] [CrossRef]
- Kumar, A.; Haery, C.; Paladugu, B.; Kumar, A.; Symeoneides, S.; Taiberg, L.; Osman, J.; Trenholme, G.; Opal, S.M.; Goldfarb, R.; et al. The Duration of Hypotension before the Initiation of Antibiotic Treatment Is a Critical Determinant of Survival in a Murine Model ofEscherichia coliSeptic Shock: Association with Serum Lactate and Inflammatory Cytokine Levels. J. Infect. Dis. 2006, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, J.; McArthur, D.A.; Miller, C.; Glenn, T.C.; Villablanca, P.; Martin, N.A.; Hovda, D.A.; Alger, J.R.; Vespa, P.M. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury*. Crit. Care Med. 2008, 36, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Ikizawa, T.; Ikeda, K.; Arita, M.; Kitajima, S.; Soga, T.; Ichijo, H.; Naguro, I. Mitochondria directly sense osmotic stress to trigger rapid metabolic remodeling via regulation of pyruvate dehydrogenase phosphorylation. J. Biol. Chem. 2023, 299, 102837. [Google Scholar] [CrossRef]
- Hamraz, M.; Abolhassani, R.; Andriamihaja, M.; Ransy, C.; Lenoir, V.; Schwartz, L.; Bouillaud, F. Hypertonic external medium represses cellular respiration and promotes Warburg/Crabtree effect. FASEB J. 2019, 34, 222–236. [Google Scholar] [CrossRef]
- Samra, Y.A.; Zaidi, Y.; Rajpurohit, P.; Raghavan, R.; Cai, L.; Kaddour-Djebbar, I.; Tawfik, A. Warburg Effect as a Novel Mechanism for Homocysteine-Induced Features of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 1071. [Google Scholar] [CrossRef]
- Tao, Y.; Jiang, P.; Wei, Y.; Wang, P.; Sun, X.; Wang, H. α-Lipoic Acid Treatment Improves Vision-Related Quality of Life in Patients with Dry Age-Related Macular Degeneration. Tohoku J. Exp. Med. 2016, 240, 209–214. [Google Scholar] [CrossRef]
- Yang, S.-H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J.W.; Liu, R. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Prog. Neurobiol. 2017, 157, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Montégut, L.; Martínez-Basilio, P.C.; Moreira, J.d.V.; Schwartz, L.; Jolicoeur, M. Combining lipoic acid to methylene blue reduces the Warburg effect in CHO cells: From TCA cycle activation to enhancing monoclonal antibody production. PLOS ONE 2020, 15, e0231770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. ; C. Rojas, J.; Gonzalez-Lima, F. Methylene Blue Prevents Neurodegeneration Caused by Rotenone in the Retina. Neurotoxicity research 2006, 9, 47–57. [Google Scholar] [PubMed]
| Characteristic | Cancer | Alzheimer disease | Macular degeneration |
|---|---|---|---|
| Extracellular osmolarity | Increases | Increases | Increases |
| Oxidative phosphorylation | Decreases in cancer cells | Decreases in neurons | Decreases in photoreceptors |
| Extracellular lactate concentration | Increases | Increases | Increases |
| Intracellular pH | Increases in cancer cells | Decreases in neurons | Decreases in photoreceptors |
| Cell ecosystem | Cancer cells + Tumor Associated Macrophages + stroma | Neurons + glial cells | Photoreceptors + Müller cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




