1. Introduction
The COVID-19 pandemic undoubtedly had great impact on the lives and health of many individuals worldwide, some of which we are only beginning to discover and understand. This was particularly true as it pertained to pregnant mothers diagnosed with the virus and the effects that this may have had on their fetuses. Evidence in professional journals worldwide indicated that COVID-19 infection during pregnancy was associated with adverse pregnancy outcomes, especially among pregnant persons with infection acquired at early gestational ages, with a need for oxygen therapy, and with more symptomatic presentation [
1]. Both higher and lower rates of preterm birth (earlier than 37 week gestation) have been reported across the literature, in addition to higher rates of preeclampsia, cesarean deliveries, and stillbirth [
2,
3,
4,
5,
6]. Studies also suggested that maternal COVID-19 infection was associated with neonatal morbidities such as hyperbilirubinemia and respiratory distress syndrome and other neonatal respiratory disorders [
5].
Our hospital, an Appalachian tertiary care center in Northeast West Virginia, did not admit it’s first pregnant mother with known COVID-19 infection until mid-September 2020, several months after some other areas of the country and well after stay-at-home orders were issued in March 2020. During the height of the pandemic period (2020-2022), 8.2% of the mothers who delivered at our institution had been infected by the COVID-19 virus. For the infants the most striking finding was an increase in large for gestation (LGA) infants to 13% and decrease in small for gestation (SGA) infants to 3% [
7].
As we moved past the pandemic period, we questioned whether there have been any lingering effects from the pandemic in terms of newborn and maternal health.
2. Materials and Methods
After IRB approval, we conducted a chart review of infants and their mothers from 3 epochs. Mother-infant pairs delivered prior to the pandemic between May-July 2018 and April-June 2019 (n=300 infants from 284 mothers) were reviewed. We examined the maternal-infant pairs with births during the height of the COVID-19 pandemic between November 14, 2020, and April 30, 2022. Mother-infant pairs from COVID-19 pandemic period were divided into two groups: infants born to mothers with COVID-19 infection during pregnancy (n=305 infants from 298 mothers), and infants born to mothers without infection (n = 300 infants from 288 mothers). Finally, we reviewed mother-infant pairs from 2023, post-pandemic period (n = 300 infants from 289 mothers).
We used Cogito SlicerDicer, which is a self-service reporting tool with the Epic-electronic medical record system (Epic Systems, Verona, WI). Using this tool, we were able to identify the total number of babies born and import the infants to appropriate data spreadsheets. We had access to key criteria such as ICD-10 codes to filter maternal and infant co-morbidities and characteristics.
Infant delivery history, postnatal treatment during the infant hospitalization, and short-term health outcomes were extracted. For all infants, the Case Mix index (CMI) derived from a modification of Medical Severity Diagnosis Related Group (MS-DRG) weights which we refer as a Research-DRG (Table 1).

MS-DRG weights, rounded to a single decimal place, that was determined by the United States’ Center for Medicare Services in 2023 [
8] were used for all epochs. Differing from MS-DRG weights & hospital CMI assignment the weight of Research-DRG 789, a weight of 1.8 was added to the assigned Research-DRG weight for any babies that died. The Research-DRG weight for every infant reviewed was assigned by a single investigator (MJP) using the criteria developed by Joya et.al. [
9]
For all mothers several maternal comorbidities were extracted including age, survival, presence of all hypertension (gestational and chronic hypertension, preeclampsia and HELLP syndrome), diabetes (gestational, type 1, and type 2), body mass index (BMI), tobacco, drug use, and hepatitis C infection. For the second and third epochs we also extracted the presence of COVID-19 infection, whether symptoms were noted as well as any COVID-19 related treatment provided. For Mothers delivering after April 15, 2022, (date that vaccine made available for low risk adults) we documented COVID-19 vaccination status.
A total of 1205 infants born to 1159 mothers at our institution during the three epochs. (four groups) This number of infants provided more than enough subjects to reach 80% power to detect a medium effect size among groups based on group at the p = 0.05 significance level. Descriptive analyses were conducted to characterize the four cohorts detailed in Table 2 and Table 3, and to explore the normative distribution of all information collected. Differences in infant and maternal outcomes and comorbidities were examined using one way ANOVA for multiple comparisons. Statistical significance was established at the p<0.05 level. SPSS version 28.0 was used to complete the analyses.
3. Results
Infant, and maternal findings for all epochs are detailed in Table 3.


Prior to the pandemic our sample of 300 infants showed characteristic typical of babies born at a tertiary academic delivery service. The C-section rate was 37% which was higher than the West Virginia state average of 34% [
10]. Though we did not delineate primary versus repeat C-sections. 29% of babies born required NICU admission which is higher that national average of 9-13% [
11], though likely similar to other tertiary NICUs associated with a high-risk delivery service. The percentage of babies born LGA and SGA was similar to regional averages at 7 to 8% [
7]. Congenital anomalies ranged from congenital small head, hypospadias, low grade hydronephrosis to complex congenital heart disease. Remaining finding are consistent with findings from tertiary delivery service.
Reviewing all epochs, NICU admission rate, hypoglycemia, and RDS [ICD-10 = P22.0] was consistent across all epochs. Maternal opiate use with resulting neonatal opiate withdrawal syndrome (NOWS) is a chronic problem in Appalachia, though our rate of 2 to 3% is lower than that in other parts of our state of West Virginia which has been noted to be 5% [
12]. Foster care of 3-5% is much higher than that noted in the US [
13].
During the pandemic period, whether or not mothers were infected, the infants seemed little affected in terms of acute medical comorbidities. Significant changes were most apparent in infants born to mother who were free from COVID-19 infections during pregnancy. We noted a significant increase in instrumented vaginal deliveries. Of more importance, the percentage of LGA infants significantly increased (6% to 13%), and the percentage of SGA infants significantly decreased (7% to 4%). Curiously, we saw fewer preterm infants born to both groups of pandemic period mothers. This is similar to the reduction in preterm births noted globally [
14]. The percentage of infants with any congenital anomalies was significantly lower in babies born to non-infected mothers during the pandemic, which we cannot easily explain.
During 2023, representing the post pandemic period, preterm births have increased modestly. Instrumented vaginal deliveries have returned to pre-pandemic rates (1%). The most important changes are a continued increase in the percentages of both SGA (13%) and LGA infants (10%). The percentage of LGA births remained higher than pre-pandemic at 10%. Strikingly the percentage of SGA infants increased to 13% from 4% during the pandemic.
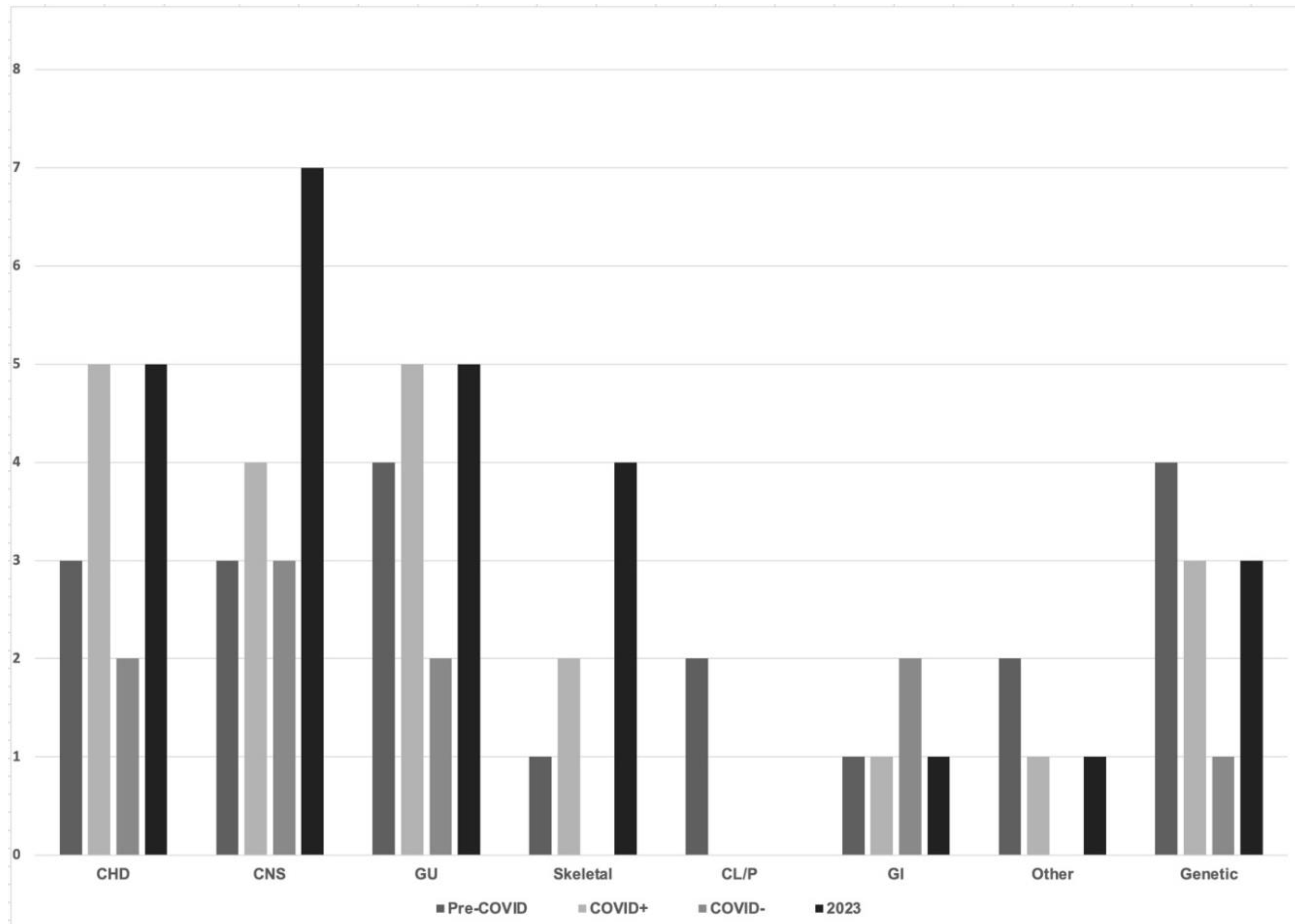
Congenital anomalies ranged from sacral dimples to lethal, complex congenital heart disease
The percentage of infants with any congenital anomalies was between 3 and 8% across all epochs and groups. We separately noted incidence of congenital small head (head circumference less than 3rd percentile for gestational age). Across all epochs this remained stable at 1%. The percentage of infants with any congenital anomalies was significantly higher in babies born to infected mothers during the pandemic. Congenital heart disease, anomalies of the central nervous system and genitourinary anomalies were the most frequently observed differences in infants across all epochs. The incidence of all congenital anomalies is not significantly different post-pandemic from those observed in the pre-pandemic period.
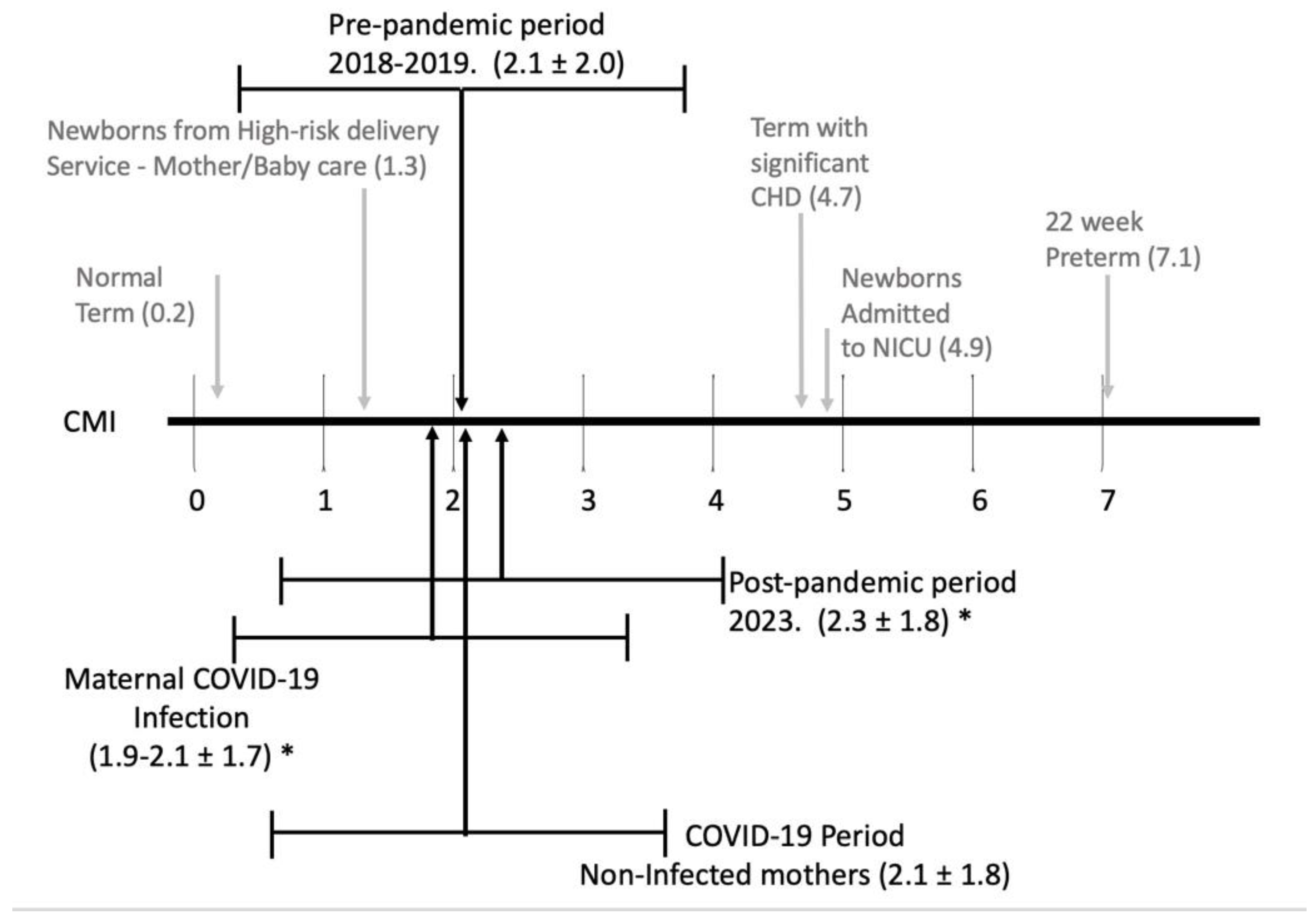
The Case Mix Index (CMI) for each epoch compared to the spectrum of CMI for infants is detailed in
Figure 2 with a breakdown of the research-DRGs and weights noted in Table 4.

The CMI in the post pandemic period was significantly higher that CMI from the pandemic period. This is likely related to significantly more late preterm, and term infants needing respiratory support (NCPAP, and/or ventilator support), and more term infants with at least one significant problem (infants affected by maternal conditions, medications, or congenital anomalies). Along with this, we noted a significant decrease in normal term infants and a trend to fewer preterm infants without major problems.
The Appalachian region of the United States, and especially the state of West Virginia have consistently ranked low for key health indicators for adults, for substance use, and drug overdose deaths. This is certainly evident in the maternal data from all epochs. The average BMI ranged from 33.3 to 34.8 kg/m
2, with 61 to 70% of these women delivering with BMI greater than 30 kg/m
2. During the pre-pandemic period, the percentage of diabetes, and maternal hypertension were higher to that noted in the country as a whole [
16,
17]. Across all epochs, tobacco use was twice that of the rest of the country [
18]. Illicit substance use (opiates, cannabis, cocaine, and methamphetamine) was similar to that noted throughout the USA [
19].
During the COVID-19 pandemic period, 288 women who had been diagnosed with COVID-19 during their pregnancies delivered babies at our institution, representing 8.2% of deliveries (305/3701). Overall, 59% of the women had symptoms ranging from mild cold-like nasal congestion to cardiorespiratory failure with 5 women needing ECMO support and two of the women dying. Their diagnosis was made 57 ± 46 days prior to delivery and 39% of their infants required NICU care. For mothers who were asymptomatic, their diagnosis was made 30 ± 57 days prior to delivery, with 8% of these asymptomatic women diagnosed upon admission to the labor and delivery service. Only 16% of the infants born to these mothers required NICU care. During this pandemic period, for mothers infected with the COVID-19 virus, the percentage presenting with hypertension showed a trend toward an increase while all types of diabetes increased significantly
During 2023, (post-pandemic period) the most striking changes have been a significant increase in hypertension (including preeclampsia and HELLP syndrome) and a trend toward more mothers with diabetes. Also, perinatal COVID-19 infections continued to occur at a rate of approximately 4% of the women though the degree of illness is subjectively less than during the pandemic period. Maternal utilization of COVID-19 vaccines was 43%, which was similar to pregnant women throughout the country though lower than the population as a whole which is close to 78% [
20].
4. Discussion
Pandemic babies are different! This phenomenon was noted by parents during the pandemic period and shared through the TikTok social media application with multiple videos. One of the TikTok user loudly exclaimed, “THESE BABIES ARE WARRIORS, I’M TALKIN’ SPARTACUS!” [
21]. Our data shows that infants are generally resilient, with increase in LGA infants and decrease in SGA infants being the major finding along with a trend toward more congenital anomalies. Being large or small for gestational age may have long-term consequences. Barker hypothesized that SGA infants have a high incidence of coronary heart disease, diabetes mellitus, hyperinsulinemia, and hypercholesterolemia as an adult [
22]. LGA infants are at an increased risk of becoming overweight and obese later in life compared to their normal-weight counterparts. Infants born greater than 4,000 g have a 50% increased risk of becoming overweight later in life. LGA infants born greater the 4500g have a 19% increase in risk of developing type 2 diabetes mellitus as an adult compared to those with birth weights between 4,000 and 4,500 grams [
22].
NICU admission rate ranged from 23% to 30% which is consistent with a high risk delivery service, as are the percentages of RDS [ICD-10: P22.0] between 7 and 11% as well as the overall percentage of infants born prior to 37 weeks at 19-28% compared to national average of 12%. The rates of neonatal hypoglycemia from our institution ranged from 16 to 21% which is higher than that noted for US infants, reported as 5 to 15% [
23]. NOWS ranged for 2 to 5% with national average of 1.2% of Medicaid eligible infants. [
19]. The need for foster care 3-5% is the highest in the country, compared to 1.3% nationally [
13,
24].
Early in the pandemic there was concern regarding the possibility of teratogenic effects of the mRNA vaccine on the developing fetus during critical windows of organogenesis. Several authors have explored this possibility found no composite increase in anomalies in infants born to mothers who received the vaccine during the first trimester versus those who did not [
25,
26,
27]. Additional reports about vaccine safety during the teratogenic window of gestation only considered congenital anomalies detected via ultrasonography rather than neonatal outcomes [
28] allowing the possibility that more subtle anomalies not identified on ultrasound could have been missed. In our cohort during the period considered to be the COVID-19 pandemic (from September 2020 to April 2022), significantly fewer infants were born with congenital anomalies to non-COVID-19 infected mothers. Vaccination status was not considered in this cohort, but rather true COVID-19 infection. The etiology for this phenomenon of increased congenital anomalies in COVID-19-infected mothers is unclear. Considerations as to the etiology of anomalies in infants born to COVID-19 infected mothers in our cohort include maternal hyperthermia which has been associated with an increase in congenital anomalies of the central nervous system such as neural tube defects, gastroschisis and cardiac anomalies [
29,
30,
31,
32]. A similar phenomenon was reported from a cohort in Iran, where CNS and genitourinary anomalies were significantly increased during the COVID-19 pandemic [
33] and this group also speculates maternal hyperthermia to be potentially causative in addition to vertical transmission of Covid-19 infection, stress and anxiety, insufficient preconception and prenatal care, neglect of fetal screening, and poverty imposed by this pandemic. These additional factors are certainly relevant to our rural, resource-limited Appalachian population. A limitation for our cohort is that timing of COVID-19 infection is not known in the mothers, thus it is not known whether hyperthermia was likely to occur during the period of the first trimester. A true TORCH-like, or intrinsic teratogenic effect of COVID-19 seems less likely given the paucity of reports on increases in congenital anomalies during the COVID-19 pandemic. The effect of the hypercoagulable state known to result from maternal COVID-19 infection was considered by Repucci et al. as a possible cause for congenital anomalies secondary to in utero vascular accidents and found not to be associated with GI or limb anomalies in their relatively small single center American cohort [
34]. Additionally, increases in microcephaly noted in the late pandemic period in a Canadian cohort was concluded to be more likely secondary to an artifact of enhanced surveillance for anomalies [
35], and no conclusion could be made as to increases in GI anomalies due to overall low incidence in a Romanian cohort [
36].
We have previously reported that the percentage of infants with microcephaly defined as head circumference less than 3% for gestational age is higher than national average; 1.02% versus 0.5% reported for the nation [
37]. In review of the congenital anomalies documented, the rate of microcephaly remained at 1% throughout all epics. We postulated that the combination of tobacco and illicit substance use was a factor in this higher rate of microcephaly and believe this is still a major factor.
The health concerns for women of childbearing age (14-44 years) in Appalachia has been well documented. The rates of obesity, tobacco, drug use, diabetes and hypertension are all above national averages [
38]. Our sample population prior to the pandemic reflects these concerns. In spite of this it seems that the infants born in our academic referral hospital fit within national averages.
The American College of Obstetricians and Gynecologists (ACOG) considers a BMI > 30 kg/m
2 as obese, with 40% of pregnant women in the US are considered obese [
39]. From all epochs 61-70% of the mothers had BMI greater than 30 kg/m
2 with >80% greater than 30 kg/m2 for mothers of LGA babies. C-section rate of 37-39% is higher than national average of 20-32% though we did not differentiate between primary and repeat C-section [
40]. All types of diabetes at 7-13% is higher than national average of 1-2%. There is increasing evidence that
suggests that intrauterine hyperglycemia may disrupt the neurocircuitry of the fetus, which may predispose the children to the development of neurocognitive and behavioral problems. This likely occurs through the induction of an exaggerated peripheral inflammatory response and neuroinflammation in the fetal brain [
41]
.
Between 21-38% % of pregnant women develop hypertension, with significant increase noted post-pandemic. These rates are higher that national averages of 8-16%.
Women with chronic hypertension have a higher incidence of superimposed preeclampsia, C-section, preterm delivery before 37 weeks' gestation, birth weight less than 2500 g, neonatal unit admission, and perinatal death. They also have a higher risk of developing cardiovascular disease later in life [
42]
. The significant increase in babies born small for gestational age likely mirrors the significant increase in mothers with hypertension during pregnancy. We have found that the rate of maternal hypertension in the post-pandemic epoch has continued to show significant increases. From 21% in the pre-pandemic epoch to 32% during the pandemic, 38% of pregnant women are diagnosed with hypertension in the post-pandemic period. Some studies now suggest that COVID-19 may have lasting impact on the cardiovascular system for some, with an increase seen in the development in either new onset hypertension or a worsening of existing hypertensive conditions [
43,
44,
45]. It remains unclear and a topic for further study whether or not these complications will resolve over time or will have a lasting effect. It is also unclear as to why effects occur, whether it is a direct effect of the virus itself or whether the indirect effects of the pandemic including social isolation, poor diet and physical activity, weight gain, psychosocial stress, and others also play a role. We are unable to delineate with certainty which of the pregnant mothers in our post-pandemic study population ever had a prior COVID infection which may be contributing to the increase in hypertension but may be a topic for further investigation. It has been well-documented that hypertension over time is associated with and is an important risk factor in development of various cardiovascular diseases and chronic kidney disease [
46]. Maternal hypertension and hypertensive disorders are also highly detrimental to the fetus, as it is a leading cause of prematurity, abnormal birth weight (either high or low for gestational age) and mortality and is being shown to put these individuals at risk for cardiovascular disease, hypertension and metabolic disease later in life [
47,
48]. This alarming trend that impacts both the mother and fetus is one that deserves further attention going forward.
Tobacco use by pregnant women in the state of West Virginia the highest in the country at approximately 23%, though the rate from our institution is lower ranging from 13-16% overall all epochs, as low as 9% (COVID-19 symptomatic mothers) and as high as 43% (COVID-19 pandemic period delivering SGA infants) (7) The prevalence of maternal smoking at any time during pregnancy in the US is 7.2% [
37]. Throughout all epochs illicit substance use of opiate, cannabis, cocaine, and methamphetamine was similar and in line with national averages [
49].
Pandemic era COVID-19 infections certainly affected the mothers with 59% having symptoms, 6 women needing significant respiratory support, 5 of these women progressing to ECMO and two dying. In the post pandemic period, perinatal COVID-19 infections were noted in about 4% of mothers giving birth. We still found that 50% showed symptoms, though none have required hospitalizations. Maternal COVID-19 vaccination rates were 43%, which is similar to U.S. vaccination rates for pregnant women in the US though this rate lags behind vaccination rate for the US population as a whole [
50].
5. Conclusions
In the pre-pandemic period, our maternal/infant data mirrors that from the entire Appalachian region. In general, showing maternal health concerns in several areas. For infants the impact of maternal substance use with NOWS and need for foster placement is higher than national averages [
51]. Although our ability to accurately track maternal COVID-19 infections came late to our region, it is clear that COVID-19 infections in women of childbearing age has been impactful. It is interesting to note that infants whose mothers were symptomatic had more than twice the rate of NICU admission as compared to those whose mothers were asymptomatic (39% vs only 18%). Factors contributing to this discrepancy is an area that may be of interest for future investigation. Fortunately, the infants have, in the short term, seemed to weather their mother’s illnesses well and have had an extremely high survival rate thus far. We are continuing to monitor our infants exposed prenatally to the COVID-19 virus as this infection continues to affect over 4% of mothers. It will be valuable to continue to follow these infants for any long-term health and developmental concerns.
Well after the pandemic period, maternal-infant health continues to be affected. For our mothers, the Increase in hypertension and diabetes during pregnancy is concerning. For the infants, being large or small for gestational age may have long-term consequences. We remain concerned about the high percentage of infants with microcephaly across all epochs. It is possible that the post-pandemic health changes in our mothers and infants may be unique to our Appalachian region though should be an alert for health care workers dealing with individuals or groups that share similar characteristics in socio-economic status, lifestyle, and limited access to basic health care needs. Moving forward, COVID-19 continues to mutate and infect our mothers. In the short term, babies seem unfazed, but long term follow up has been showing changes in gut microbiome [
52] and concerns about some developmental delays, mainly speech and possibly gross motor [
53].
Author Contributions
Kelsey Haarbauer, MD is an assistant professor of Pediatrics at West Virginia University School of Medicine and an attending neonatologist in the NICU at WVUMedicine Children’s Hospital She completed her pediatric residency & neonatal-perinatal fellowship at WVUMedicine Children’s Hospital. Her research interest is care of infants with BPD. Her contributions include Conceptualization; Methodology; Investigation; Validation, Analysis; Data Curation; Writing – Original Draft Preparation; Writing – Review & Editing. Rebecca Burke, MD, PhD is an assistant Professor at Penn State University College of Medicine and attending neonatologist at the Milton J Hershey Medical Center. She is double boarded in Neonatal-perinatal medicine & Genetics. Her contributions include Conceptualization; Methodology; Investigation; Validation, Analysis; Data Curation; Writing – Review & Editing. M Cody Smith, MD is an assistant professor of Pediatrics at West Virginia University School of Medicine and an attending neonatologist in the NICU at WVUMedicine Children’s Hospital He went to medical school at WVU School of Medicine and completed his pediatric residency & neonatal-perinatal fellowship at WVUMedicine Children’s Hospital. His research interests are care of infants with BPD and management of infants with Neonatal opiate withdrawal syndrome. His contributions include Investigation; Validation, Analysis; Data Curation; Writing – Review & Editing. Audrey N Miller, MD is an assistant professor of Pediatrics at Ohio State University College of Medicine and an attending neonatologist in the NICU at Nationwide Children’s Hospital She completed her pediatric residency & neonatal-perinatal fellowship at WVUMedicine Children’s Hospital. Her research interest is care of infants with BPD. Her contributions include Investigation; Data Curation; Writing – Review & Editing. Patricia N Moran MD is a fellow in neonatal-perinatal medicine at The University of Virginia. She went to medical school at WVU School of Medicine and completed her pediatric residency at WVUMedicine Children’s Hospital. Her contributions include Investigation; Writing – Review & Editing. Alicia A Moise MD is associate professor of Pediatrics at University of Oklahoma and an attending neonatologist at Oklahoma Children’s Hospital. Currently She is medical director of the ECMO program at her hospital. Her contributions include Conceptualization; Methodology; Investigation; Data Curation; Validation, Analysis; Writing – Review & Editing. Lesley Cottrell PhD is a professor of Pediatrics at West Virginia University School of Medicine Joint Faculty Member, Social & Behavioral Health Department, School of Public Health Co-Director, Prevention Research Center & Director of the Pediatric Research Unit for the Department of Pediatrics. Her contributions include Validation, Formal Analysis; Data Curation; Writing – Review & Editing. Mark J Polak, MD is the senior author for this manuscript. is a professor of Pediatrics at West Virginia University School of Medicine and an attending neonatologist in the NICU at WVUMedicine Children’s Hospital He went to medical school at WVU School of Medicine and completed his pediatric residency at WVUMedicine Children’s Hospital and his neonatal-perinatal fellowship at the University of Florida. He has a long history of laboratory, & clinical research. His contributions include Conceptualization; Visualization; Methodology; Validation; Formal Analysis; Data Curation; Writing – Review & Editing; Supervision; Project Administration.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of West Virginia University School of Medicine (WVU IRB# 2011163947 approved 25-Nov-2020)
Informed Consent Statement
informed consent was not required.
Data Availability Statement
At this time, the patient data is unavailable due to privacy or ethical restrictions (HIPAA).
Conflicts of Interest
All authors declare no conflict of interest in this project.
References
- Dubey, P.; Reddy, S.Y.; Manuel, S.; Dwivedi, A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107–100107. [Google Scholar] [CrossRef] [PubMed]
- Novoa, R.H.; Quintana, W.; Llancarí, P.; Urbina-Quispe, K.; Guevara-Ríos, E.; Ventura, W. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med. Infect. Dis. 2020, 39, 101919–101919. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, K. R. Birth and Infant Outcomes Following Laboratory-Confirmed SARS-CoV-2 Infection in Pregnancy — SET-NET, 16 Jurisdictions, –October 14, 2020. MMWR. 2020 69: 1635–1640. 29 March.
- Norman M, Navér L, Söderling J, Ahlberg M, Hervius Askling H, Aronsson B, Byström E, Jonsson J, Sengpiel V, Ludvigsson JF, Håkansson S, Stephansson O. Association of Maternal SARS-CoV-2 Infection in Pregnancy With Neonatal Outcomes. JAMA. 2021 325:2076-2086.
- Calvert, C.; Brockway, M. (.; Zoega, H.; Miller, J.E.; Been, J.V.; Amegah, A.K.; Racine-Poon, A.; Oskoui, S.E.; Abok, I.I.; Aghaeepour, N.; et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat. Hum. Behav. 2023, 7, 529–544. [Google Scholar] [CrossRef]
- Moran, T.; Moise, A.; Miller, A.; Burke, R.; Cottrell, L.; Haarbauer, K.; Smith, M.C.; Polak, M. Changes in Large for Gestation and Small for Gestation Births During the COVID-19 Era. 119, 35. [CrossRef]
- Joya, R.M.; Cottrell, L.; Kiefer, A.; Polak, M.J. Diagnosis-Related Group Weight and Derived Case Mix Index to Assess the Complexity among Twins. Am. J. Perinatol. 2022, 39, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- https://www.cms. 2024.
- https://www.cdc.gov/nchs/pressroom/states/westvirginia/wv.
- Pineda, R.; Knudsen, K.; Breault, C.C.; Rogers, E.E.; Mack, W.J.; Fernandez-Fernandez, A. NICUs in the US: levels of acuity, number of beds, and relationships to population factors. J. Perinatol. 2023, 43, 796–805. [Google Scholar] [CrossRef]
- Umer, A.; Loudin, S.; Maxwell, S.; Lilly, C.; Stabler, M.E.; Cottrell, L.; Hamilton, C.; Breyel, J.; Mullins, C.; John, C. Capturing the statewide incidence of neonatal abstinence syndrome in real time: the West Virginia experience. Pediatr. Res. 2018, 85, 607–611. [Google Scholar] [CrossRef] [PubMed]
- https://www.aspe.hhs.gov/sites/default/files/2021-08/infant-foster-care-brief.
- Calvert, C.; Brockway, M. (.; Zoega, H.; Miller, J.E.; Been, J.V.; Amegah, A.K.; Racine-Poon, A.; Oskoui, S.E.; Abok, I.I.; Aghaeepour, N.; et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat. Hum. Behav. 2023, 7, 529–544. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Obstetrics. Obesity in Pregnancy Obstet Gynecol 2021. e: 127.
- https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy.
- Khedagi AM, Bello NA. Hypertensive Disorders of Pregnancy Card Clin 2021. 7: 39.
- https://www.cdc.gov/nchs/products/databriefs/db458.
- 8: A, Substance Use During Pregnancy F1000 Res 2016 5, 1000.
- Regan AK, Kaur R, Nosek M, et.al. 1: Prev Med Rep 2022 29, 2022.
- Loveandpositiveenergy. Build like warriors #bigpandemicbabies. TikTok. Published , 2021. www.tiktok.com. 13 July.
- Barker, D.J. Fetal programming of coronary heart disease. Trends Endocrinol. Metab. 2002, 13, 364–368. [Google Scholar] [CrossRef]
- Edwards T, Harding J Clinical Aspects of Neonatal Hypoglycemia: A Mini Review. 1: Front Pediatr 2021 8, 2021.
- Grouse G, Ghertner R, Madden E, Radel L. Foster Care Entry Rates Grew Faster for Infants than for Children of Other Ages, 2011–2018. 2021. https://www.aspe.hhs.gov/sites/default/files/2021-08/infant-foster-care-brief.
- Woestenberg, P.J.; de Feijter, M.; Bergman, J.E.H.; Lutke, L.R.; Passier, A.J.L.M.; Kant, A.C. Maternal first trimester COVID-19 vaccination and risk of major non-genetic congenital anomalies. Birth Defects Res. 2023, 115, 1746–1757. [Google Scholar] [CrossRef]
- Santos J, Miller M, Branda ME, Mehta RA and Theiler RN Maternal COVID-19 vaccination status and association with neonatal congenital anomalies. Front Pediatr 2024 12:1355502.
- Ding, C.; Liu, Y.; Pang, W.; Zhang, D.; Wang, K.; Chen, Y. Associations of COVID-19 vaccination during pregnancy with adverse neonatal and maternal outcomes: A systematic review and meta-analysis. Front. Public Heal. 2023, 11, 1044031. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, R.S.; Mormol, J.; Trawick, E.; Perry, M.F.; Allen, E.C.; Millan, D.; Miller, E.S. Association of COVID-19 Vaccination During Early Pregnancy With Risk of Congenital Fetal Anomalies. JAMA Pediatr. 2022, 176, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Luteijn, J.M.; Brown, M.J.; Dolk, H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum. Reprod. 2013, 29, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Botto LD, Panichello JD, Browne ML, Krikov S, Feldkamp ML, Lammer E, Shaw GM; National Birth Defects Prevention Study. Congenital heart defects after maternal fever. Am J Obstet and Gynecol. 2014 210:359.e1-359.e11.
- Graham, J.M., Jr. Update on the gestational effects of maternal hyperthermia. Birth Defects Res. 2020, 112, 943–952. [Google Scholar] [CrossRef]
- Suarez L, Felkner M, Hendricks K. The effect of fever, febrile illnesses, and heat exposures on the risk of neural tube defects in a Texas-Mexico border population. Birth Defects Res A Clin Mol Teratol 2004 70:815-9.
- Heidarzadeh M, Taheri M, Mazaheripour Z, Abbasi-Khameneh F. The incidence of congenital anomalies in infants before and during the Covid-19 pandemic. Ital J Pediatr. 2022 15;48:174.
- Reppucci, M.; Kaizer, A.; Prendergast, C.; Acker, S.; Mandell, E.; Euser, A.; Diaz-Miron, J. Incidence of congenital complications related to COVID-19 infection during pregnancy. J. Neonatal-Perinatal Med. 2023, 16, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Auger N, Arbour L, Lewin A, Brousseau É, Healy-Profitós J, Luu TM. Congenital anomalies during Covid-19: artifact of surveillance or a real TORCH? Eur J Epidemiol. 2024 Apr 9. Epub ahead of print.
- Brandibur TE, Kundnani NR, Boia M, Nistor D, Velimirovici DM, Mada L, Manea AM, Boia ER, Neagu MN, Popoiu CM. Does COVID-19 Infection during Pregnancy Increase the Appearance of Congenital Gastrointestinal Malformations in Neonates? Biomedicines. 2023 21;11:3105.
- Moore CA, Moise A, Cottrell L, Polak MJ. Incidence of Congenital Microcephaly is Increased With Fetal Exposure to Tobacco, Opioids, and Cannabis. 3: WVMJ 2023 118, 2023.
- Pederson, A. Maternal Mortality Rates in Appalachia 2022 The Pitt Journal https://pitjournal.unc. 2023. [Google Scholar]
- ACOG. Obesity in Pregnancy ACOG Practice Bulletin, Number 230 Obstet Gynecol 2021. e: 137.
- https://www.cdc.gov/nchs/data/vsrr/vsrr021.
- Rodolaki K, Pergialiotius V, Lakovidou N, et. al. The impact of maternal diabetes on the future health and neurodevelopment of the offspring: a review of the evidence. 1: Front Endocrin 2023 14, 2023.
- Agrawal, A, Wenger NK. Hypertension During Pregnancy. 6: Curr Hypertens Rep 2020 22, 2020.
- 43. Delalić, .; Jug, J.; Prkačin, I. Arterial hypertension following COVID-19: A retrospective study of patients in a Central European tertiary care center. Acta Clin. Croat. [CrossRef]
- Zhang, V.; Fisher, M.; Hou, W.; Zhang, L.; Duong, T.Q. Incidence of New-Onset Hypertension Post-COVID-19: Comparison With Influenza. Hypertension 2023, 80, 2135–2148. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiol 2022;73:682-687.
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W.; LaMarca, B. Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R521–R528. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Best, K.E.; Pearce, M.S.; Waugh, J.; Robson, S.C.; Bell, R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur. J. Epidemiology 2013, 28, 1–19. [Google Scholar] [CrossRef]
- https://www.cdc.
- Shephard, H.M.; Manning, S.E.; Nestoridi, E.; Darling, A.M.; Brown, C.M.; Hatch, M.; Ahnger-Pier, K.; Pagnano, S.; Mather, D.; Yazdy, M.M. Inequities in COVID-19 Vaccination Coverage Among Pregnant Persons, by Disaggregated Race and Ethnicity — Massachusetts, May 2021–October 2022. Mmwr. Morb. Mortal. Wkly. Rep. 2023, 72, 1052–1056. [Google Scholar] [CrossRef]
- https://www.cdc.gov/nchs/fastats/births.
- Querdasi FR, Vogel SC, Thomason ME, et.al. 1: A comparison of the infant gut microbiome before versus after the start of the covid-19 pandemic Sci Rep 2023 13, 2023.
- Giesbrecht, G.F.P.; Lebel, C.; Dennis, C.-L.; Silang, K.B.; Xie, E.B.M.; Tough, S.; McDonald, S.; Tomfohr-Madsen, L.P. Risk for Developmental Delay Among Infants Born During the COVID-19 Pandemic. J. Dev. Behav. Pediatr. 2023, 44, e412–e420. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).