1. Introduction
Liraglutide, a glucagon-like peptide 1 (GLP-1) analogue used to treat type 2 diabetes, increases the postprandial insulin level in a glucose-dependent manner and reduces glucagon secretion, leading to improved postprandial glucose metabolism. In addition, liraglutide delays gastric emptying and induces weight loss through reductions in appetite and energy intake [
1,
2,
3]. Moreover, findings from a recent clinical trial suggest the potential of liraglutide as a new treatment modality for bile acid (BA) diarrhea[
4]. The acute effects of liraglutide on blood glucose levels in a fasted condition and the mechanisms by which liraglutide reduces the frequency of stool in BA diarrhea, however, remain unclear.
Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine derived from tryptophan (Trp). In the periphery, circulating 5-HT is primarily produced in enterochromaffin (EC) cells of the gastrointestinal tract through microbial metabolites, including short-chain fatty acids and secondary BAs[
5]. 5-HT is required to maintain normal intestinal peristalsis, and the increased 5-HT in the colon is critically involved in the pathogenesis of inflammatory bowel diseases[
6], irritable bowel syndrome[
7], and post-cholecystectomy diarrhea[
8]. Moreover, 5-HT is involved in glucose metabolism and energy homeostasis[
5,
9]. We therefore hypothesized that liraglutide may alter tryptophan metabolites and decrease 5-HT in the colon, which may be involved in the improvement of BA diarrhea and glucose-reducing effects of liraglutide in the fasted state.
To determine the acute effects of liraglutide on blood glucose levels in the fasted condition and the mechanisms by which liraglutide improves BA diarrhea, we examined the effects of liraglutide on BAs in the liver and feces, tryptophan metabolites in the colon, blood glucose, plasma insulin, 5-HT, and fibroblast growth factor 21 (FGF21), and gene expression involved in BA transport in the ileum of mice deprived of food after intraperitonal administration of the drug.
2. Results
2.1. Effects of Liraglutide on Body Weight, Blood Glucose, Plasma Insulin, Serotonin, FGF21 Levels in Mice Deprived of Food after Intraperitonal Administration of the Drug
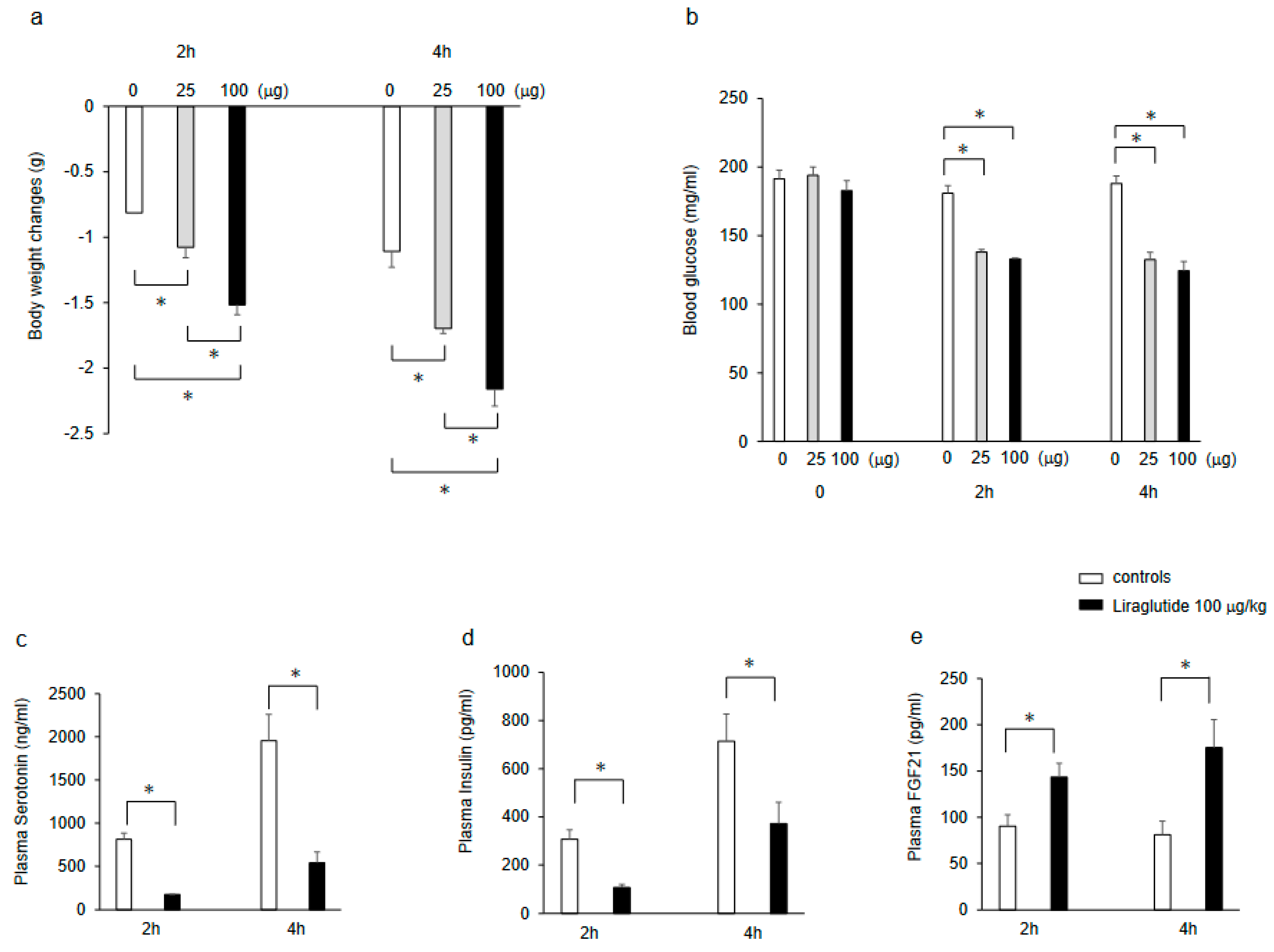
Intraperitoneal injection of liraglutide (25 and 100 μg/kg) significantly decreased body weight in a dose-dependent manner compared with saline controls in mice that were food-deprived for 2 h and 4 h after the injection (
Figure 1a). In addition, intraperitoneal injection of liraglutide (25 and 100 μg/kg) significantly decreased blood glucose levels, but there were no significant differences between the doses of 25 μg/kg and 100 μg/kg liraglutide (
Figure 1b). Moreover, intraperitoneal injection of liraglutide (100 μg/kg) significantly decreased plasma 5-HT (
Figure 1c) and insulin (
Figure 1d) levels, whereas it significantly increased plasma FGF21 levels (
Figure 1e) compared with the saline controls in mice that were food-deprived for 2 h and 4 h after the injection.
2.2. Effects of Liraglutide on Tryptophan Metabolites in the Colon of Mice
The administration of liraglutide at 100 μg/kg significantly decreased L-tryptophan (Trp), 5-HT, 5-hydroxy indole-3-acetic acid (5-HIAA), L- kynurenine (KYN), and xanthurenic acid (XA) in the colon of mice that were food-deprived for 2 h after the injection, whereas it significantly increased indole-3-propionic acid (IPA;
Table 1).
2.3. Effects of Liraglutide on Bile Acids in the Feces and Liver of Mice
The administration of 100 μg/kg liraglutide significantly decreased total bile acids (TBA) and the primary BAs including cholic acid (CA), chenodeoxycholic acid (CDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), glycocholic acid (GCA), and β-muricholic acid (β-MCA) in the colon of mice that were food-deprived for 2 h after the injection, whereas it did not decrease the secondary BAs, including deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), taurodeoxycholic acid (TDCA), and lithocholic acid (LCA) in the feces (
Table 2).
The administration of 100 μg/kg liraglutide significantly decreased TBA and the primary BAs including CA, TCA, and β-MCA in the liver of mice that were food-deprived for 2 h after the injection, whereas it did not decrease DCA, TDCA, and LCA in the liver (
Table 3).
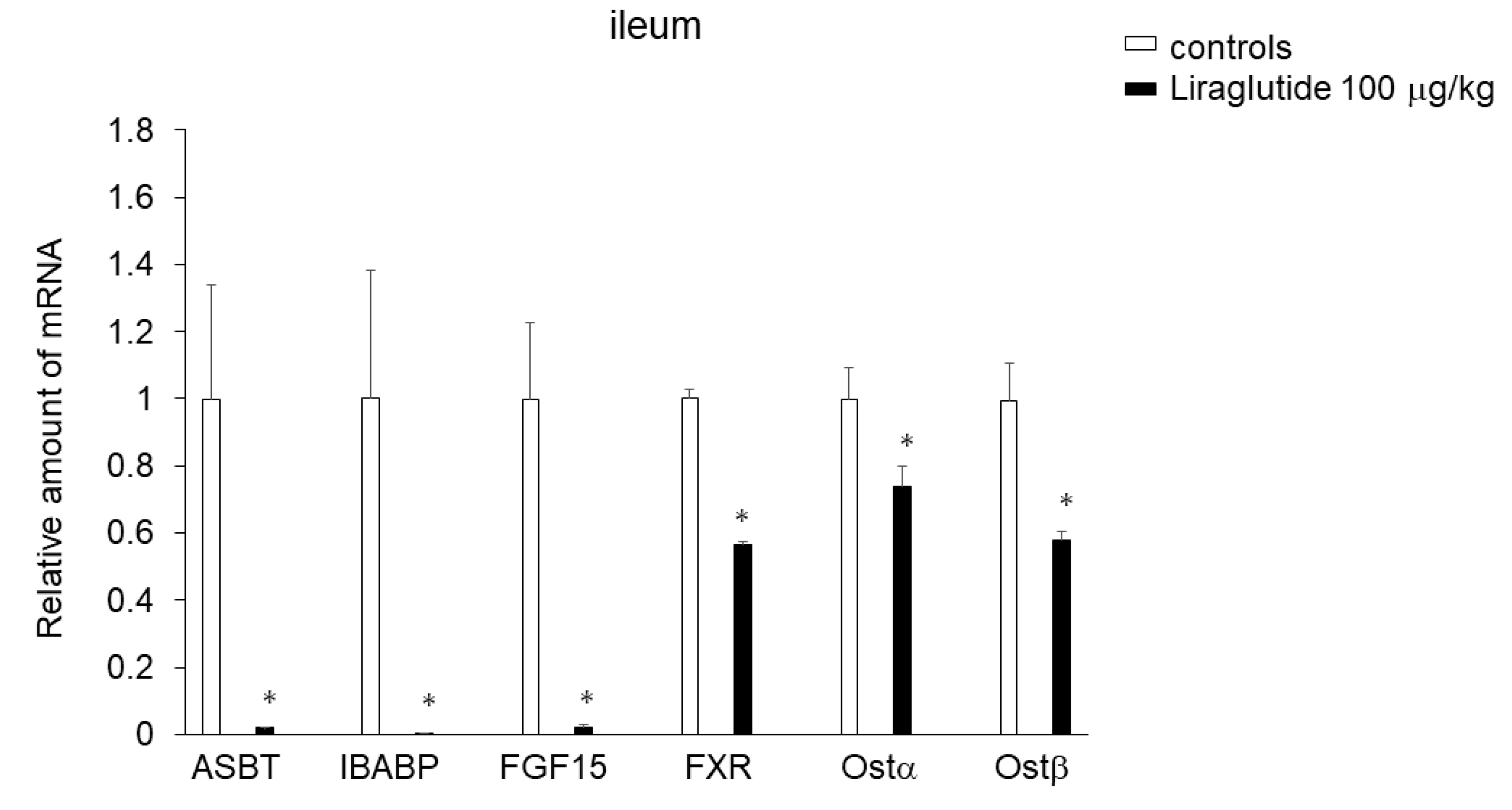
2.4. Effects of Liraglutide on Expression of Genes Involved in BA Transport in the Ileum
The administration of liraglutide (100 μg/kg) dramatically decreased the expression of apical sodium-dependent bile acid transporter (Asbt; Slc10a2), which mediates BA uptake across the apical brush border member in the ileum, ileal BA binding protein (IBABP), and FGF15 in the ileum compared with saline controls in mice that were food-deprived for 2 h after the injection. Moreover, liraglutide significantly decreased expression of the BA-activated nuclear receptor farnesoid X receptor (FXR) or the heteromeric organic solute transporter Ostα/β, which induces BA excretion, in the ileum (
Figure 2).
3. Discussion
The results of the present study revealed that liraglutide acutely decreased total BAs, especially the primary BAs, and 5-HT in the colon of mice deprived of food after the drug administration. The BA sequestrant colesevelam reduces stool frequency in BA diarrhea[
10]. A recent clinical trial comparing the effects of liraglutide and colesevelam on BA diarrhea demonstrated the superiority of liraglutide in reducing stool frequency[
4]. Our findings indicate that the liraglutide-induced decreases in the primary BAs and 5-HT in the colon may contribute to the mechanisms by which liraglutide reduces stool frequency in BA diarrhea.
GLP-1 released from enteroendocrine cells in the small intestine in response to nutrient metabolites reportedly stimulates 5-HT secretion via GLP-1 receptors in enterochromaffin cells of the small intestine and colon[
11]. Our results, however, demonstrated that liraglutide decreased Trp, 5-HT, and 5-HIAA in the colon, leading to decreases in plasma 5-HT levels in mice deprived of food after administration of the drug. The liraglutide-induced decreases in total BAs, especially the primary BAs, might contribute to the suppression of gut-derived 5-HT secretion.
Genetic ablation of ABST, which induces BA uptake in the enterocytes, decreases FGF15 expression in the ileum and induces the synthesis of primary BAs[
12,
13]. Our findings revealed that liraglutide acutely inhibited the expression of ASBT, IBABP, and FGF15 in the ileum. The decreased expression of ASBT, IBABP and FGF15 in the ileum may be a compensatory response to decreases in the primary BAs. These findings suggest that the liraglutide-induced suppression of primary BAs in the liver may be due to pathways other than the gut-derived FGF15-mediated hepatic BAs synthesis.
In the present study, despite decreases in the plasma insulin levels, liraglutide acutely decreased blood glucose levels in the food-deprived mice, suggesting that the acute decreases in blood glucose levels induced by liraglutide are independent of feeding and insulin secretion. Because BA sequestrants, including colesevelam, have beneficial effects on glycemic control in subjects with type 2 diabetes and diabetic rodents[
14], the liraglutide-induced decreases in the total BAs, especially the primary BAs, may contribute to the insulin-independent glucose-reducing effect of liraglutide in the fasted state.
The inhibition of intestinal ASBT induced by liraglutide may also contribute to the decrease in blood glucose levels and the increase in plasma FGF21 levels. ASBT is a potential regulator of glucose metabolism[
15,
16]. Genetic or pharmacologic inhibition of ASBT decreases blood glucose levels associated with decreased plasma insulin and increased FGF21 levels in mice[
13,
15,
16].
In addition, the decreases in 5-HT induced by liraglutide may contribute to the decrease in blood glucose levels in mice deprived of food after the drug administration. Genetic and/or pharmacologic inhibition of tryptophan hydroxylase 1 (TPH1), which induces the decreases in serum and colonic mucosal 5-HT, can improve glucose tolerance without affecting insulin sensitivity[
9]. Increased peripheral 5-HT synthesis induced by nutrients such as by overeating, a high-fat diet, and a high carbohydrate diet, or altered microbiota composition have been suggested as the pathophysiologic mechanisms of obesity-related diseases including metabolic syndrome, non-alcoholic fatty liver disease, type 2 diabetes, and cardiovascular disease[
5].
Moreover, the increase in IPA induced by liraglutide may contribute to the decrease in blood glucose levels in mice deprived of food after the drug administration. IPA is a microbial product from tryptophan absorbed from the gut into the bloodstream[
17]. Type 2 diabetes and obesity likely decrease IPA levels in the serum and/or colonic mucosa[
18,
19,
20], and IPA supplementation exerts beneficial effects on body weight and glucose metabolism disorders[
20]. The increase in IPA induced by liraglutide may therefore have beneficial effects on body weight and glucose metabolism.
These findings suggest that the liraglutide-induced decrease in the primary BAs and alterations of Trp metabolites in the colon may be involved in the improvement of BA diarrhea and/or the insulin-independent glucose-reducing effects of liraglutide in the fasted state.
4. Materials and Methods
4.1. General Procedures
Six-week-old male C57BL6N mice were individually housed in cages with free access to water and chow pellets in a light- and temperature-controlled environment (12 h on/12 h off, lights on at 08:00; 20–22°C) for 1 week before the experiment.
In the first experiment, 7-wk-old mice were intraperitoneally injected with saline or liraglutide (25 and/or 100 μg/kg) and then deprived of food after the treatment. At 2 h and 4 h later, body weights and blood glucose levels were measured.
In the second experiment, 7-wk-old mice were intraperitoneally injected with saline or liraglutide (100 μg/kg) and then deprived of food after the treatment. At 2 h and 4 h later, the animals were decapitated, and blood was obtained for the measurement of plasma insulin, 5-HT, FGF21, and total BA levels. The liver, ileum and colon were dissected for determining mRNA levels, BAs, and tryptophan’s metabolites at 2 h later.
The doses of liraglutide used were described previously [
21,
22]. The experiment was performed between 12:00-17:00.
The animal studies were conducted in accordance with the institutional guidelines for animal experiments at Tohoku University Graduate School of Medicine and all experimental protocols were approved by the institutional ethics committee at Tohoku University.
4.2. Blood Chemistry.
Whole blood was mixed with EDTA-2Na (2 mg/ml) and aprotinin (500 kIU/ml) to determine the plasma levels of FGF21, 5-HT, and insulin. Plasma insulin, 5-HT, and FGF21 levels were measured by enzyme-linked immunosorbent assay (mouse Insulin ELISA Kit [TMB], AKRIN-011T, Shibayagi, Gunma, Japan, mouse 5-HT; BA E-5900, Labor Diagnostika Nord, Nordhorn, Germany, and mouse FGF21 ELISA kit) as described previously[
23,
24]. Blood glucose levels were measured using glucose strips (blood glucose monitoring system; Accu-Check, Roche Diagnostics, Tokyo, Japan).
4.3. BA Analysis
BA analysis was performed using a facility service at LSI Medience Corporation, contract clinical trial company (Tokyo, Japan) as described previously[
24]. Briefly, approximately 100 mg of liver and colon tissue was transferred to disruptor tubes supplied by Yasui Kikai (Osaka, Japan) and shaken with iron cones cooled in liquid nitrogen. The tissue powders were suspended with 1 mL of water and 4 mL of methanol. After mixing using a shaker for 15 min, the samples were centrifuged at 1000 × g for 15 min at room temperature. The supernatants were analyzed by liquid chromatography-tandem mass spectrometry (Nexera X2 LC0AD, 8050, Shimadzu, Kyoto, Japan) equipped with reverse phase LC column (InfinityLab Poroshell 120 EC-C18 2.7 mm, 2.1 mm ×150 mm, Agilent Technologies, Santa Clara, CA). The data were analyzed by LabSolutions (Shimadzu, Kyoto, Japan). The peak areas were normalized by internal standards, and each bile acid concentration was obtained using a standard curve.
4.4. Tryptophan and Its Metabolites Analysis
Tryptophan and its metabolites were subjected to analysis by LSI Medience Corporation, a contracted laboratory based in Tokyo, Japan. Briefly, approximately 100 mg of colon tissues were introduced into sample disruptor tubes provided by Yasui Kikai (Osaka, Japan). Subsequently, these tubes were agitated with iron cones that had been pre-cooled in liquid nitrogen. The resulting sample powders were suspended in 500 µL of methanol and vigorously shaken for 15 minutes, after which centrifugation was carried out at 20,000 × g for 3 minutes. The supernatants, constituting 40 µL, were meticulously transferred to 2 mL microtubes. Internal standards were then introduced and combined with the supernatants, followed by the addition of 1,000 µL of a 2% formic acid solution to induce protein precipitation. Afterward, we purified the analytes from the supernatant using solid-phase extraction (OASIS MCX, Waters, Milford, MA) and analyzed them using liquid chromatography-tandem mass spectrometry (Ultivo, Agilent, Santa Clara, CA) with a reverse-phase LC column (ACQUITY UPLC HSS T3, 1.8 µm, 2.1 mm × 50 mm, Waters, Milford, MA). The data were processed with Mass Hunter software (Agilent, Santa Clara, CA). We normalized the peak areas using internal standards and determined the concentration of each analyte using a standard curve.
4.5. Real-Time Quantitative Reverse Transcription–Polymerase Chain Reaction (RT–PCR)
Total RNA was isolated from mouse liver using the RNeasy Midi kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA synthesis was performed using a Super Script III First-Strand Synthesis System for RT-PCR Kit (Invitrogen, Rockville, MD) with 1 μg total RNA. cDNA synthesized from total RNA was evaluated in a real-time PCR quantitative system (LightCycler Nano Instrument Roche Diagnostics, Mannheim, Germany).
The relative amount of mRNA was calculated using β-actin mRNA as the invariant control. Data are shown as fold-change of the mean value of the control group as described previously [
22,
23]
.
4.6. Statistical Methods
Data are presented as mean ± SEM (n= 6). Comparisons between two groups were performed using Student’s t-test. Comparison between more than two groups were performed using analysis of variance with Bonferroni's correction for multiple comparisons. A P value of less than 0.05 was considered statistically significant.
Author Contributions
K.N. designed the study, performed the experiments, interpreted all analyses, generated all figures and tables, and wrote the manuscript. T.K. performed the experiments and interpreted all analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant in-Aid for Scientific Research.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Kårhus, M.L.; Brønden, A.; Forman, J.L.; Haaber, A.; Knudsen, E.; Langholz, E.; Dragsted, L.O.; Hansen, S.H.; Krakauer, M.; Vilsbøll, T.; et al. Safety and efficacy of liraglutide versus colesevelam for the treatment of bile acid diarrhoea: a randomised, double-blind, active-comparator, non-inferiority clinical trial. Lancet Gastroenterol. Hepatol. 2022, 7, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K. The Regulatory Role of the Central and Peripheral Serotonin Network on Feeding Signals in Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 1600. [Google Scholar] [CrossRef] [PubMed]
- Koopman, N.; Katsavelis, D.; Hove, A.S.T.; Brul, S.; Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.; Konrad, P.; Mędrek-Socha, M.; Kaczka, A.; Błońska, A.; Zajdel, R.; Chojnacki, C.; Gasiorowska, A. The Variability of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 2550. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Wu, X.; Jing, H.; Zhang, S.; Hu, Z.; Rao, L.; Chang, Q.; Wang, L.; Zhang, Z. Gut microbiota alteration after cholecystectomy contributes to post-cholecystectomy diarrhea via bile acids stimulating colonic serotonin. Gut. Microbes 2023, 15, 2168101. [Google Scholar] [CrossRef] [PubMed]
- Martin, A., M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Sun, E.W.; Jessup, C.F.; Wesselingh, S.L.; Khan, W.I.; Rogers, G.B.; Steinberg, G.R.; et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA. 2019, 116, 19802–19804. [Google Scholar] [CrossRef]
- Borup, C.; Vinter-Jensen, L.; Jørgensen, S.P.G.; Wildt, S.; Graff, J.; Gregersen, T.; Zaremba, A.; Borup Andersen, T.; Nøjgaard, C.; Timm, H.B.; et al. Efficacy and safety of colesevelam for the treatment of bile acid diarrhoea: a double-blind, randomised, placebo-controlled, phase 4 clinical trial. Lancet Gastroenterol. Hepatol. 2023, 8, 321–331. [Google Scholar] [CrossRef]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E.J. Bile acids in glucose metabolism in health and diseases. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Lundåsen, T.; Andersson, E.-M.; Snaith, M.; Lindmark, H.; Lundberg, J.; Östlund-Lindqvist, A.-M. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLoS One 2012, 7, e37787. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Bile acid sequestrants for glycemia control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complications 2017, 31, 918–927. [Google Scholar] [PubMed]
- Ge, M.-X.; Niu, W.-X.; Ren, J.-F.; Cai, S.-Y.; Yu, D.-K.; Liu, H.-T.; Zhang, N.; Zhang, Y.X.; Wang, Y.C.; Shao, R.G.; et al. A novel ASBT inhibitor, IMB17-15, repressed nonalcoholic fatty liver disease development in high-fat diet-fed Syrian golden hamsters. Acta Pharmacol. Sin. 2019, 40, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Haywood, J.; Craddock, A.L.; Belinsky, M.G.; Kruh, G.D.; Dawson, P.A. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc. Natl. Acad. Sci. USA. 2008, 105, 3891–3896. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto. J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Sun, S.; Xie, Y.; Pan, C.; Li, M.; Li, C.; Liu, Y.; Xu, Z.; Liu, W.; et al. Indolepropionic acid reduces obesity-induced metabolic dysfunction through colonic barrier restoration mediated via tuft cell-derived IL-25. FEBS J. 2022, 289, 5985–6004. [Google Scholar] [CrossRef]
- Nonogaki, K.; Suzuki, M.; Sanuki, M.; Wakameda, M.; Tamari, T. The contribution of serotonin 5 HT2C and melanocortin-4 receptors to the satiety signaling of glucagon-like peptide 1 and liraglutide, a glucagon-like peptide 1 receptor agonist, in mice. Biochem. Biophysic. Res. Communi. 2011, 411, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Kaji, T. The acute anorexic effect of liraglutide, a GLP-1 receptor agonist, does not require functional leptin receptor, serotonin, and hypothalamic POMC and CART activities in mice. Diabetes Res. Clin. Practice 2016, 120, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Kaji, T. Whey protein isolate inhibits hepatic FGF21 production, which precedes weight gain, hyperinsulinemia and hyperglycemia in mice fed a high-fat diet. Sci. Rep. 2020, 10, 15784. [Google Scholar] [CrossRef]
- Nonogaki, K.; Kaji, T. Ingestion of whey protein and β-conglycinin exerts opposite effects on intestinal FGF15 and serotonin secretion in mice. Frontier Endocrinol. 2023, 14, 1080790. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).