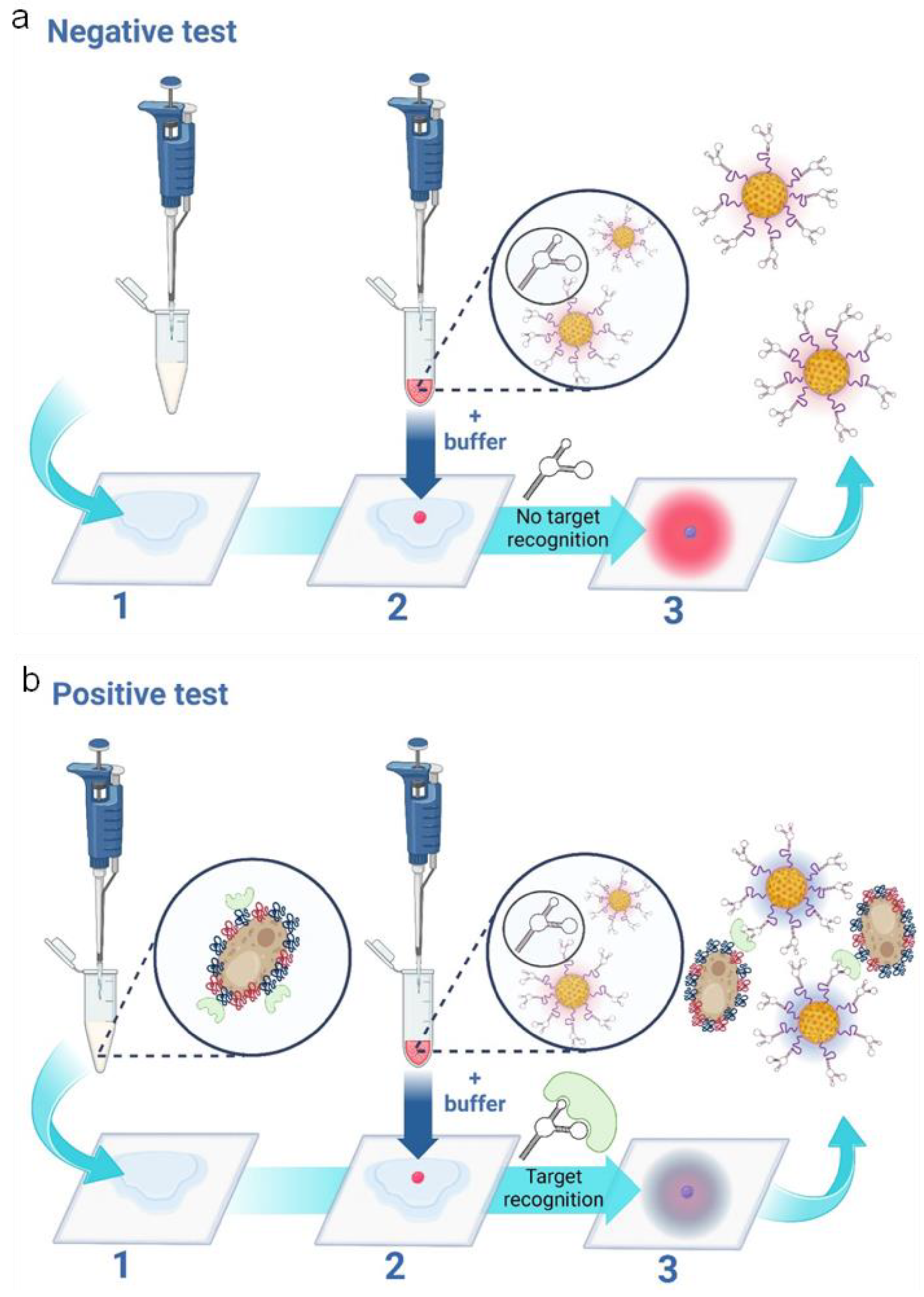
Assay Principle
The assay principle is schematically presented in
Figure 1 depicting the cases of both negative (
Figure 1A) and positive tests (
Figure 1B), respectively for candida albicans. The assay features three main steps: 1- Addition of vaginal fluid samples to wearable sample pads; 2- addition of the nanoparticle gel to the sample pad, followed by the addition of the running buffer and 3- a colorimetric result based on the aggregation state of the nanoparticles. In the case of a negative test result (the absence of candida albicans), the sample pad turns bright pink, as the red nanoparticles remain free of aggregation. In the case of a positive test result (the presence of candida albicans), the sample pad turns dark blue. This is caused by aptamer-target induced nanoparticle aggregation. I.e. nanoparticle aggregation results in a redshift (or blue color change) on the plasmonic spectrum.[
25]
Typical lateral flow assembly (LFA) platforms utilize the glass fiber diagnostic pad as the platform’s conjugate pad. The conjugate pad has three main functions: preserving dried nanoparticles; releasing them upon sample wetting and providing the first interaction between the target and labelled bioreceptor. [
22] Typically, conjugate pads of lateral flow assays incorporate buffering agents to maximize nanoparticle stability and to completely release them upon rewetting by the sample. [
22] Once the conjugate pad buffer has been chosen, the nanoparticle conjugates are loaded onto the conjugate pad by either immersion or air jet dispensing. Air jet dispensing requires a costly and well-calibrated dispensing apparatus, and the immersion method may have non-uniform coverage, leading to sensor-to-sensor variability. [
22] Furthermore, within LFA construction, the conjugate pad requires drying in hot air (which is typically fixed at 37 °C), or vacuum drying. Drying is critical for maintaining the stability of the dried nanoparticle-bioreceptor conjugates. [
22] Improper conjugate pad drying can greatly impact the nanoparticle release efficiency from the conjugate pad. Much like the LFA, our method is disposable, cheap, rapid and convenient. However, our method avoids the complexity of precise assembly and chemical/bioreceptor modification required within the conventional LFA. Our method repurposes the LFA conjugate pad as a user-friendly, discrete and wearable substrate (with slight modification) for instant candida albicans detection. Unlike conventional LFAs, our wearable substrate does not require pre-treatment or careful nanoparticle/ bioreceptor functionalization onto conjugate pads. Furthermore, the wearable substrate is not subject to strict storage conditions or shelf-life limitations. To the best of our knowledge, current LFAs do not offer detection of candida albicans from vaginal fluid and they are not yet wearable. We believe that our platform has significant clinical translational potential because our wearable substrate can be adhered directly to existing sanitary napkins for testing with a user-friendly nanoparticle gel-based solution and buffer before sanitary pad disposal.
Initial Concept and Running Buffer Selection
The type and concentration of running buffer can greatly influence the overall pH and ionic strength. Ionic strength and pH can affect the interaction between the receptor and target as well as affecting potential nonspecific binding. This can greatly impact the sensitivity, specificity and reproducibility of a biomolecular detection platform. The performance of various running buffers was assessed within our platform to determine which buffer would show the best distinction between positive and negative samples. Running buffers were selected for our platform based on commonly reported buffers within LFA platforms. LFA buffers were selected due to the performance for enabling the efficient release of the nanoparticles from the conjugate pads.
Some of these buffers include: phosphate buffer (pH range between 5.8 and 8); Tris (pH range between 7.5 and 9) and HEPES (pH range between 6.8 and 8.2). Common detergents such as SDS are typically used within the sample pad portion of LFA platforms. Detergents may assist with minimizing nonspecific binding (disrupting weak ionic and hydrophobic bonds) and with facilitating the flow of the detection labels along the different pads. Consequently, we tested SDS to observe its effect for our platform. Colloidal nanoparticle suspension stability is generally affected by the ionic strength of the solution. As such, buffers containing borate may be used due to their low ionic strength.
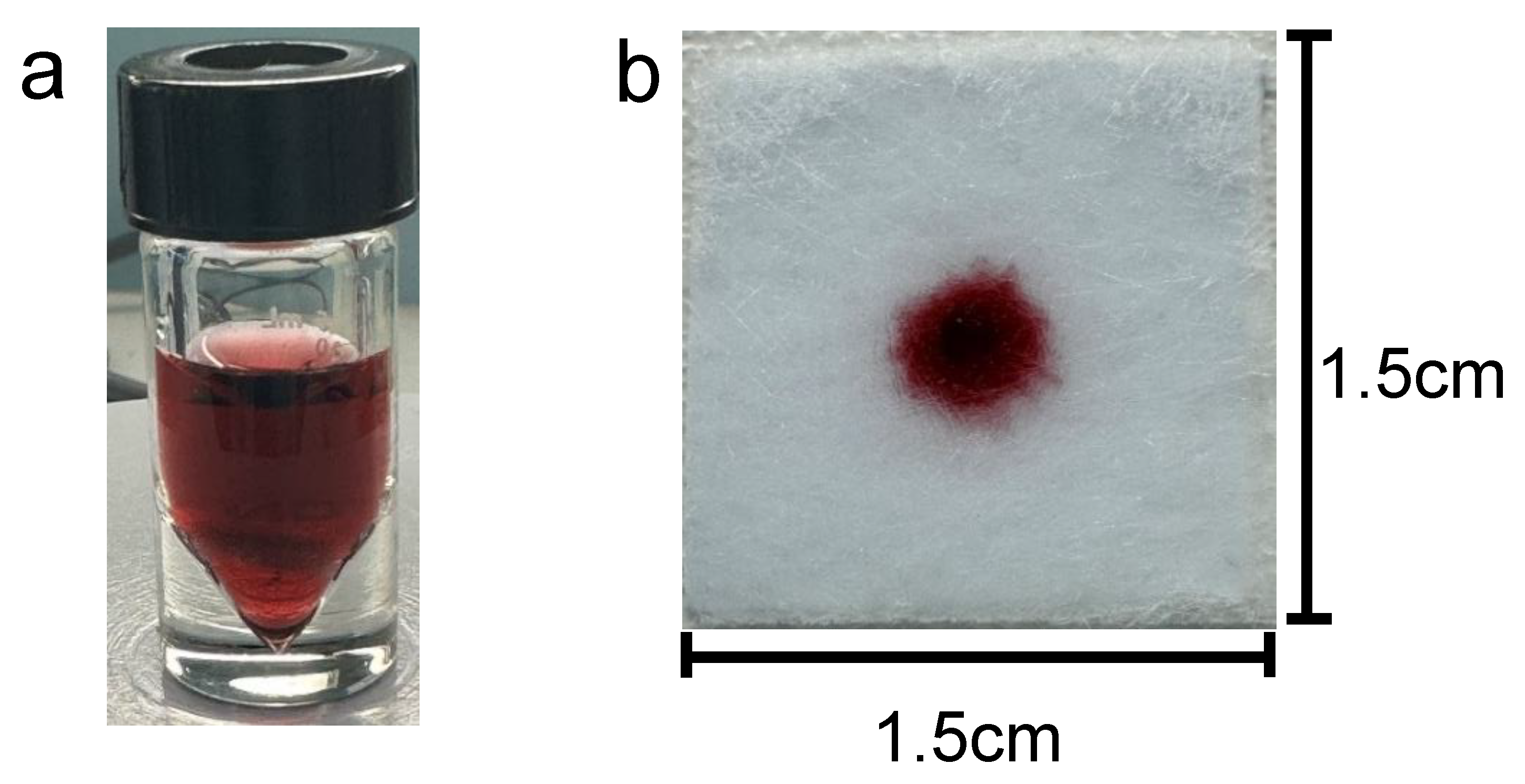
Figure 2 A shows the prepared gold nanoparticles prior to centrifugation and prior to addition to the gel.
Figure 2b shows 5 μL of nanoparticle gel solution that is dispensed onto the substrate containing 100 μL of previously soaked vaginal fluid simulant solution, prior to running buffer addition. Prior to the running buffer, the nanoparticles remain confined to the gel matrix and do not disperse across the substrate. We believe that this greatly improves manual handling, allowing for more uniform distribution of nanoparticles by the user. The results of various buffer testing are displayed in
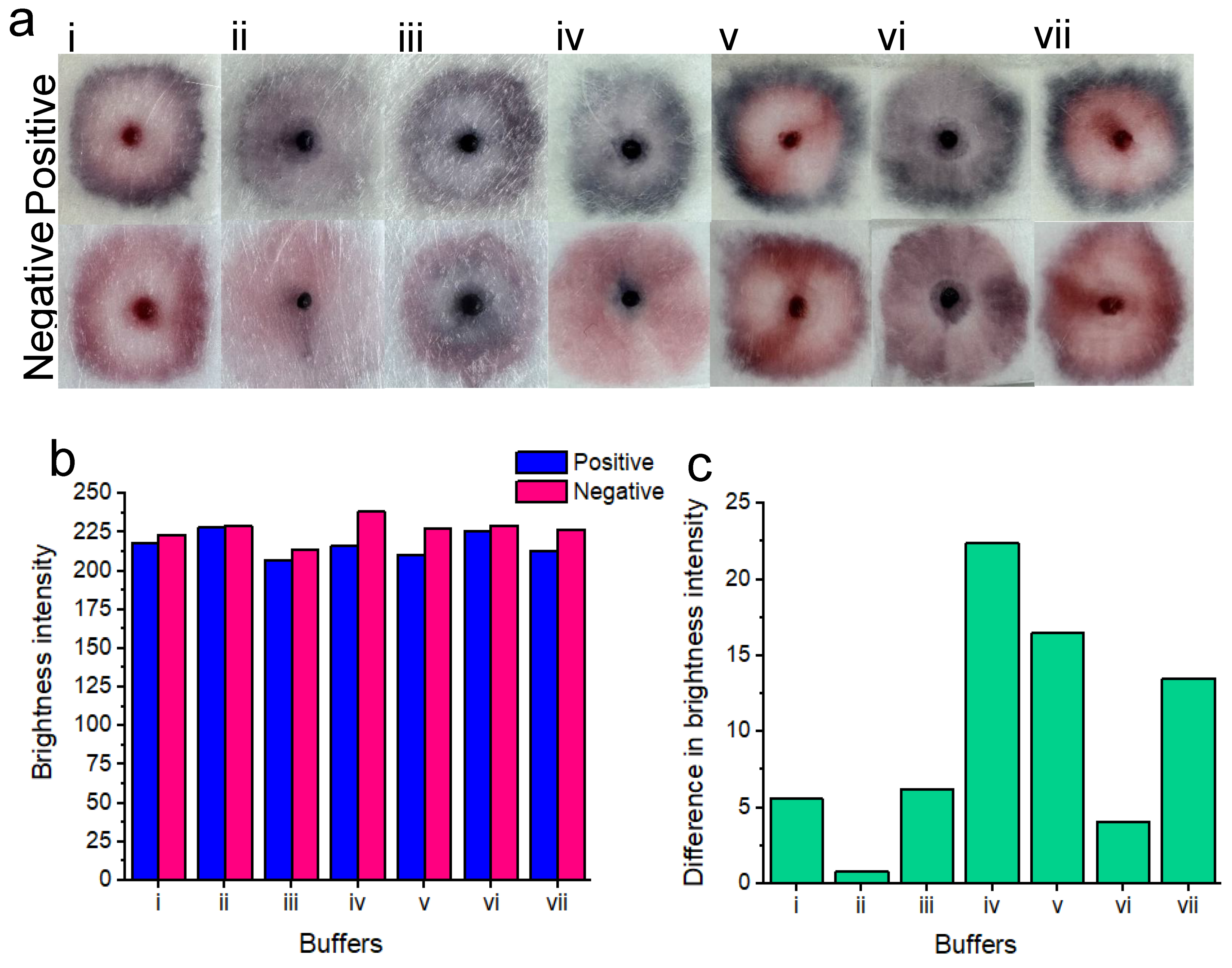
Figure 3. A concentration of 7.0×10
7 candida albicans cells per ml was tested to ensure the best distinction could be made between positive and negative tests for each tested buffer. Results of buffer testing are described as follows, describing the outer portion, inner portion and nanoparticle gel conditions (in terms of naked-eye clarity) of each of the tests.
SDS buffer i)- the distinction was unclear between positive and negative samples. Positive samples showed a pattern of nanoparticle aggregation in the form of a dark pink outer ring. Whereas, the center of the positive test showed a pink unaggregated portion as well as a pink coloration of the nanoparticle gel. Negative tests showed a bright pink outer ring, a pink inner color as well as a pink coloration of the nanoparticle gel.
Sodium borate/ sucrose/ NaCl/ Tween/ Sodium azide buffer ii)- the distinction was unclear between positive and negative samples. Positive samples showed a slight pattern of nanoparticle aggregation in the form of a thin dark/purple outer ring. The center of the test showed slightly aggregated purple nanoparticles as well as a dark purple color of aggregated nanoparticles within the nanoparticle gel. The negative test showed no definitive nanoparticle ring, however, the inner portion of the test was a pale pink color. The nanoparticle gel of the negative test resembled the same aggregated purple color as seen within the positive test.
Tris-HCl/ MgCl2/ NaCl and ethanol buffer iii)- distinction was unclear between positive and negative tests. Positive samples showed a purple outer ring of aggregated nanoparticles, while the inner portion of the test showed a feint, aggregated dark blue color. The nanoparticle gel of the positive test showed a dark blue to black color of aggregated nanoparticles. The negative test showed a pink outer ring of unaggregated nanoparticles, whereas, the center of the test showed significant non-specific nanoparticle aggregation. The nanoparticle gel of the negative test showed a dark blue to black color of aggregated nanoparticles.
Sodium borate/ KCl/ Tween buffer iv)- a very clear distinction between positive and negative samples was observed. Positive samples showed a dark purple to blue outer ring of aggregated nanoparticles. The inner portion of the test displayed a slightly aggregated, purple nanoparticle color, whereas the nanoparticle gel showed a dark purple to black color of aggregated nanoparticles. The negative test did not show a ring of nanoparticles. However, the inner portion of the test showed a relatively homogeneous portion of pink unaggregated nanoparticles. However, the nanoparticle gel of the negative test displayed a dark purple to blue color, suggesting some nonspecific nanoparticle aggregation.
Phosphate buffer v)- a distinction was evident between the positive and negative tests. Positive samples showed a dark purple to blue outer ring of aggregated nanoparticles. The inner portion of the test showed a bright pink portion of unaggregated nanoparticles, however. The nanoparticle gel remained free of aggregated nanoparticles, showing a bright pink to red color. The negative test showed a dark pink color of nanoparticles of “borderline” aggregation status. The inner portion of the test showed dispersed nanoparticles that remained free of aggregation. As with the positive test, the nanoparticle gel remained free of visible aggregation and was pink to red in color.
Tris-HCL buffer vi)- no clear distinction between positive and negative tests was observed. Positive samples showed a dark blue ring of aggregated nanoparticles, while the middle portion of the test showed slightly aggregated purple nanoparticles. The nanoparticle gel showed an aggregated dark purple color. The negative test did not display an aggregated ring portion of nanoparticles, however, the inner portion of the test showed some non-specific nanoparticle aggregation in the form of a purple to pink color. The nanoparticle gel showed nanoparticle aggregation in the form of a dark purple color.
HEPES buffer vii)- a distinction was observed between positive and negative tests. Positive samples showed a distinct dark blue color of aggregated nanoparticles as an outer ring. Whereas, the inner portion of the test showed zero nanoparticle aggregation in the form of a bright pink color. The nanoparticle gel remained unaggregated in the form of a bright pink to red color. In the case of the negative test, the outer portion of the test displayed a dark pink color, possibly indicating potentially and slightly aggregated nanoparticles. The inner portion of the test remained aggregation free and pink. The nanoparticle gel also remained unaggregated and bright pink to red in color.
Figure 3 b displays the red channel brightness intensity of each buffer for the positive and negative samples after image processing. Notably, all buffers displayed a lower red channel brightness intensity for the positive tests compared to the negative tests. c) shows the difference in brightness intensity (after image processing) between positive and negative samples, for each of the buffers. Notably, buffer iv) (Sodium borate/ KCl/ Tween-20) showed the greatest difference in red channel brightness intensity between positive and negative results. I.e. The positive result was “blue” which was low in red channel brightness intensity and the negative result was “pink” which was high in red channel brightness intensity.
The final buffer chosen for subsequent experiments was the sodium borate/ KCl/ Tween buffer. This buffer provided the clearest distinction between the positive and negative result (at this tested concentration), with minimal nonspecific nanoparticle aggregation within the negative control. I.e. this buffer provided an overall relatively homogeneous purple to blue color for a positive result and an overall homogeneous (ignoring the nanoparticle gel portion) bright pink color for the negative result. Importantly at this proof-of-concept stage, buffer optimization has not yet been performed. We acknowledge that different buffering conditions may be better suited for our platform. However, we note that buffer optimization studies would require testing with a broad range of target concentrations, reaction times, buffer concentrations, reagent ratios, etc. Buffer performance may be assessed based on platform rapidity, sensitivity, specificity and ease of distinction based on naked-eye differentiation between positive and negative samples. Moreover, the “ideal” choice of buffer may be subjective because one buffer may provide a better sensitivity for example, however, it may not yield the optimum distinction (between positive and negative targets) for ease of naked-eye readability. Full optimization of these parameters is beyond the scope of this current proof-of-concept testing stage. Rather, the platform was only optimized in terms of speed and visual distinction between positive and negative samples at this stage. However, we did optimize the buffer volume and we determined that 45 μL was ideal for the 1.5cm × 1.5cm sample pads that had been previously soaked in 100 μL of vaginal fluid simulant solution. This buffer volume was not so large that it diluted the colorimetric results or overflowed the sample pads, and it was not so small that it could not release the nanoparticles from the gel.
Substrate Selection
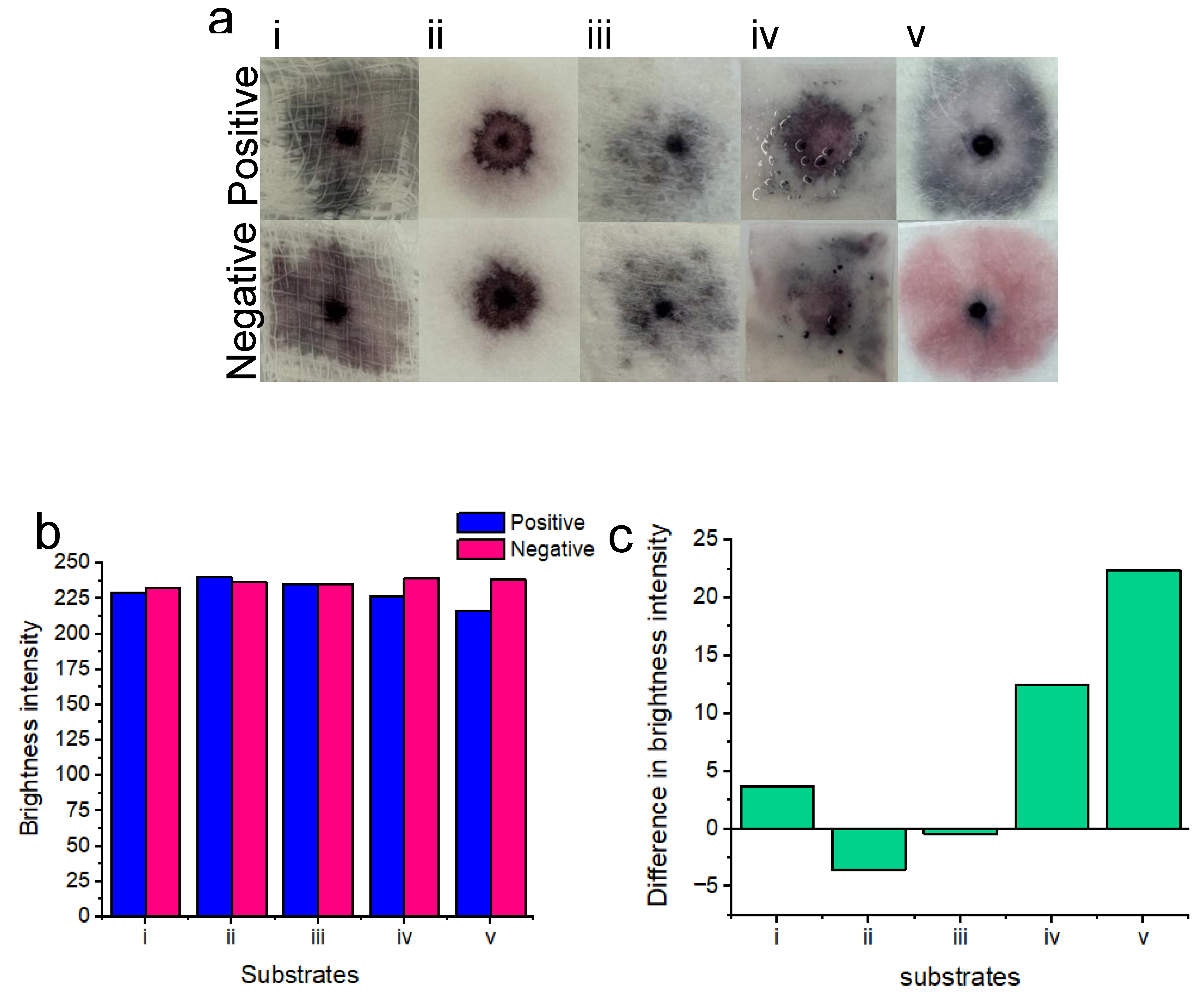
The choice of substrate was crucial for the success of the platform at this stage of testing (
Figure 4). The final substrate was chosen for all future experiments based on its ability to showcase visually distinctive positive results, compared to the negative results. Five substrates were tested including: gauze swabs; cellulose fiber sample pads; sanitary pads; chromatography paper and glass fiber pads. These substrates were selected due to availability, as well as being low-cost, absorbent (for vaginal fluid samples), light weight, cheap, flexible and potentially wearable. A concentration of 7.0×10
7 candida albicans cells per ml was tested to ensure the best distinction could be made between positive and negative tests for each tested substrate. Results of the substrate testing describing the clarity of the naked-eye distinction between positive and negative samples are as follows:
Gauze i): There was no clear distinction between positive and negative samples. Positive samples turned dark purple upon nanoparticle aggregation, whereas negative samples turned a similar color.
Cellulose fiber ii): There was no distinction between positive and negative samples. Both samples turned purple on this substrate. We believe that this was possibly due to nanoparticles drying out within this substrate.
Sanitary pad iii): There was no distinction between positive and negative samples when testing with sanitary pad substrates. Significant non-specific nanoparticle aggregation was exhibited between both positive and negative samples. We believe that this may be attributed to significant drying of the nanoparticle solutions.
Chromatography paper iv): There was no distinction between positive and negative samples when testing on chromatography paper. Upon this substrate, nanoparticle gel became disrupted possibly due to the buffer solution spreading the nanoparticles across the surface of the substrate rather than within the substrate.
Glass fiber v): There was a very clear distinction between positive and negative results when testing on the glass fiber substrate. Positive samples showed significant nanoparticle aggregation and turned dark blue, whereas negative samples remained a bright pink color and free of visible aggregation.
Image processing for red channel brightness intensity for each of the substrates is shown in
Figure 4 b. Gauze i), chromatography paper iv) and glass fiber v) showed an increase in red channel brightness intensity for the negative results in comparison to the positive results. However, cellulose fiber ii) and sanitary pad substrates iii) unexpectedly showed less red channel brightness intensity for negative results when compared to positive results. Image processing showed that the greatest difference in red channel brightness intensity between positive and negative results was achieved with the glass fiber substrate. Consequently, glass fiber was selected for all subsequent experiments due to its performance for differentiating positive “blue” tests from negative “pink” tests.
Figure 4c shows the difference in brightness intensity (after image processing) between positive and negative samples, for each of the substrates. Negative values for ii) and iii) indicate decreased red channel brightness intensity for the negative samples.
Semiquantitative Candida Yeast Cell Detection
To demonstrate a proof-of-concept for c. albicans detection, the platform was tested with four different concentrations of c. albicans cells across three replicates (
Figure 5a). The sodium borate/ KCl/ Tween-20 buffer combination was chosen for all following experiments due to its superior performance in initial testing. Initially, a stock solution of 7.0×10
7 c. albicans cells per ml (in vaginal fluid simulant solution) was created via optical density (OD
600) analysis.
Then, the stock solution was diluted (1:1) in vaginal fluid simulant solution to an eventual concentration of 4.4×106 c. albicans cells per ml. Each of the concentrations were compared to the blank solution comprised of vaginal fluid simulant solution only. The results are displayed after 20 minutes. 20 minutes was deemed the optimal time to visualize all the colorimetric results at lower concentrations, however, at higher concentrations, a result starts to appear within 5 minutes. Notably, upon optimization of manual buffer addition to the nanoparticle gel, we believe that our results will reflect indistinguishable replicates. Increasing the concentration of c. albicans cells led to increased nanoparticle aggregation and therefore an increase in the intensity of the observed “blue” color. All five concentrations of c. albicans cells differed in color compared to the blank solution that remained pink. The results indicated that the platform was able to detect c. albicans cells within vaginal fluid simulation after 20 minutes.
Image processing was performed for each of the concentrations and replicates (
Figure 5 b) and red channel brightness intensity is shown. As the concentration of candida albicans fungal cells decreased, there was an increase in red channel brightness intensity due to decreased nanoparticle aggregation.
Figure 5 c shows the difference in red channel brightness intensity for each of the concentrations in comparison to the blank test. The higher the concentration of candida albicans cells, the greater the difference in red channel brightness intensity is from the blank solution. The results of
Figure 5c represent an average of each of the individual concentrations from
Figure 5b. We note that manual handling effects can impact the results. I.e. if careful handling is not given to adding the running buffer dropwise to the nanoparticle gel, the particles may be unevenly displaced across the sample pad, showing an uneven nanoparticle aggregation pattern for positive tests. Notably, once the result has developed, it is also visible for at least two months after testing. I.e. once the platform has been allowed 20 minutes of development time, the colorimetric result is “locked in” and this does not significantly change in brightness intensity for the tested candida albicans concentrations or the blank sample. For this proof-of-concept testing, we note that colorimetric naked eye detection is subjective. I.e. a result that may appear “positive” to one person may be perceived as “negative” to another person. As such, we chose to ignore reporting on a limit of detection here, instead, opting to show the lowest concentration of tested candida albicans cells that may still be perceived as a “positive” result in reference to the negative blank results. We believe that our results are within the range of clinical feasibility concerning the available number of c.albicans cells that are present within symptomatic vulvovaginal candidiasis cases. [
18] However, we will improve upon this proof-of-concept detection limit with further optimization.
Nanoparticle Specificity and Dry Sample Testing
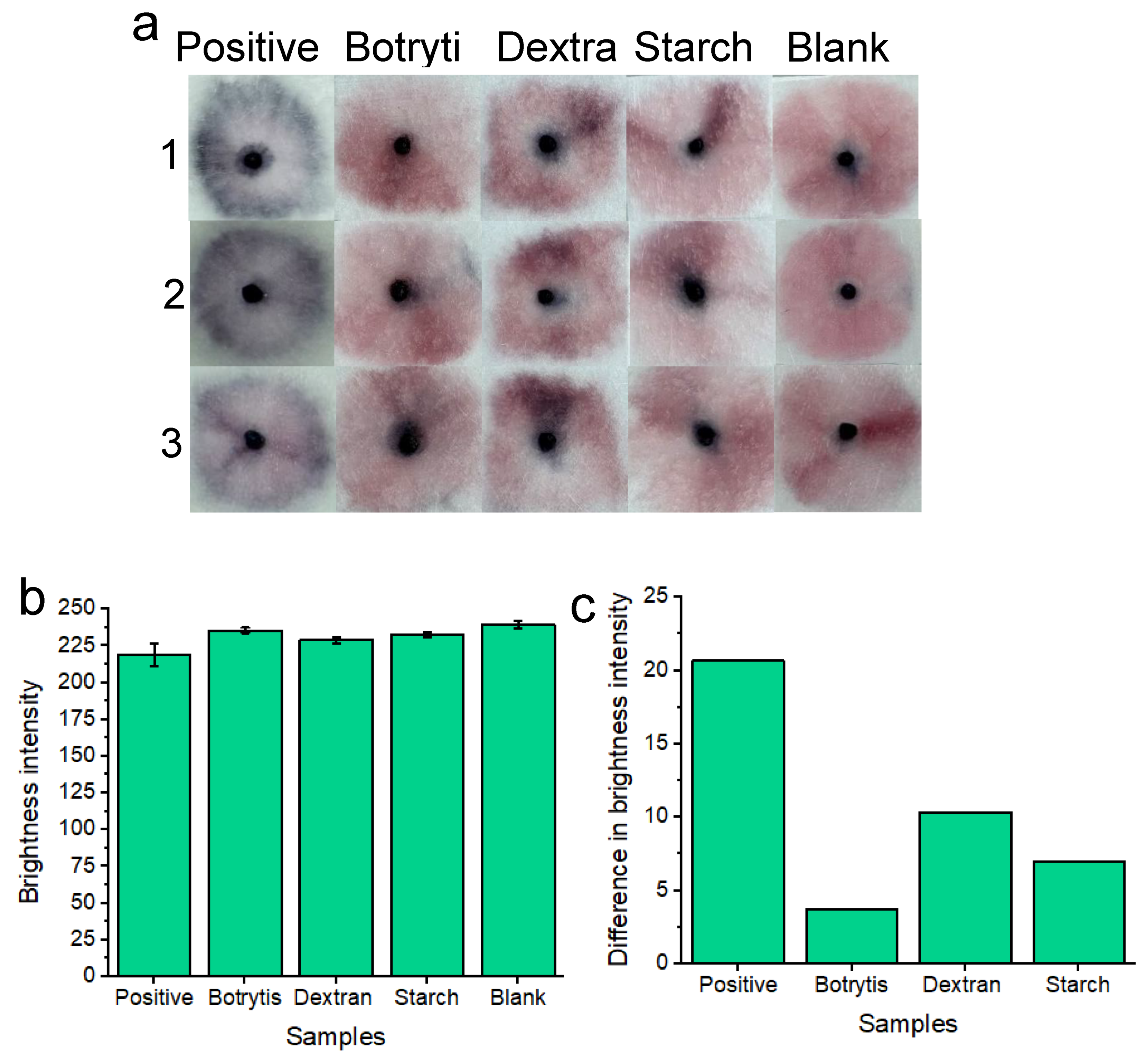
Nanoparticle specificity testing (
Figure 6 a) was conducted following our previous work [
16] by using other available glucan-containing substances such as dextran ([C
6H
10O
5]
n), starch ([C
6H
10O
5]
n) and the Botrytis Cinerea fungus. For this purpose, our aptamer-conjugated gold nanoparticles were expected to only aggregate upon specific recognition of candida albicans glucan molecules. A concentration of 7.0×10
7 candida albicans cells per ml was tested to ensure the best distinction could be made between positive and negative tests for each specificity test. Both Dextran and starch were diluted in vaginal fluid simulant solution to give respective concentrations of 50 mg/mL. This was to ensure a high concentration of glucan molecules for each of the reagents. At concentrations exceeding 50 mg/mL, solubility was an issue that would unfairly challenge our platform due to matrix effects aiding in nanoparticle aggregation, rather than glucan composition alone. As per our prior work [
16] an excess of botrytis cinerea mycelium (50 mg) was harvested and was sonicated for addition to samples of vaginal fluid simulant solutions for assay specificity testing. Selecting Botrytis cinerea was useful for testing the specificity of our AD1 aptamer-nanoparticle conjugates for two reasons: Firstly, the fungal mycelium provides a significant biological challenge for nanoparticles in solution as nanoparticles may non-specifically aggregate upon the fungal mass. And secondly, it is crucial to test the AD1 aptamer specificity within other glucan molecules as our aptamer is designed to recognize the specific microenvironment of candida albicans BDG molecules only. 100 μL of each respective solution. (containing starch, dextran or botrytis) was used per test on our sample pads. Three individual replicates (1, 2 and 3) were conducted to show reproducibility of the results. Results are shown after 20 minutes of development time. Each of the non-specific glucan-containing species (dextran, starch and botrytis) showed a similar “pink” result compared to the blank samples. Image processing of red channel brightness intensity is shown in
Figure 6b (with error bars) for three individual replicates of each experiment.
Figure 6C shows the difference in red channel brightness intensity between each of the tested samples compared to the blank sample. The results represent the average of the three replicates for each experiment from
Figure 6b. The positive “blue” tests differ significantly from the blank “pink” negative test in terms of red channel brightness intensity. Botrytis, dextran and starch each show a minimal difference in red channel brightness intensity from the blank samples. Therefore, the nanoparticles did not significantly non-specifically aggregate under these specificity testing conditions. This implies that our platform is highly specific for the detection of c. albicans BDG molecules only.
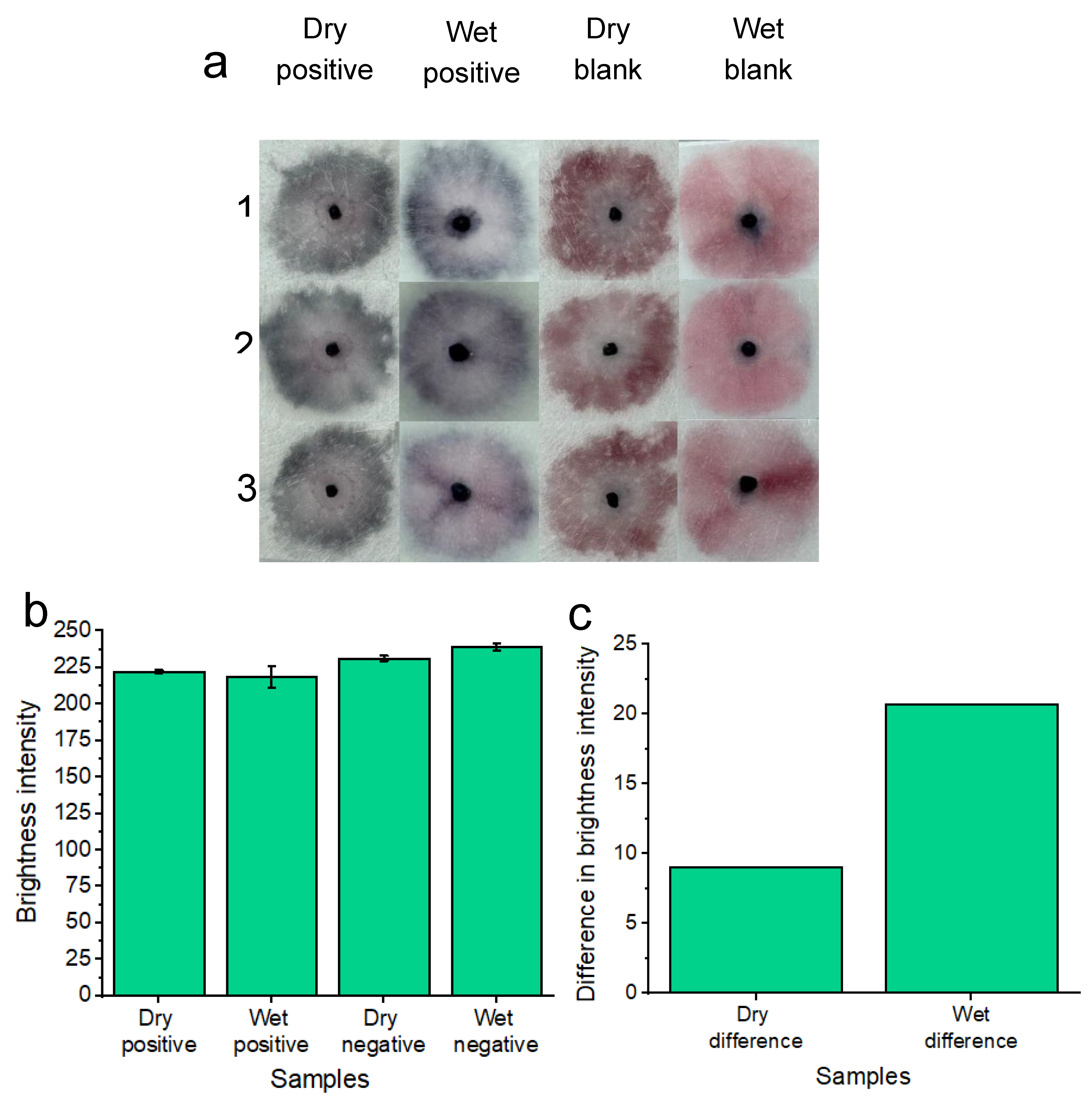
In addition to testing the platform with freshly supplied (wet) candida albicans fungal samples in vaginal fluid simulant solution, dried samples were also tested (
Figure 7a). As part of the drying process, samples were added to the pads and were allowed to dry for 1.5 hours at 37°C before nanoparticle gel addition and subsequent running buffer addition. This dry testing was important to assess given that within a commercial platform, patients would not always be testing on fresh and wet samples of vaginal fluid. i.e. patient vaginal fluid secretions may flow onto the substrate, but the patient may not decide to test the wearable for candida albicans until some time has elapsed throughout their day. Three replicates (1,2 and 3) are shown for the concentration of 7.0×10
7 candida albicans cells per ml in reference to the blank solution. A concentration of 7.0×10
7 candida albicans cells per ml was tested to ensure the best distinction could be made between positive and negative tests across the dry and wet sample conditions. Results are shown after 20 minutes of development time. The positive samples showed a distinct “blue” color in reference to the “pink” blank tests. Results indicated that the platform is feasible under dry testing conditions for detecting 7.0×10
7 candida albicans cells per ml. However, for future work. Other lower concentrations of candida albicans cells will be tested across different drying times to simulate times throughout the day that a patient may decide to test after removing the wearable sample pads. For comparison, the previous results of wet sample testing are included. Image processing for measuring red channel brightness intensity was also conducted (
Figure 7b). Three individual replicates are shown with error bars. The results of
Figure 7c show a comparison between dry and wet sample positive tests compared to their respective blank samples. The results indicate that testing under wet sample conditions gives the best distinction between positive and negative samples, however, a distinction between positive and negative samples can still be made when testing dry samples.