Submitted:
24 May 2024
Posted:
24 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Exosomes and Their Functions
1.2. The Biogenesis of Exosomes
- Exosomal membrane proteins directly activate membrane receptors of target cells without being internalized,
- Exosomal membrane proteins are cleaved by proteases, and the resultant soluble fragments bind to membrane receptors of the target cells and
- Exosomes are internalized into the target cells, via endocytosis, phagocytosis, or direct fusion with the plasma membrane of the target cells.
1.3. The Cargo of Exosomes
- (a)
- Their protein cargo consists of the most commonly expressed endosomal proteins incorporated during the biogenesis of the MVBs (Alix, TSG101, heat shock proteins Hsc/Hsp 70 and 90), the vesicular transport and fusion proteins (Rab GTPases, SNAREs, annexins, and flotillin), and the proteins that interact with the target cell integrins and tetraspanins. Apart from the above, however, the protein composition of each exosome is diverse and depends on the cell type of origin and its physiologic or pathologic state [11].
- (b)
- Their lipid cargo includes cholesterol, sphingolipids (e.g., ceramide), and glycerophospholipids [15].
- (c)
- Their nucleic acid cargo includes DNA, mRNA, and non-coding RNAs (ncRNAs). The latter subdivided into long non-coding RNAs (lncRNAs) and short ncRNAs -including microRNAs (miRNAs)-react with other nucleic acids and proteins of the target cell and dynamically modify its gene expression and protein translation.
1.4. Exosomal miRNAs: Biogenesis and Function as Modulators of Gene Expression
2. The Role of Exosomes and Exosomal miRNAs in the CNS
2.1. Cell-to-Cell Communication
2.2. Neurogenesis
2.3. Synaptic Plasticity
2.4. Stress Response
2.5. Mitochondrial Function in Brain
3. Stress-Induced Changes in CNS Exosomal Functions
3.1. Neurogenesis
3.2. Neuroinflammation
3.3. Stress-Induced Epigenetic Modifications
4. The Role of Exosomes in the Pathogenesis of Mental Disorders
4.1. Schizophrenia
4.2. Major Depressive Disorder
4.3. Bipolar Disorder
4.4. Alzheimer Disease
4.5. Huntington’s Disease
4.6. Critical View of Neuropsychiatric Diseases That Share Similarities in Alterations of miRNA Levels
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AHN | adult hippocampal neurogenesis |
| Alix protein | ALG2-interacting protein X |
| ATXN1 | Ataxin 1 |
| Aβ | beta-amyloid |
| BACE1 | β-site APP-cleaving enzyme 1 |
| BBB | Blood-Brain Barrier |
| BD | Bipolar disorder |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMECs | Brain Macrovascular Endothelial Cells |
| C3 | complement component 3 |
| ccf-mtDNA | circulating cell-free mitochondrial DNA |
| CCNA2 | Cyclin A2 |
| CCR2 | C-C motif chemokine receptor 2 |
| CHEK1 | Checkpoint Kinase 1 |
| circRNAs | Circular RNAs |
| CNS | Central Nervous System |
| COX6A2 | Cytochrome c Oxidase Subunit 6A2 |
| CREB | cAMP-response element binding protein |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAMP | damage-associated molecular pattern |
| Drosha-DGCR8 | DiGeorge syndrome Critical Region gene 8 |
| ECT | Electron Transport Chain |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| EV | Extracellular vesicle |
| FADD protein | Fas-associated death domain protein |
| GABA | Gamma-aminobutyric acid |
| GAC | glutaminase C |
| HD | Huntington’s’s disease |
| Hsp70 | heat-shock protein 70 |
| HTT | Huntingtin |
| IFNα | Interferon alpha |
| IL1R2 | Interleukin 1 receptor type II |
| IL-1β | Interleukin-1β |
| ILV | Intraluminal vesicle |
| KLF4 | Krüppel-like factor 4 |
| lncRNA | long non-coding RNA |
| MAP1b | microtubule-associated protein 1B |
| MAPK pathway | Mitogen-activated protein kinase |
| MDD | Major Depressive Disorder |
| MFN2 | mitofusin 2 |
| mHTT | mutant Huntingtin |
| miRNA | microRNA |
| MV | microvesicle |
| MVB | Multivesicular body |
| ncRNA | non-coding RNA |
| NF-Κb | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NMDAR-C | N-methyl D-aspartate receptor C |
| OXPHOS | Oxidative Phosphorylation |
| P2RX7 | Purinergic Receptor P2X 7 |
| PGC-1α | peroxisome proliferator-activated receptor-γ coactivator-1α |
| PrP | Prion Protein |
| RISC | RNA-mediated silencing complex |
| ROS | reactive oxygen species |
| SCZ | Schizophrenia |
| SNARE protein | SNAP Receptor protein |
| TBP | TATA-binding protein |
| TNF-α | Tumor Necrosis Factor-alpha |
| TRBP | Transactivation-Responsive RNA Binding Protein |
| TSG101 | tumor susceptibility gene 101 protein |
| Wnt pathway | Wingless-related integration site |
References
- Lee Y, El Andaloussi S, Wood MJA. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012 Oct 15;21(R1):R125-134. [CrossRef]
- Zduriencikova M, Gronesova P, Cholujova D, Sedlak J. Potential biomarkers of exosomal cargo in endocrine signaling. Endocr Regul. 2015 Jul;49(3):141–50. [CrossRef]
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009 Aug;9(8):581–93. [CrossRef]
- Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017 May 16;8(1):15287.
- Isola AL, Chen S. Exosomes: The Messengers of Health and Disease. Curr Neuropharmacol. 2017;15(1):157–65.
- Makrygianni EA, Chrousos GP. Extracellular Vesicles and the Stress System. Neuroendocrinology. 2023;113(2):120–67.
- Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell & Bioscience. 2019 Feb 15;9(1):19.
- Maligianni I, Yapijakis C, Bacopoulou F, Chrousos G. The Potential Role of Exosomes in Child and Adolescent Obesity. Children (Basel). 2021 Mar 6;8(3):196.
- Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 9(1):1703244.
- Vlachakis D, Mitsis Τ, Nicolaides N, Efthimiadou A, Giannakakis A, Bacopoulou F, et al. Functions, pathophysiology and current insights of exosomal endocrinology (Review). Molecular Medicine Reports. 2021 Jan 1;23(1):1–1.
- Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Current Opinion in Cell Biology. 2009 Aug 1;21(4):575–81.
- Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016 Mar;17(3):160–72.
- Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019 Oct 1;129(10):4041–9. [CrossRef]
- Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, et al. Signaling Pathways in Exosomes Biogenesis, Secretion and Fate. Genes (Basel). 2013 Mar 28;4(2):152–70. [CrossRef]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013 Feb 18;200(4):373–83. [CrossRef]
- Gallo A, Tandon M, Alevizos I, Illei GG. The Majority of MicroRNAs Detectable in Serum and Saliva Is Concentrated in Exosomes. PLOS ONE. 2012 Mar 9;7(3):e30679.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–97.
- Yapijakis C. Regulatory Role of MicroRNAs in Brain Development and Function. Adv Exp Med Biol. 2020;1195:237–47.
- Sohel MH. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achievements in the Life Sciences. 2016 Dec 1;10(2):175–86.
- Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3.
- Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. IJMS. 2016 Oct 13;17(10):1712.
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009 Feb;10(2):126–39.
- Mouillet-Richard S, Baudry A, Launay JM, Kellermann O. MicroRNAs and depression. Neurobiology of Disease. 2012 May 1;46(2):272–8.
- Gregory RI, Shiekhattar R. MicroRNA Biogenesis and Cancer. Cancer Research. 2005 May 2;65(9):3509–12. [CrossRef]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006 Mar 1;20(5):515–24. [CrossRef]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral Sphingomyelinase 2 (nSMase2)-dependent Exosomal Transfer of Angiogenic MicroRNAs Regulate Cancer Cell Metastasis*. Journal of Biological Chemistry. 2013 Apr 12;288(15):10849–59. [CrossRef]
- Frank F, Sonenberg N, Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010 Jun;465(7299):818–22.
- Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci. 2015;9:476.
- Nowak JS, Michlewski G. miRNAs in development and pathogenesis of the nervous system. Biochem Soc Trans. 2013 Aug;41(4):815–20.
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010 Aug;466(7308):835–40.
- Gao W, Lu X, Liu L, Xu J, Feng D, Shu Y. MiRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol Ther. 2012 Mar;13(5):330–40.
- Altick AL, Baryshnikova LM, Vu TQ, von Bartheld CS. Quantitative Analysis of Multivesicular Bodies (MVBs) in the Hypoglossal Nerve: Evidence that Neurotrophic Factors do not use MVBs for Retrograde Axonal Transport. J Comp Neurol. 2009 Jun 20;514(6):641–57.
- Glebov K, Löchner M, Jabs R, Lau T, Merkel O, Schloss P, et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia. 2015 Apr;63(4):626–34.
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011 Feb;46(2):409–18.
- Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U, et al. Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by miRNAs in Astrocyte-Derived Exosomes. Stem Cells Int. 2017;2017:1719050.
- Zheng T, Pu J, Chen Y, Guo Z, Pan H, Zhang L, et al. Exosomes Secreted from HEK293-APP Swe/Ind Cells Impair the Hippocampal Neurogenesis. Neurotox Res. 2017 Jul;32(1):82–93.
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009 Apr;12(4):399–408.
- Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, et al. Characterization of Small RNAs in Aplysia Reveals a Role for miR-124 in Constraining Synaptic Plasticity through CREB. Neuron. 2009 Sep 24;63(6):803–17. [CrossRef]
- Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010 Apr 5;189(1):127–41. [CrossRef]
- Le MTN, Xie H, Zhou B, Chia PH, Rizk P, Um M, et al. MicroRNA-125b Promotes Neuronal Differentiation in Human Cells by Repressing Multiple Targets. Molecular and Cellular Biology. 2009 Oct 1;29(19):5290–305. [CrossRef]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006 Jan;439(7074):283–9.
- Bátiz LF, Castro MA, Burgos PV, Velásquez ZD, Muñoz RI, Lafourcade CA, et al. Exosomes as Novel Regulators of Adult Neurogenic Niches. Front Cell Neurosci. 2015;9:501.
- Lafourcade C, Ramírez JP, Luarte A, Fernández A, Wyneken U. MiRNAs in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity. J Exp Neurosci. 2016;10(Suppl 1):1–9.
- Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014 Aug;42(14):9195–208.
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010 Dec;15(12):1176–89.
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011 Jan 15;69(2):180–7. [CrossRef]
- Bahrini I, Song J hoon, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep. 2015 Jan 23;5(1):7989. [CrossRef]
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Developmental Neurobiology. 2010;70(5):289–97. [CrossRef]
- Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte–Neuron Communication. PLOS Biology. 2013 Jul 9;11(7):e1001604.
- Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia. 2016 Jun;64(6):896–910.
- Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, et al. Synapsin I Is an Oligomannose-Carrying Glycoprotein, Acts As an Oligomannose-Binding Lectin, and Promotes Neurite Outgrowth and Neuronal Survival When Released via Glia-Derived Exosomes. J Neurosci. 2011 May 18;31(20):7275–90.
- Sbai O, Ould-Yahoui A, Ferhat L, Gueye Y, Bernard A, Charrat E, et al. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia. 2010;58(3):344–66.
- Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: Role of signaling kinases. Developmental Neurobiology. 2007;67(13):1815–29.
- Kato T. Neurobiological basis of bipolar disorder: Mitochondrial dysfunction hypothesis and beyond. Schizophrenia Research. 2017 Sep 1;187:62–6. [CrossRef]
- Rosolen D, Nunes-Souza E, Marchi R, Tofolo MV, Antunes VC, Berti FCB, et al. MiRNAs Action and Impact on Mitochondria Function, Metabolic Reprogramming and Chemoresistance of Cancer Cells: A Systematic Review. Biomedicines. 2023 Feb 24;11(3):693. [CrossRef]
- Macgregor-Das AM, Das S. A microRNA’s journey to the center of the mitochondria. American Journal of Physiology-Heart and Circulatory Physiology. 2018 Aug;315(2):H206–15.
- Duarte FV, Palmeira CM, Rolo AP. The Emerging Role of MitomiRs in the Pathophysiology of Human Disease. Adv Exp Med Biol. 2015;888:123–54.
- Pearce WJ. Mitochondrial influences on smooth muscle phenotype. American Journal of Physiology-Cell Physiology. 2024 Feb;326(2):C442–8.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009 Jul;5(7):374–81.
- Gayen M, Bhomia M, Balakathiresan N, Knollmann-Ritschel B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int J Mol Sci. 2020 Mar 27;21(7):2312.
- Fan C, Li Y, Lan T, Wang W, Long Y, Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Molecular Therapy. 2022 Mar 2;30(3):1300–14.
- Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacol. 2020 May;45(6):1050–8.
- Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013 Oct 30;7:182.
- Dozio V, Sanchez JC. Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J Extracell Vesicles. 2017;6(1):1302705.
- Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008 Jun;29(3):287–91.
- Dalvi P, Sun B, Tang N, Pulliam L. Immune activated monocyte exosomes alter microRNAs in brain endothelial cells and initiate an inflammatory response through the TLR4/MyD88 pathway. Sci Rep. 2017 Aug 30;7(1):9954.
- Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019 Mar 28;9(1):1–11.
- Xian X, Cai LL, Li Y, Wang RC, Xu YH, Chen YJ, et al. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. Journal of Nanobiotechnology. 2022 Mar 9;20(1):122.
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010 Sep 17;329(5998):1537–41. [CrossRef]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008 Apr 15;17(8):1156–68. [CrossRef]
- Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Primers. 2015 Nov 12;1:15067.
- Borroto-Escuela DO, Tarakanov AO, Bechter K, Fuxe K. IL1R2, CCR2, and CXCR4 May Form Heteroreceptor Complexes with NMDAR and D2R: Relevance for Schizophrenia. Front Psychiatry. 2017;8:24.
- Du Y, Yu Y, Hu Y, Li XW, Wei ZX, Pan RY, et al. Genome-Wide, Integrative Analysis Implicates Exosome-Derived MicroRNA Dysregulation in Schizophrenia. Schizophrenia Bulletin. 2019 Oct 24;45(6):1257–66.
- Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr Bull. 2019 Jun 18;45(4):742–51.
- Goetzl EJ, Srihari VH, Guloksuz S, Ferrara M, Tek C, Heninger GR. Neural cell-derived plasma exosome protein abnormalities implicate mitochondrial impairment in first episodes of psychosis. FASEB J. 2021 Feb;35(2):e21339. [CrossRef]
- Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev. 2015 Jan;48:10–21. [CrossRef]
- Kanellopoulos AK, Mariano V, Spinazzi M, Woo YJ, McLean C, Pech U, et al. Aralar Sequesters GABA into Hyperactive Mitochondria, Causing Social Behavior Deficits. Cell. 2020 Mar 19;180(6):1178-1197.e20. [CrossRef]
- Khadimallah I, Jenni R, Cabungcal JH, Cleusix M, Fournier M, Beard E, et al. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2022 Feb;27(2):1192–204.
- Tan G, Wang L, Liu Y, Zhang H, Feng W, Liu Z. The alterations of circular RNA expression in plasma exosomes from patients with schizophrenia. J Cell Physiol. 2021 Jan;236(1):458–67.
- He K, Guo C, Guo M, Tong S, Zhang Q, Sun H, et al. Identification of serum microRNAs as diagnostic biomarkers for schizophrenia. Hereditas. 2019 Jun 27;156(1):23.
- Xu C, Zhang Y, Zheng H, Loh HH, Law PY. Morphine modulates mouse hippocampal progenitor cell lineages by upregulating miR-181a level. Stem Cells. 2014 Nov;32(11):2961–72.
- Gruzdev SK, Yakovlev AA, Druzhkova TA, Guekht AB, Gulyaeva NV. The Missing Link: How Exosomes and miRNAs can Help in Bridging Psychiatry and Molecular Biology in the Context of Depression, Bipolar Disorder and Schizophrenia. Cell Mol Neurobiol. 2019 Aug;39(6):729–50.
- Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015 Apr;16(4):201–12.
- Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, et al. Investigation of miR-1202, miR-135a, and miR-16 in Major Depressive Disorder and Antidepressant Response. Int J Neuropsychopharmacol. 2017 Jun 6;20(8):619–23.
- Camkurt MA, Acar Ş, Coşkun S, Güneş M, Güneş S, Yılmaz MF, et al. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res. 2015 Oct;69:67–71.
- Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, et al. Identification of Differential MicroRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder. PLOS ONE. 2015 Mar 12;10(3):e0121975. [CrossRef]
- Kuang WH, Dong ZQ, Tian LT, Li J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res. 2018;51(7):e7212. [CrossRef]
- Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun. 2017 May 22;8:15497. [CrossRef]
- Enatescu VR, Papava I, Enatescu I, Antonescu M, Anghel A, Seclaman E, et al. Circulating Plasma Micro RNAs in Patients with Major Depressive Disorder Treated with Antidepressants: A Pilot Study. Psychiatry Investig. 2016 Sep;13(5):549–57.
- Capitano F, Camon J, Licursi V, Ferretti V, Maggi L, Scianni M, et al. MicroRNA-335-5p modulates spatial memory and hippocampal synaptic plasticity. Neurobiol Learn Mem. 2017 Mar;139:63–8.
- Li Y, Li S, Yan J, Wang D, Yin R, Zhao L, et al. miR-182 (microRNA-182) suppression in the hippocampus evokes antidepressant-like effects in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:96–103.
- Chiu RWK, Chan LYS, Lam NYL, Tsui NBY, Ng EKO, Rainer TH, et al. Quantitative Analysis of Circulating Mitochondrial DNA in Plasma. Clinical Chemistry. 2003 May 1;49(5):719–26.
- Zhang M, Zhang B, Guo Y, Zhang L, Yang S, Yin L, et al. Alteration of circulating mitochondrial DNA concentration after irradiation. Adv Exp Med Biol. 2013;765:371–7.
- Ogata H, Higasa K, Kageyama Y, Tahara H, Shimamoto A, Takekita Y, et al. Relationship between circulating mitochondrial DNA and microRNA in patients with major depression. Journal of Affective Disorders. 2023 Oct 15;339:538–46.
- Fries GR, Carvalho AF, Quevedo J. The miRNome of bipolar disorder. J Affect Disord. 2018 Jun;233:110–6.
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, et al. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015 May;20(5):573–84.
- Azevedo JA, Carter BS, Meng F, Turner DL, Dai M, Schatzberg AF, et al. The microRNA network is altered in anterior cingulate cortex of patients with unipolar and bipolar depression. J Psychiatr Res. 2016 Nov;82:58–67.
- Mokhtari MA, Sargazi S, Saravani R, Heidari Nia M, Mirinejad S, Hadzsiev K, et al. Genetic Polymorphisms in miR-137 and Its Target Genes, TCF4 and CACNA1C, Contribute to the Risk of Bipolar Disorder: A Preliminary Case-Control Study and Bioinformatics Analysis. Dis Markers. 2022 Sep 22;2022:1886658.
- Ceylan D, Tufekci KU, Keskinoglu P, Genc S, Özerdem A. Circulating exosomal microRNAs in bipolar disorder. J Affect Disord. 2020 Feb 1;262:99–107.
- Wang K, Long B, Jiao JQ, Wang JX, Liu JP, Li Q, et al. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun. 2012 Apr 17;3(1):781.
- Andreazza AC, Young LT. The neurobiology of bipolar disorder: identifying targets for specific agents and synergies for combination treatment. Int J Neuropsychopharmacol. 2014 Jul;17(7):1039–52.
- Shrestha A, Mukhametshina RT, Taghizadeh S, Vásquez-Pacheco E, Cabrera-Fuentes H, Rizvanov A, et al. MicroRNA-142 is a multifaceted regulator in organogenesis, homeostasis, and disease. Dev Dyn. 2017 Apr;246(4):285–90.
- Fries GR, Lima CNC, Valvassori SS, Zunta-Soares G, Soares JC, Quevedo J. Preliminary investigation of peripheral extracellular vesicles’ microRNAs in bipolar disorder. J Affect Disord. 2019 Aug 1;255:10–4.
- Smalheiser NR, Lugli G, Rizavi HS, Torvik VI, Turecki G, Dwivedi Y. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS One. 2012;7(3):e33201.
- Gilicze AB, Wiener Z, Tóth S, Buzás E, Pállinger É, Falcone FH, et al. Myeloid-derived microRNAs, miR-223, miR27a, and miR-652, are dominant players in myeloid regulation. Biomed Res Int. 2014;2014:870267.
- Maussion G, Yang J, Yerko V, Barker P, Mechawar N, Ernst C, et al. Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers. PLoS One. 2012;7(6):e39301.
- Kohli MA, Salyakina D, Pfennig A, Lucae S, Horstmann S, Menke A, et al. Association of Genetic Variants in the Neurotrophic Receptor–Encoding Gene NTRK2 and a Lifetime History of Suicide Attempts in Depressed Patients. Arch Gen Psychiatry. 2010 Apr;67(4):348–59.
- Soria Lopez JA, González HM, Léger GC. Alzheimer’s disease. Handb Clin Neurol. 2019;167:231–55.
- Gao G, Zhao S, Xia X, Li C, Li C, Ji C, et al. Glutaminase C Regulates Microglial Activation and Pro-inflammatory Exosome Release: Relevance to the Pathogenesis of Alzheimer’s Disease. Front Cell Neurosci. 2019;13:264.
- Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Translational Medicine. 2020 Sep 1;9(9):1068–84.
- Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer’s Disease. Front Neurosci. 2020;14:564.
- Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin-loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau-mediated neurofibrillary tangles. Drug Delivery. 2020 Jan 1;27(1):745–55.
- Cai Q, Tammineni P. Mitochondrial Aspects of Synaptic Dysfunction in Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2017;57(4):1087–103.
- John A, Kubosumi A, Reddy PH. Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells. 2020 May 28;9(6):1345.
- Herrera F, Tenreiro S, Miller-Fleming L, Outeiro TF. Visualization of cell-to-cell transmission of mutant huntingtin oligomers. PLoS Curr. 2011 Feb 11;3:RRN1210.
- Yapijakis C. Ancestral Concepts of Human Genetics and Molecular Medicine in Epicurean Philosophy. In: Petermann HI, Harper PS, Doetz S, editors. History of Human Genetics: Aspects of Its Development and Global Perspectives [Internet]. Cham: Springer International Publishing; 2017 [cited 2023 Sep 14]. p. 41–57. [CrossRef]
- Vella LJ, Sharples RA, Nisbet RM, Cappai R, Hill AF. The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008 Mar 1;37(3):323–32. [CrossRef]
- Pearce MMP, Spartz EJ, Hong W, Luo L, Kopito RR. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun. 2015 Apr 13;6(1):6768. [CrossRef]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009 Feb;11(2):219–25. [CrossRef]
- Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The Bifunctional microRNA miR-9/miR-9* Regulates REST and CoREST and Is Downregulated in Huntington’s’s Disease. J Neurosci. 2008 Dec 31;28(53):14341–6.
- Sinha M, Ghose J, Das E, Bhattarcharyya NP. Altered microRNAs in STHdhQ111/HdhQ111 cells: miR-146a targets TBP. Biochemical and Biophysical Research Communications. 2010 Jun 4;396(3):742–7.
- Hoss AG, Labadorf A, Latourelle JC, Kartha VK, Hadzi TC, Gusella JF, et al. miR-10b-5p expression in Huntington’s’s disease brain relates to age of onset and the extent of striatal involvement. BMC Medical Genomics. 2015 Mar 1;8(1):10.
- Dong X, Cong S. MicroRNAs in Huntington’s’s Disease: Diagnostic Biomarkers or Therapeutic Agents? Front Cell Neurosci. 2021 Aug 6;15:705348.
- Yang T, Nie Z, Shu H, Kuang Y, Chen X, Cheng J, et al. The Role of BDNF on Neural Plasticity in Depression. Front Cell Neurosci. 2020 Apr 15;14:82.
- Zhan-Qiang H, Hai-Hua Q, Chi Z, Miao W, Cui Z, Zi-Yin L, et al. miR-146a aggravates cognitive impairment and Alzheimer disease-like pathology by triggering oxidative stress through MAPK signaling. Neurologia (Engl Ed). 2021 Mar 11;S0213-4853(21)00022-0.
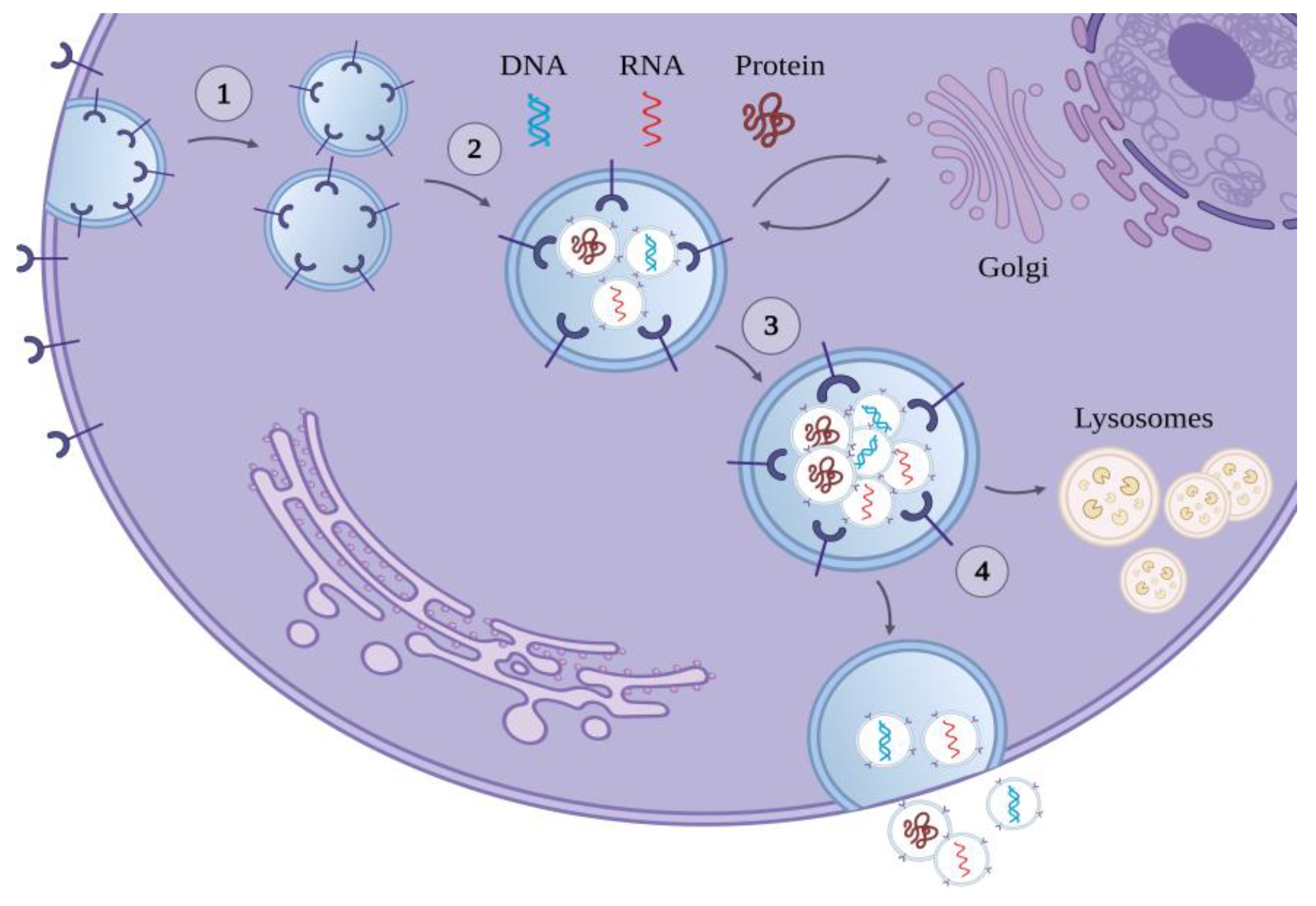
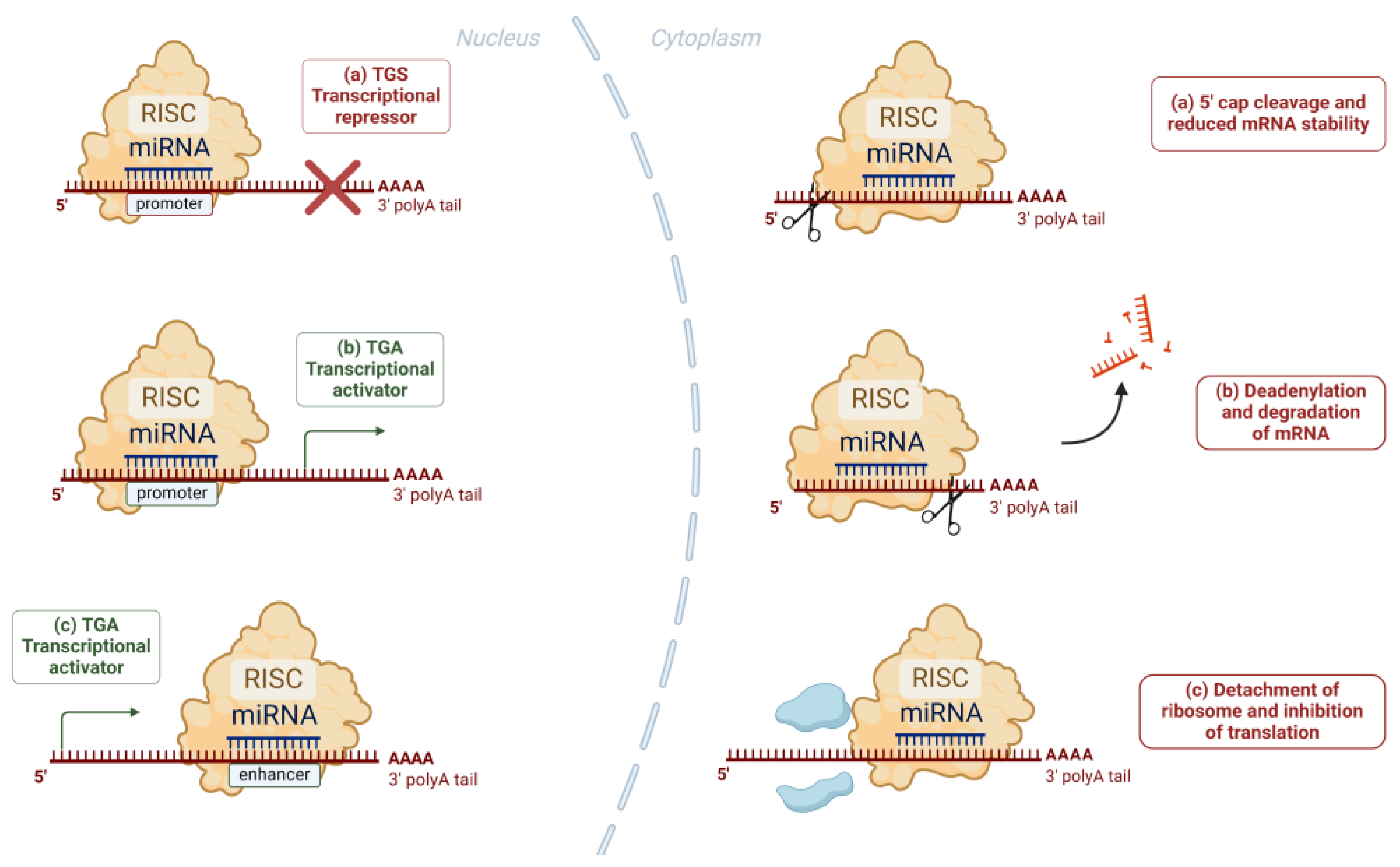
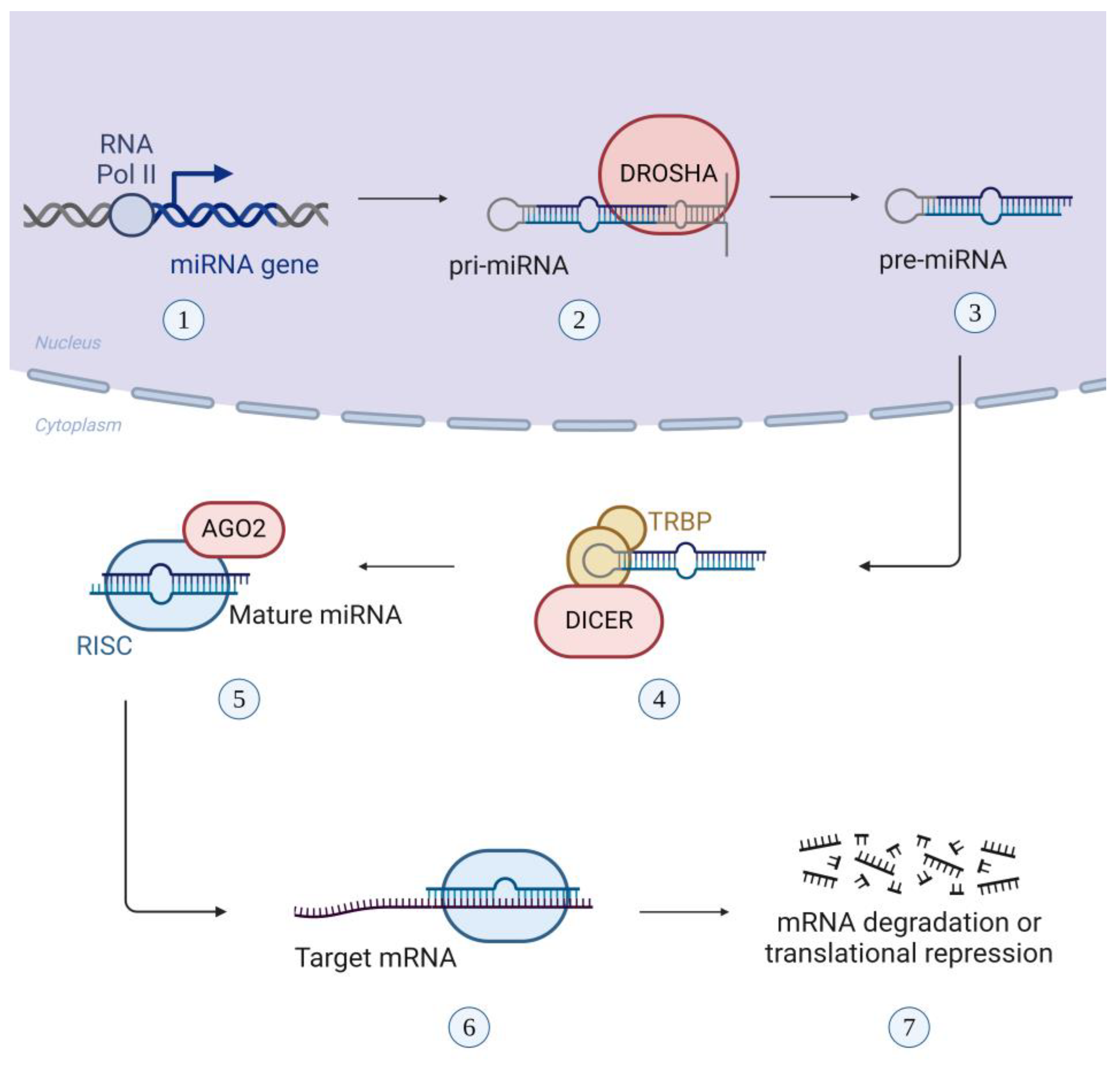
| Disease | miRNA | Alteration |
|---|---|---|
| Alzheimer’s disease | miR-15a | ↑ [114] |
| miR-132 | ↓ [114] | |
| miR-146a-5pa | ↑ [125] | |
| Huntington’s disease | miR-9 | ↓ [120] |
| miR-10b-5p | ↑ [122] | |
| miR-128 | ↓ [114] | |
| miR-146a | ↓ [121] | |
| miR-196a | ↑ [123] | |
| miR-214 | ↑ [123] | |
| Bipolar Disorder | miR-34a | ↑ [96]/ ↓ [97] |
| miR-137 | ↑ [98] | |
| miR-142-3p | ↓ [99] | |
| miR-185-5p | ↑ [99] | |
| miR-484 | ↓ [99] | |
| miR-652-3p | ↓ [99] | |
| Major Depressive Disorder | let-7d | ↑ [86] |
| miR-24-3p | ↑ [88] | |
| miR-26a | ↑ [89] | |
| miR-34a-5p | ↑ [86] | |
| miR-135a | ↓ [83] | |
| miR-182 | ↑ [91] | |
| miR-187-5p | Positive correlation with ccf-mtDNA copy number [94] | |
| miR-221-3p | ↑ [86] | |
| miR-335-5p | ↑ [90] | |
| miR-425-3p | ↑ [88] | |
| miR-451a | ↑ [85] | |
| miR-939-5p | Positive correlation with ccf-mtDNA copy number [94] | |
| miR-1202 | ↓ [84] | |
| miR-4707-3p | Positive correlation with ccf-mtDNA copy number [94] | |
| miR-6068 | Positive correlation with ccf-mtDNA copy number [94] | |
| miR-7110-5p | Positive correlation with ccf-mtDNA copy number [94] | |
| Schizophrenia | miR-34a-5p + miR-499a | ↑ [80] |
| miR-137 | ↑ [78] | |
| miR-206 | ↑ [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





