Submitted:
04 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Motivation
3. Materials and Methods
4. Results
5. Conclusion
6. Discussion
7. Acknowledgment
8. Ethical Statement
9. Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
Author Contributions
Funding
Conflicts of Interest
References
- Marijic, J.; Neelankavil, J.P. Semaglutide: A New Medical Swiss Army Knife? Journal of Cardiothoracic and Vascular Anesthesia 2024, 38, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Cowart, K. Oral Semaglutide: First-in-Class Oral GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes Mellitus. Annals of Pharmacotherapy 2019, 54, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Frontiers in Endocrinology 2019, 10, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proceedings of the National Academy of Sciences 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Han, J.; Fu, J.; Yang, Q.; Zhou, F.; Chen, X.; Li, C.; Yin, J. Rational design and biological evaluation of gemfibrozil modified Xenopus GLP-1 derivatives as long-acting hypoglycemic agents. European Journal of Medicinal Chemistry 2020, 198, 112389. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, D.S. New Drug for Type 2 Diabetes. AJN, American Journal of Nursing 2020, 120, 25. [Google Scholar] [CrossRef]
- Ahrén, B.; Atkin, S.L.; Charpentier, G.; Warren, M.L.; Wilding, J.P.H.; Birch, S.; Holst, A.G.; Leiter, L.A. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes, Obesity and Metabolism 2018, 20, 2210–2219. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jun, H.S. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014, 63, 9–19. [Google Scholar] [CrossRef]
- Li, W. Strengthening Semaglutide-GLP-1R Binding Affinity via a Val27-Arg28 Exchange in the Peptide Backbone of Semaglutide: A Computational Structural Approach. Journal of Computational Biophysics and Chemistry 2021, 20, 495–499. [Google Scholar] [CrossRef]
- Wang, W.; Volkow, N.D.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Xu, R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nature Medicine 2024, 30, 168–176. [Google Scholar] [CrossRef]
- Li, W. Designing Insulin Analogues with Lower Binding Affinity to Insulin Receptor than That of Insulin Icodec 2024. [CrossRef]
- Li, W. Delving Deep into the Structural Aspects of the BPro28-BLys29 Exchange in Insulin Lispro: A Structural Biophysical Lesson 2020.
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; Jensen, T.J.; Liisberg, K.; Lindegaard, M.L.; Abhayaratna, W.; Ahmed, F.Z.; Ben-Gal, T.; Chopra, V.; Ezekowitz, J.A.; Fu, M.; Ito, H.; Lelonek, M.; Melenovský, V.; Merkely, B.; Núñez, J.; Perna, E.; Schou, M.; Senni, M.; Sharma, K.; van der Meer, P.; Von Lewinski, D.; Wolf, D.; Shah, S.J. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. New England Journal of Medicine 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H. Semaglutide in weight management: Author’s reply. The Lancet 2019, 394, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, J.D.; Pamulapati, L.G.; Carter, N.; Malloy, K.; Dixon, D.L.; Sisson, E.M. Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist. Diabetes Technology & Therapeutics 2020, 22, 10–18. [Google Scholar]
- Kanters, S.; Wilkinson, L.; Vrazic, H.; Sharma, R.; Lopes, S.; Popoff, E.; Druyts, E. Comparative efficacy of once-weekly semaglutide versus SGLT-2 inhibitors in patients inadequately controlled with one to two oral antidiabetic drugs: a systematic literature review and network meta-analysis. BMJ Open 2019, 9, e023458. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Verma, S.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Jon Jensen, T.; Rasmussen, S.; Erlang Marstrand, P.; Petrie, M.C.; Shah, S.J.; Ito, H.; Schou, M.; Melenovský, V.; Abhayaratna, W.; Kitzman, D.W. Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients With Heart Failure With Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial. Circulation 2024, 149, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of Glucose Homeostasis by GLP-1. In Progress in Molecular Biology and Translational Science; Elsevier, 2014; pp. 23–65.
- Bucheit, J.D.; Pamulapati, L.G.; Carter, N.; Malloy, K.; Dixon, D.L.; Sisson, E.M. Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist. Diabetes Technology & Therapeutics 2020, 22, 10–18. [Google Scholar]
- Granhall, C.; Donsmark, M.; Blicher, T.M.; Golor, G.; Sondergaard, F.L.; Thomsen, M.; Bakdal, T.A. Safety and Pharmacokinetics of Single and Multiple Ascending Doses of the Novel Oral Human GLP-1 Analogue, Oral Semaglutide, in Healthy Subjects and Subjects with Type 2 Diabetes. Clinical Pharmacokinetics 2018, 58, 781–791. [Google Scholar] [CrossRef]
- Garg, S.K.; Kaur, G.; Haider, Z.; Rodriquez, E.; Beatson, C.; Snell-Bergeon, J. Efficacy of Semaglutide in Overweight and Obese Patients with Type 1 Diabetes. Diabetes Technology & Therapeutics 2024, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Europe, T.L.R.H. Semaglutide and beyond a turning point in obesity pharmacotherapy. The Lancet Regional Health Europe 2024, 37, 100860. [Google Scholar] [CrossRef]
- Fuji, H.; Qi, F.; Qu, L.; Takaesu, Y.; Hoshino, T. Prediction of Ligand Binding Affinity to Target Proteins by Molecular Mechanics Theoretical Calculation. Chemical and Pharmaceutical Bulletin 2017, 65, 461–468. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nature Structural & Molecular Biology 2003, 10, 980–980. [Google Scholar]
- Li, W. Half-a-century Burial of ρ, θ and φ in PDB 2021. [CrossRef]
- Li, W. Visualising the Experimentally Uncharted Territories of Membrane Protein Structures inside Protein Data Bank 2020.
- Li, W. A Local Spherical Coordinate System Approach to Protein 3D Structure Description 2020.
- Li, W. Structurally Observed Electrostatic Features of the COVID-19 Coronavirus-Related Experimental Structures inside Protein Data Bank: A Brief Update 2020.
- Li, W. How do SMA-linked mutations of SMN1 lead to structural/functional deficiency of the SMA protein? PLOS ONE 2017, 12, e0178519. [Google Scholar] [CrossRef]
- Li, W. Extracting the Interfacial Electrostatic Features from Experimentally Determined Antigen and/or Antibody-Related Structures inside Protein Data Bank for Machine Learning-Based Antibody Design 2020.
- Li, W. Structural Identification of the Electrostatic Hot Spots for Severe Acute Respiratory Syndrome Coronavirus Spike Protein to Be Complexed with Its Receptor ACE2 and Its Neutralizing Antibodies 2020.
- Li, W. Calcium Channel Trafficking Blocker Gabapentin Bound to the -2–1 Subunit of Voltage-Gated Calcium Channel: A Computational Structural Investigation 2020.
- Li, W. Inter-Molecular Electrostatic Interactions Stabilizing the Structure of the PD-1/PD-L1 Axis: A Structural Evolutionary Perspective 2020.
- Li, W. Structural and Functional Consequences of the SMA-Linked Missense Mutations of the Survival Motor Neuron Protein: A Brief Update. In Novel Aspects on Motor Neuron Disease; IntechOpen, 2019.
- Li, W. Designing Nerve Growth Factor Analogues to Suppress Pain Signal Transduction Mediated by the p75NTR-NGF-TrkA Complex: A Structural and Biophysical Perspective 2024. [CrossRef]
- Aroda, V.R.; Rosenstock, J.; Terauchi, Y.; Altuntas, Y.; Lalic, N.M.; Villegas, E.C.M.; Jeppesen, O.K.; Christiansen, E.; Hertz, C.L.; Haluzík, M. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 1724–1732. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsletter On Protein Crystallography 2002, 40, 82–92. [Google Scholar]
- Blüher, M.; Rosenstock, J.; Hoefler, J.; Manuel, R.; Hennige, A.M. Dose–response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: a randomised clinical trial. Diabetologia 2023, 67, 470–482. [Google Scholar] [CrossRef]
- Lau, J.; Bloch, P.; Schäffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; Strauss, H.M.; Gram, D.X.; Knudsen, S.M.; Nielsen, F.S.; Thygesen, P.; Reedtz-Runge, S.; Kruse, T. Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. Journal of Medicinal Chemistry 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Beutel, T.R.; Trujillo, J.M. Oral semaglutide in type 2 diabetes. Journal of Diabetes and its Complications 2020, 34, 107520. [Google Scholar] [CrossRef]
- Pieber, T.R.; Bode, B.; Mertens, A.; Cho, Y.M.; Christiansen, E.; Hertz, C.L.; Wallenstein, S.O.R.; Buse, J.B.; Akın, S.; Aladağ, N.; Arif, A.A.; Aronne, L.J.; Aronoff, S.; Ataoglu, E.; Baik, S.H.; Bays, H.; Beckett, P.L.; Berker, D.; Bilz, S.; Bode, B.; Braun, E.W.; Buse, J.B.; Canani, L.H.S.; Cho, Y.M.; Chung, C.H.; Colin, I.; Condit, J.; Cooper, J.; Delgado, B.; Eagerton, D.C.; Ebrashy, I.N.E.; Hefnawy, M.H.M.F.E.; Eliaschewitz, F.G.; Finneran, M.P.; Fischli, S.; Fließer-Görzer, E.; Geohas, J.; Godbole, N.A.; Golay, A.; de Lapertosa, S.G.; Gross, J.L.; Gulseth, H.L.; Helland, F.; Høivik, H.O.; Issa, C.; Kang, E.S.; Keller, C.; Khalil, S.H.A.; Kim, N.H.; Kim, I.J.; Klaff, L.J.; Laimer, M.; LaRocque, J.C.; Lederman, S.N.; Lee, K.W.; Litchfield, W.R.; Manning, M.B.; Mertens, A.; Morawski, E.J.; Murray, A.V.; Nicol, P.R.; O’Connor, T.M.; Oğuz, A.; Ong, S.; özdemir, A.; Palace, E.M.; Palchick, B.A.; Pereles-Ortiz, J.; Pieber, T.; Prager, R.; Preumont, V.; Riffer, E.; Rista, L.; Rudofsky, G.; Sarı, R.; Scheen, A.; Schultes, B.; Seo, J.A.; Shelbaya, S.A.; Sivalingam, K.; Sorli, C.H.; Stäuble, S.; Streja, D.A.; T’Sjoen, G.; Tetiker, T.; Gaal, L.V.; Vercammen, C.; Warren, M.L.; Weinstein, D.L.; Weiss, D.; White, A.; Winnie, M.; Wium, C.; Yavuz, D. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. The Lancet Diabetes & Endocrinology 2019, 7, 528–539. [Google Scholar]
- Zhang, X.; Belousoff, M.; Danev, R.; Sexton, P.; Wootten, D. Semaglutide-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in Complex with Gs protein, 2021. [CrossRef]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. In Methods in Molecular Biology; Springer US, 2020; pp. 239–255.
- Vangone, A.; Bonvin, A.M. Contacts-based prediction of binding affinity in protein-protein complexes. eLife 2015, 4. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: a web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, p. btw514.
- Li, W.; Vottevor, G. Towards a Truly General Intermolecular Binding Affinity Calculator for Drug Discovery & Design 2023. [CrossRef]
- Carris, N.W.; Wallace, S.; DuCoin, C.G.; Mhaskar, R.; Stern, M.; Bunnell, B. Discontinuing semaglutide after weight loss: strategy for weight maintenance and a possible new side effect. Canadian Journal of Physiology and Pharmacology 2024. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W.; Smith, B.A. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Therapeutic Advances in Endocrinology and Metabolism 2021, 12, 204201882199732. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, J.; Lan, B.; Gao, Y.; Zhao, J. Targeting off-target effects: endoplasmic reticulum stress and autophagy as effective strategies to enhance temozolomide treatment. OncoTargets and Therapy 2019, Volume 12, 1857–1865. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Frontiers in Endocrinology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2022, 16, 102427. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Towards a General Intermolecular Binding Affinity Calculator 2022.
- Li, W. High-Throughput Extraction of Interfacial Electrostatic Features from GLP-1-GLP-1R Complex Structures: A GLP-1-GLP-1R-Based Mini GIBAC Perspective 2024. [CrossRef]
- Li, W.; Shi, G. How CaV1.2-bound verapamil blocks Ca2+ influx into cardiomyocyte: Atomic level views. Pharmacological Research 2019, 139, 153–157. [Google Scholar] [CrossRef]
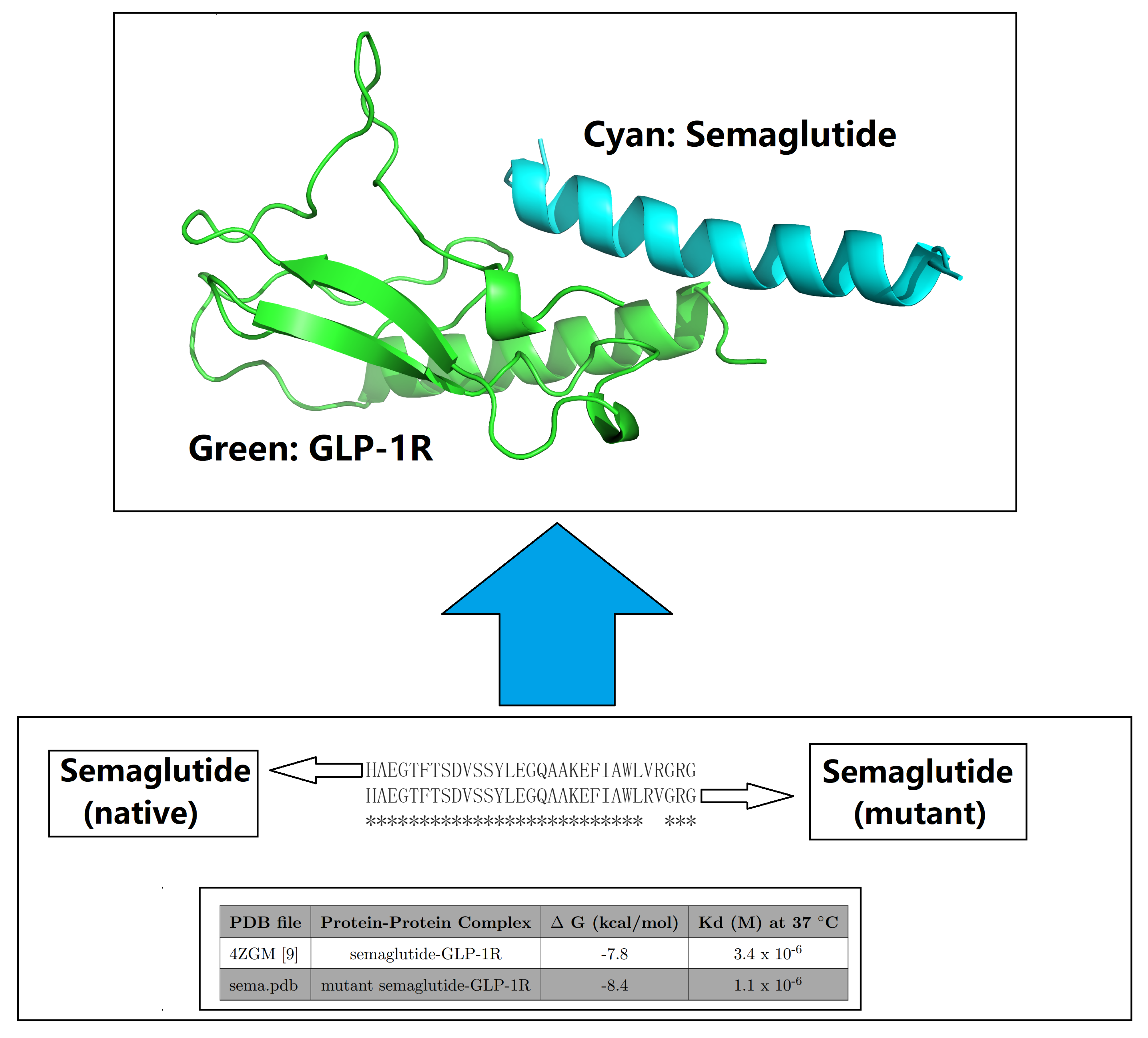
| PDB ID | Structure Title (release date from newest to oldest) |
|---|---|
| 7KI0 | Semaglutide-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in Complex with Gs protein |
| PDB ID | Structure Title (release date from newest to oldest) |
|---|---|
| 7KI0 | Semaglutide-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in Complex with Gs protein |
| 7KI1 | Taspoglutide-bound Glucagon-Like Peptide-1 (GLP-1) Receptor in Complex with Gs Protein |
| 4ZGM | Crystal structure of Semaglutide peptide backbone in complex with the GLP-1 receptor extracellular domain |
| No. | Muta1 | Muta2 | Muta3 | Muta4 | Min | Max | Mean | Std |
|---|---|---|---|---|---|---|---|---|
| 1 | G13B_A | I20B_Q | L23B_Q | V24B_N | 5.3E-08 | 2.2E-07 | 1.337E-07 | 4.778E-08 |
| 2 | G13B_A | I20B_N | L23B_R | V24B_N | 6.5E-08 | 2.4E-07 | 1.344E-07 | 4.996E-08 |
| 3 | G13B_A | I20B_N | L23B_Q | V24B_T | 6.6E-08 | 2.2E-07 | 1.376E-07 | 4.199E-08 |
| 4 | G13B_A | I20B_T | L23B_Q | V24B_N | 8.0E-08 | 3.1E-07 | 1.404E-07 | 5.478E-08 |
| 5 | G13B_A | I20B_Q | L23B_Q | V24B_T | 6.8E-08 | 2.0E-07 | 1.407E-07 | 3.779E-08 |
| 6 | G13B_A | I20B_S | L23B_R | V24B_T | 6.1E-08 | 2.5E-07 | 1.408E-07 | 5.527E-08 |
| 7 | G13B_A | I20B_Q | L23B_R | V24B_N | 3.0E-08 | 3.2E-07 | 1.461E-07 | 7.095E-08 |
| 8 | G13B_A | I20B_T | L23B_R | V24B_N | 8.3E-08 | 2.1E-07 | 1.467E-07 | 3.690E-08 |
| 9 | G13B_A | I20B_N | L23B_R | V24B_Q | 6.3E-08 | 2.9E-07 | 1.487E-07 | 5.848E-08 |
| 10 | G13B_A | I20B_Q | L23B_R | V24B_Q | 8.6E-08 | 2.5E-07 | 1.489E-07 | 5.170E-08 |
| 11 | G13B_A | I20B_Q | L23B_Q | V24B_Q | 6.3E-08 | 2.4E-07 | 1.505E-07 | 5.269E-08 |
| 12 | G13B_A | I20B_S | L23B_R | V24B_N | 4.4E-08 | 3.5E-07 | 1.520E-07 | 6.568E-08 |
| 13 | G13B_A | I20B_T | L23B_R | V24B_T | 9.4E-08 | 2.2E-07 | 1.545E-07 | 4.188E-08 |
| 14 | G13B_A | I20B_N | L23B_Q | V24B_N | 7.7E-08 | 2.2E-07 | 1.559E-07 | 4.164E-08 |
| 15 | G13B_A | I20B_S | L23B_R | V24B_Q | 7.7E-08 | 3.0E-07 | 1.571E-07 | 6.401E-08 |
| 16 | G13B_A | I20B_S | F19B_Q | V24B_N | 3.5E-08 | 2.8E-07 | 1.583E-07 | 6.648E-08 |
| 17 | G13B_A | I20B_N | L23B_Q | V24B_Q | 8.2E-08 | 2.9E-07 | 1.602E-07 | 5.879E-08 |
| 18 | G13B_A | I20B_N | F19B_Q | V24B_N | 5.0E-08 | 2.9E-07 | 1.634E-07 | 7.035E-08 |
| 19 | G13B_A | I20B_T | F19B_Q | V24B_Q | 9.7E-08 | 2.9E-07 | 1.653E-07 | 4.839E-08 |
| 20 | G13B_A | I20B_N | L23B_R | V24B_T | 8.0E-08 | 3.4E-07 | 1.662E-07 | 8.233E-08 |
| 21 | G13B_A | I20B_S | L23B_Q | V24B_Q | 7.8E-08 | 3.4E-07 | 1.682E-07 | 7.062E-08 |
| 22 | G13B_A | I20B_Q | F19B_Q | V24B_T | 3.6E-08 | 3.3E-07 | 1.686E-07 | 7.653E-08 |
| 23 | G13B_A | I20B_S | L23B_Q | V24B_N | 6.2E-08 | 3.0E-07 | 1.703E-07 | 6.652E-08 |
| 24 | G13B_A | E18B_T | I20B_Q | V24B_Q | 8.4E-08 | 2.7E-07 | 1.706E-07 | 5.558E-08 |
| 25 | G13B_A | I20B_T | F19B_Q | V24B_N | 6.3E-08 | 2.9E-07 | 1.711E-07 | 7.268E-08 |
| 26 | Y10B_A | I20B_Q | L23B_Q | V24B_Q | 7.3E-08 | 3.8E-07 | 1.725E-07 | 8.816E-08 |
| 27 | G13B_A | I20B_S | F19B_Q | V24B_T | 6.2E-08 | 3.2E-07 | 1.725E-07 | 6.884E-08 |
| 28 | G13B_A | I20B_T | L23B_R | V24B_Q | 5.5E-08 | 3.0E-07 | 1.725E-07 | 6.902E-08 |
| 29 | G13B_A | I20B_T | F19B_Q | V24B_T | 6.5E-08 | 3.3E-07 | 1.765E-07 | 7.216E-08 |
| 30 | G13B_A | I20B_T | L23B_Q | V24B_T | 9.2E-08 | 3.4E-07 | 1.765E-07 | 7.525E-08 |
| 31 | G13B_A | I20B_Q | L23B_R | V24B_T | 8.3E-08 | 3.7E-07 | 1.778E-07 | 7.613E-08 |
| 32 | G13B_A | I20B_S | L23B_Q | V24B_T | 7.8E-08 | 3.6E-07 | 1.785E-07 | 6.839E-08 |
| 33 | G13B_A | I20B_T | L23B_Q | V24B_Q | 9.1E-08 | 2.9E-07 | 1.791E-07 | 4.888E-08 |
| 34 | G13B_A | I20B_Q | K17B_S | V24B_N | 7.5E-08 | 2.9E-07 | 1.822E-07 | 7.042E-08 |
| 35 | G13B_A | I20B_Q | K17B_S | V24B_T | 8.2E-08 | 2.9E-07 | 1.826E-07 | 5.543E-08 |
| 36 | G13B_A | I20B_Q | S9B_A | V24B_N | 7.7E-08 | 3.5E-07 | 1.836E-07 | 6.655E-08 |
| 37 | G13B_A | E18B_T | I20B_Q | V24B_N | 9.5E-08 | 2.7E-07 | 1.842E-07 | 5.523E-08 |
| 38 | Y10B_A | I20B_Q | L23B_R | V24B_N | 8.1E-08 | 4.7E-07 | 1.851E-07 | 9.983E-08 |
| 39 | G13B_A | I20B_Q | F19B_Q | V24B_N | 6.0E-08 | 3.1E-07 | 1.869E-07 | 6.942E-08 |
| 40 | G13B_A | I20B_Q | F19B_Q | V24B_Q | 7.3E-08 | 3.4E-07 | 1.870E-07 | 7.308E-08 |
| 41 | Y10B_A | I20B_N | L23B_R | V24B_Q | 8.9E-08 | 3.7E-07 | 1.890E-07 | 7.203E-08 |
| 42 | G13B_A | I20B_Q | S9B_A | V24B_T | 9.1E-08 | 3.6E-07 | 1.895E-07 | 7.000E-08 |
| 43 | G13B_A | I20B_Q | S9B_A | L23B_R | 8.6E-08 | 4.6E-07 | 1.900E-07 | 8.860E-08 |
| 44 | G13B_A | I20B_N | F19B_Q | V24B_Q | 6.8E-08 | 3.4E-07 | 1.909E-07 | 6.762E-08 |
| 45 | G13B_A | I20B_S | F19B_Q | V24B_Q | 8.8E-08 | 3.5E-07 | 1.926E-07 | 7.739E-08 |
| 46 | Y10B_A | I20B_Q | L23B_R | V24B_T | 9.9E-08 | 3.5E-07 | 1.934E-07 | 7.962E-08 |
| 47 | Y10B_A | I20B_Q | L23B_R | V24B_Q | 6.6E-08 | 3.3E-07 | 1.951E-07 | 7.414E-08 |
| 48 | G13B_A | I20B_Q | K17B_S | V24B_Q | 8.3E-08 | 3.6E-07 | 1.962E-07 | 6.561E-08 |
| 49 | Y10B_A | I20B_T | L23B_R | V24B_T | 1.1E-07 | 3.4E-07 | 1.970E-07 | 6.689E-08 |
| 50 | G13B_A | I20B_T | K17B_S | V24B_N | 1.1E-07 | 3.0E-07 | 1.975E-07 | 5.524E-08 |
| 52 | Y10B_A | I20B_N | L23B_R | V24B_T | 1.1E-07 | 3.5E-07 | 1.985E-07 | 6.784E-08 |
| 53 | G13B_A | I20B_Q | S9B_A | V24B_Q | 9.0E-08 | 3.0E-07 | 1.985E-07 | 6.548E-08 |
| 54 | G13B_A | I20B_S | F19B_Q | L23B_R | 1.2E-07 | 3.1E-07 | 1.990E-07 | 5.077E-08 |
| 55 | G13B_A | I20B_N | K17B_S | V24B_N | 1.2E-07 | 4.4E-07 | 2.015E-07 | 7.264E-08 |
| 56 | G13B_A | I20B_S | F19B_Q | L23B_Q | 7.7E-08 | 4.1E-07 | 2.023E-07 | 9.647E-08 |
| 57 | G13B_A | I20B_S | L23B_R | K17B_S | 1.1E-07 | 2.8E-07 | 2.030E-07 | 4.736E-08 |
| 58 | Y10B_A | I20B_T | L23B_R | V24B_N | 8.9E-08 | 4.4E-07 | 2.034E-07 | 7.708E-08 |
| 59 | G13B_A | I20B_N | K17B_S | V24B_Q | 1.0E-07 | 5.2E-07 | 2.035E-07 | 8.707E-08 |
| 60 | Y10B_A | I20B_S | L23B_R | V24B_T | 8.6E-08 | 3.5E-07 | 2.038E-07 | 7.595E-08 |
| 61 | G13B_A | I20B_N | S9B_A | L23B_R | 1.2E-07 | 3.8E-07 | 2.050E-07 | 8.300E-08 |
| 62 | G13B_A | I20B_N | F19B_Q | V24B_T | 8.4E-08 | 4.1E-07 | 2.067E-07 | 7.351E-08 |
| 63 | G13B_A | I20B_N | L23B_R | K17B_S | 1.1E-07 | 3.8E-07 | 2.070E-07 | 8.228E-08 |
| 64 | G13B_A | E18B_T | I20B_Q | L23B_Q | 1.1E-07 | 3.4E-07 | 2.080E-07 | 7.317E-08 |
| 65 | G13B_A | I20B_N | L23B_Q | K17B_S | 1.1E-07 | 3.6E-07 | 2.080E-07 | 7.557E-08 |
| 66 | G13B_A | I20B_T | F19B_Q | L23B_R | 6.7E-08 | 3.5E-07 | 2.097E-07 | 9.136E-08 |
| 67 | G13B_A | I20B_S | K17B_S | V24B_N | 8.6E-08 | 3.6E-07 | 2.098E-07 | 7.261E-08 |
| 68 | Y10B_A | I20B_N | F19B_Q | V24B_Q | 7.6E-08 | 3.9E-07 | 2.098E-07 | 8.400E-08 |
| 69 | Y10B_A | I20B_N | L23B_R | V24B_N | 1.1E-07 | 3.4E-07 | 2.100E-07 | 6.696E-08 |
| 70 | G13B_A | I20B_T | S9B_A | L23B_R | 8.9E-08 | 3.8E-07 | 2.104E-07 | 7.158E-08 |
| 71 | Y10B_A | I20B_T | L23B_R | V24B_Q | 1.2E-07 | 3.2E-07 | 2.105E-07 | 5.698E-08 |
| 72 | G13B_A | I20B_Q | L23B_R | K17B_S | 8.2E-08 | 7.9E-07 | 2.120E-07 | 1.610E-07 |
| 73 | G13B_A | I20B_T | K17B_S | V24B_Q | 9.2E-08 | 4.2E-07 | 2.121E-07 | 7.875E-08 |
| 74 | G13B_A | I20B_N | E18B_T | V24B_N | 1.3E-07 | 3.8E-07 | 2.125E-07 | 7.181E-08 |
| 75 | G13B_A | I20B_T | L23B_R | K17B_S | 9.5E-08 | 4.5E-07 | 2.128E-07 | 9.408E-08 |
| 76 | G13B_A | E18B_T | I20B_S | V24B_T | 1.0E-07 | 3.5E-07 | 2.135E-07 | 7.081E-08 |
| 77 | G13B_A | I20B_Q | F19B_W | V24B_Q | 8.9E-08 | 3.9E-07 | 2.149E-07 | 8.920E-08 |
| 78 | Y10B_A | I20B_Q | L23B_Q | V24B_N | 6.7E-08 | 4.5E-07 | 2.161E-07 | 1.064E-07 |
| 79 | Y10B_A | I20B_N | L23B_Q | V24B_Q | 9.7E-08 | 3.3E-07 | 2.168E-07 | 5.626E-08 |
| 80 | G13B_A | I20B_N | E18B_T | L23B_R | 1.1E-07 | 4.0E-07 | 2.175E-07 | 9.118E-08 |
| 81 | G13B_A | I20B_Q | V24B_Q | E12B_A | 1.0E-07 | 3.9E-07 | 2.175E-07 | 8.175E-08 |
| 82 | Y10B_A | I20B_S | L23B_Q | V24B_Q | 8.7E-08 | 3.4E-07 | 2.179E-07 | 7.437E-08 |
| 83 | G13B_A | I20B_N | F19B_Q | L23B_R | 8.9E-08 | 4.4E-07 | 2.179E-07 | 9.986E-08 |
| 84 | Y10B_A | I20B_Q | K17B_S | V24B_T | 1.1E-07 | 4.0E-07 | 2.195E-07 | 7.970E-08 |
| 85 | G13B_A | I20B_S | S9B_A | V24B_Q | 9.9E-08 | 3.2E-07 | 2.199E-07 | 5.489E-08 |
| 86 | G13B_A | I20B_Q | L23B_Q | K17B_S | 7.5E-08 | 3.6E-07 | 2.208E-07 | 7.533E-08 |
| 87 | Y10B_A | I20B_T | L23B_Q | V24B_N | 1.0E-07 | 4.7E-07 | 2.210E-07 | 9.037E-08 |
| 88 | Y10B_A | I20B_Q | F19B_Q | V24B_N | 6.8E-08 | 5.7E-07 | 2.218E-07 | 1.294E-07 |
| 89 | G13B_A | I20B_S | F19B_W | V24B_N | 1.3E-07 | 3.7E-07 | 2.225E-07 | 7.376E-08 |
| 90 | Y10B_A | I20B_Q | F19B_Q | V24B_Q | 9.9E-08 | 4.3E-07 | 2.244E-07 | 8.656E-08 |
| 91 | G13B_A | E18B_T | I20B_Q | L23B_R | 8.6E-08 | 3.9E-07 | 2.248E-07 | 9.776E-08 |
| 92 | G13B_A | I20B_T | S9B_A | V24B_Q | 1.4E-07 | 3.7E-07 | 2.250E-07 | 6.653E-08 |
| 93 | Y10B_A | I20B_S | L23B_R | V24B_N | 1.4E-07 | 3.5E-07 | 2.260E-07 | 6.344E-08 |
| 94 | G13B_A | I20B_T | F19B_Q | L23B_Q | 6.0E-08 | 4.6E-07 | 2.263E-07 | 1.111E-07 |
| 95 | G13B_A | I20B_N | E18B_T | V24B_Q | 1.0E-07 | 4.0E-07 | 2.265E-07 | 7.700E-08 |
| 96 | Y10B_A | I20B_Q | F19B_Q | V24B_T | 8.5E-08 | 3.2E-07 | 2.267E-07 | 6.989E-08 |
| 97 | Y10B_A | I20B_T | L23B_Q | V24B_Q | 9.6E-08 | 4.5E-07 | 2.268E-07 | 9.045E-08 |
| 98 | G13B_A | I20B_N | E18B_T | V24B_T | 1.2E-07 | 4.4E-07 | 2.270E-07 | 8.572E-08 |
| 99 | G13B_A | E18B_T | I20B_T | L23B_Q | 1.0E-07 | 4.9E-07 | 2.270E-07 | 1.064E-07 |
| 100 | Y10B_A | I20B_N | F19B_Q | V24B_N | 9.0E-08 | 4.4E-07 | 2.277E-07 | 9.537E-08 |
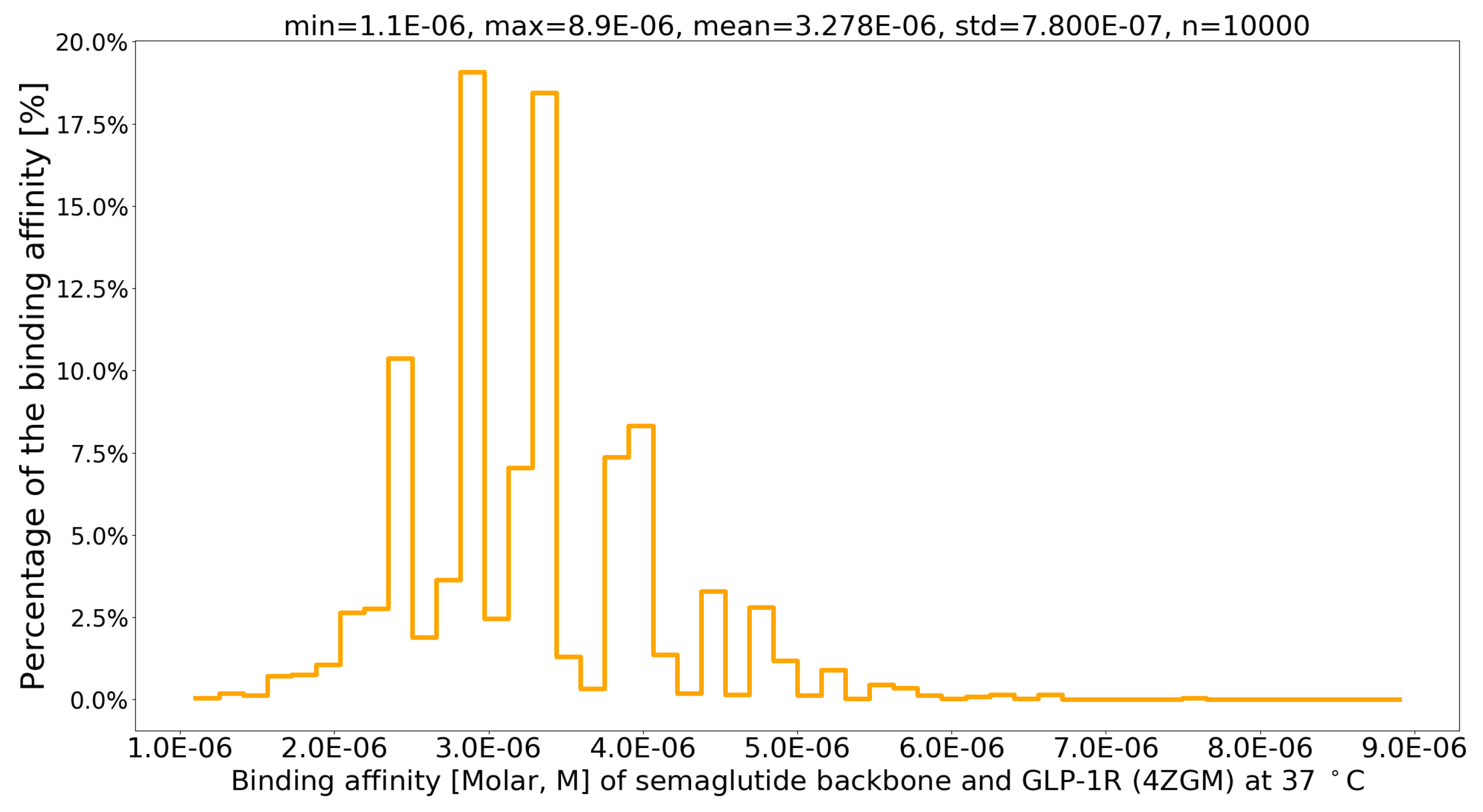
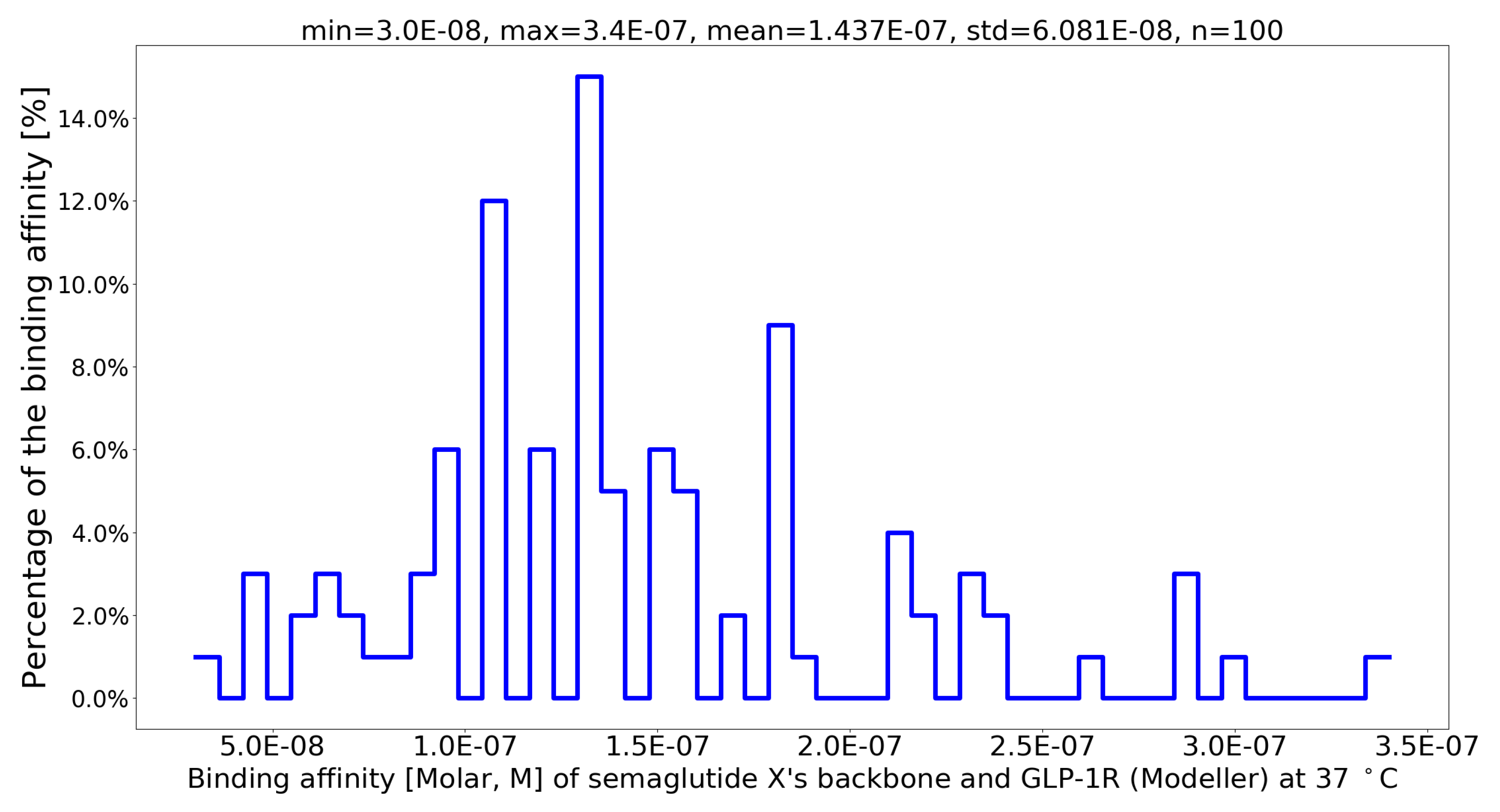
| PDB file | Protein-Protein Complex | G (kcal/mol) | Kd (M) at 37 °C | Fold to 4ZGM |
|---|---|---|---|---|
| 4ZGM [39] | semaglutide-GLP-1R [39] | -7.8 | 3.4 × 10-6 | 1 |
| sema.pdb [9] | Val27-Arg28 exchange [9] | -8.4 | 1.1 × 10-6 | 3.09 |
| semx.pdb | G13B_A I20B_Q L23B_R V24B_N | -10.7 | 3.0 × 10-8 | 113.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).