Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
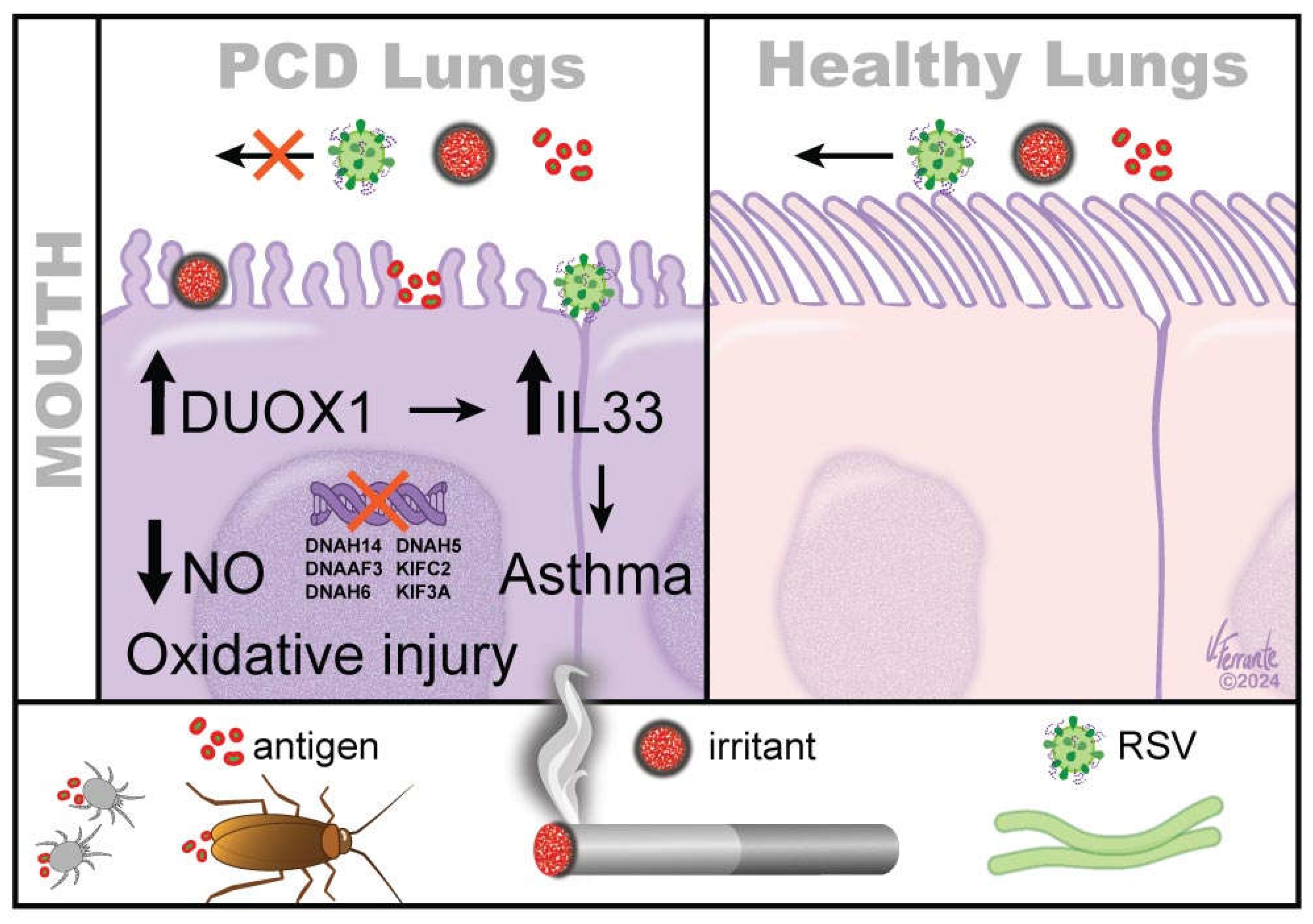
Abstract
Keywords:
1. Introduction
1.1. Antigen Stasis in Cilia as Predisposition for Asthma
2. The Majority of PCD Patients Have Asthma
3. In Epithelial Cells, Cilia Can also Serve as Sensory Cilia, and Loss of Epithelia Can Affect Epithelial Signaling and Airway Smooth Muscle Relaxation
4. Secondary Ciliopathy
5. Antigen Stasis Leading to Oxidant Generation Is Increased in the PCD Airway Epithelium and May Contribute to both Ciliopathies and Asthma Pathogenesis. Antioxidant Treatment May Be Beneficial for PCD Management
6. PCD Genes and Asthma Risk
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leigh, M.W.; Horani, A.; Kinghorn, B.; O’Connor, M.G.; Zariwala, M.A.; Knowles, M.R. Primary Ciliary Dyskinesia (PCD): A genetic disorder of motile cilia. Transl Sci Rare Dis 2019, 4, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; Seifert, B.A.; Truty, R.; Zariwala, M.A.; Ameel, K.; Zhao, Y.; Nykamp, K.; Gaston, B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: a genetic database analysis. Lancet Respir Med 2022, 10, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Zariwala, M.A.; Ferkol, T.; Davis, S.D.; Sagel, S.D.; Dell, S.D.; Rosenfeld, M.; Olivier, K.N.; Milla, C.; Daniel, S.J.; et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol 2016, 51, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Noone, P.G.; Leigh, M.W.; Sannuti, A.; Minnix, S.L.; Carson, J.L.; Hazucha, M.; Zariwala, M.A.; Knowles, M.R. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med 2004, 169, 459–467. [Google Scholar] [CrossRef]
- Walker, W.T.; Liew, A.; Harris, A.; Cole, J.; Lucas, J.S. Upper and lower airway nitric oxide levels in primary ciliary dyskinesia, cystic fibrosis and asthma. Respir Med 107, 380-386.
- Wallmeier, J.; Al-Mutairi, D.A.; Chen, C.T.; Loges, N.T.; Pennekamp, P.; Menchen, T.; Ma, L.; Shamseldin, H.E.; Olbrich, H.; Dougherty, G.W. , et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet 2014, 46, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Gaston, B.; Smith, L.A.; Davis, M.D.; Saunders, J.; Daniels, I.; Horani, A.; Brody, S.L.; Giddings, O.; Zhao, Y.; Marozkina, N. Antigen stasis and airway nitrosative stress in human primary ciliary dyskinesia. Am J Physiol Lung Cell Mol Physiol 2024, 326, L468–l476. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.; Habibovic, A.; Veith, C.; Janssen-Heininger, Y.M.; Dixon, A.E.; Geiszt, M.; van der Vliet, A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J Allergy Clin Immunol 2016, 137, 1545–1556.e1511. [Google Scholar] [CrossRef]
- van der Vliet, A.; Danyal, K.; Heppner, D.E. Dual oxidase: a novel therapeutic target in allergic disease. Br J Pharmacol 2018, 175, 1401–1418. [Google Scholar] [CrossRef]
- Habibovic, A.; Hristova, M.; Heppner, D.E.; Danyal, K.; Ather, J.L.; Janssen-Heininger, Y.M.; Irvin, C.G.; Poynter, M.E.; Lundblad, L.K.; Dixon, A.E.; et al. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI Insight 2016, 1, e88811. [Google Scholar] [CrossRef]
- Dweik, R.A.; Comhair, S.A.; Gaston, B.; Thunnissen, F.B.; Farver, C.; Thomassen, M.J.; Kavuru, M.; Hammel, J.; Abu-Soud, H.M.; Erzurum, S.C. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A 2001, 98, 2622–2627. [Google Scholar] [CrossRef]
- Herbert, C.A.; King, C.M.; Ring, P.C.; Holgate, S.T.; Stewart, G.A.; Thompson, P.J.; Robinson, C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol 1995, 12, 369–378. [Google Scholar] [CrossRef]
- Degano, B.; Valmary, S.; Serrano, E.; Brousset, P.; Arnal, J.F. Expression of nitric oxide synthases in primary ciliary dyskinesia. Hum Pathol 2011, 42, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Chee, C.B.; Gaston, B.; Lilly, C.M.; Gerard, C.; Drazen, J.M.; Stamler, J.S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A 1994, 91, 10089–10093. [Google Scholar] [CrossRef]
- Smith, C.M.; Fadaee-Shohada, M.J.; Sawhney, R.; Baker, N.; Williams, G.; Hirst, R.A.; Andrew, P.W.; O’Callaghan, C. Ciliated cultures from patients with primary ciliary dyskinesia do not produce nitric oxide or inducible nitric oxide synthase during early infection. Chest 2013, 144, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Jourd’heuil, D.; Jourd’heuil, F.L.; Kutchukian, P.S.; Musah, R.A.; Wink, D.A.; Grisham, M.B. Reaction of Superoxide and Nitric Oxide with Peroxynitrite: IMPLICATIONS FOR PEROXYNITRITE-MEDIATED OXIDATION REACTIONSIN VIVO *. Journal of Biological Chemistry 2001, 276, 28799–28805. [Google Scholar] [CrossRef] [PubMed]
- Marozkina, N.V.; Gaston, B. Nitrogen chemistry and lung physiology. Annu Rev Physiol 2015, 77, 431–452. [Google Scholar] [CrossRef]
- Price, M.E.; Sisson, J.H. Redox regulation of motile cilia in airway disease. Redox Biol 2019, 27, 101146. [Google Scholar] [CrossRef]
- Piacentini, G.L.; Bodini, A.; Peroni, D.; Rigotti, E.; Pigozzi, R.; Pradal, U.; Boner, A.L. Nasal nitric oxide for early diagnosis of primary ciliary dyskinesia: practical issues in children. Respir Med 2008, 102, 541–547. [Google Scholar] [CrossRef]
- van der Vliet, A.; Eiserich, J.P.; Shigenaga, M.K.; Cross, C.E. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med 1999, 160, 1–9. [Google Scholar] [CrossRef]
- Wan, W.Y.; Hollins, F.; Haste, L.; Woodman, L.; Hirst, R.A.; Bolton, S.; Gomez, E.; Sutcliffe, A.; Desai, D.; Chachi, L.; et al. NADPH Oxidase-4 Overexpression Is Associated With Epithelial Ciliary Dysfunction in Neutrophilic Asthma. Chest 2016, 149, 1445–1459. [Google Scholar] [CrossRef]
- Cook, D.P.; Thomas, C.M.; Wu, A.Y.; Rusznak, M.; Zhang, J.; Zhou, W.; Cephus, J.-Y.; Gibson-Corley, K.N.; Polosukhin, V.V.; Norlander, A.E.; et al. Cystic Fibrosis Reprograms Airway Epithelial IL-33 Release and Licenses IL-33-Dependent Inflammation. Am J Respir Crit Care Med 2023, 207, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Bergeron, C.; Boulet, L.P.; Cockcroft, D.W.; Côté, A.; Davis, B.E.; Leigh, R.; Myers, I.; O’Byrne, P.M.; Sehmi, R. Sounding the alarmins-The role of alarmin cytokines in asthma. Allergy 2023, 78, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, N.; Santamaria, F.; Allegorico, A.; Fainardi, V.; Borrelli, M.; Ferraro, V.A.; Proietti, E.; Parisi, G.F.; Romagnoli, V.; Lucca, F.; et al. Primary ciliary dyskinesia: A multicenter survey on clinical practice and patient management in Italy. Pediatr Pulmonol 2023, 58, 1127–1135. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Bar-On, O.; Nir, V.; West, N.; Dizitzer, Y.; Mussaffi, H.; Prais, D. Reversible Bronchial Obstruction in Primary Ciliary Dyskinesia. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Seidl, E.; Gatt, D.; Wee, W.B.; Wilson, D.; Ratjen, F.; Grasemann, H. Bronchodilator responsiveness in children with primary ciliary dyskinesia. ERJ Open Res 2024, 10. [Google Scholar] [CrossRef] [PubMed]
- Jarjour, N.N.; Erzurum, S.C.; Bleecker, E.R.; Calhoun, W.J.; Castro, M.; Comhair, S.A.; Chung, K.F.; Curran-Everett, D.; Dweik, R.A.; Fain, S.B.; et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med 2012, 185, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Wright, R.J.; Ivanova, A.; Israel, E.; LaVange, L.M.; Akuthota, P.; Carr, T.F.; Denlinger, L.C.; Fajt, M.L.; Kumar, R.; et al. The Precision Interventions for Severe and/or Exacerbation-Prone (PrecISE) Asthma Network: An overview of Network organization, procedures, and interventions. J Allergy Clin Immunol 2022, 149, 488–516.e489. [Google Scholar] [CrossRef]
- Joseph, P. Castlen, E.M.M.J.Z., Beth Eubanks Julia Meyer,Charles Clem, Benjamin Gaston, Nadzeya Marozkina. Prevalence of Bronchial Hyperreactivity and Asthma in Patients with Primary Ciliary Dyskinesia: A Preliminary Study. PCD on the move: abstract 2024.
- Strippoli, M.P.; Frischer, T.; Barbato, A.; Snijders, D.; Maurer, E.; Lucas, J.S.; Eber, E.; Karadag, B.; Pohunek, P.; Zivkovic, Z.; et al. Management of primary ciliary dyskinesia in European children: recommendations and clinical practice. Eur Respir J 2012, 39, 1482–1491. [Google Scholar] [CrossRef]
- Marozkina, N.; Smith, L.; Zhao, Y.; Zein, J.; Chmiel, J.F.; Kim, J.; Kiselar, J.; Davis, M.D.; Cunningham, R.S.; Randell, S.H.; Gaston, B. Somatic cell hemoglobin modulates nitrogen oxide metabolism in the human airway epithelium. Sci Rep 2021, 11, 15498. [Google Scholar] [CrossRef]
- Straub, A.C.; Lohman, A.W.; Billaud, M.; Johnstone, S.R.; Dwyer, S.T.; Lee, M.Y.; Bortz, P.S.; Best, A.K.; Columbus, L.; Gaston, B.; Isakson, B.E. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature 2012, 491, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Gaston, B.; Reilly, J.; Drazen, J.M.; Fackler, J.; Ramdev, P.; Arnelle, D.; Mullins, M.E.; Sugarbaker, D.J.; Chee, C.; Singel, D.J.; et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A 1993, 90, 10957–10961. [Google Scholar] [CrossRef] [PubMed]
- Kobzik, L.; Bredt, D.S.; Lowenstein, C.J.; Drazen, J.; Gaston, B.; Sugarbaker, D.; Stamler, J.S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol 1993, 9, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Angelo, M.; Singel, D.J.; Stamler, J.S. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A 2006, 103, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Gaston, B.; Drazen, J.M.; Jansen, A.; Sugarbaker, D.A.; Loscalzo, J.; Richards, W.; Stamler, J.S. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J Pharmacol Exp Ther 1994, 268, 978–984. [Google Scholar] [PubMed]
- Marozkina, N.; Bosch, J.; Cotton, C.; Smith, L.; Seckler, J.; Zaman, K.; Rehman, S.; Periasamy, A.; Gaston, H.; Altawallbeh, G.; et al. Cyclic compression increases F508 Del CFTR expression in ciliated human airway epithelium. American Journal of Physiology-Lung Cellular and Molecular Physiology 2019, 317, L247–L258. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, L.; Wijers, C.; Gupta, R.; Marozkina, N.; Li, C.; Gaston, B. A novel, noninvasive assay shows that distal airway oxygen tension is low in cystic fibrosis, but not in primary ciliary dyskinesia. Pediatr Pulmonol 2019, 54, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, A.J.; Arshad, S.H.; Bont, L.; Brunwasser, S.M.; Cherian, T.; Englund, J.A.; Fell, D.B.; Hammitt, L.L.; Hartert, T.V.; Innis, B.L.; et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine 2020, 38, 2435–2448. [Google Scholar] [CrossRef] [PubMed]
- Hartert, T.V.; Wu, P.; Brunwasser, S.M. Respiratory syncytial virus and asthma: untying the Gordian knot. Lancet Respir Med 2021, 9, 1092–1094. [Google Scholar] [CrossRef]
- Wittig, H.J.; Glaser, J. The relationship between bronchiolitis and childhood asthma; a follow-up study of 100 cases of bronchiolitis. J Allergy 1959, 30, 19–23. [Google Scholar] [CrossRef]
- Harris, E. RSV Infection During Infancy Tied to Asthma Later. JAMA 2023, 329, 1731–1731. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Kulkarni, H.; Radhakrishnan, P.; Rutman, A.; Bankart, M.J.; Williams, G.; Hirst, R.A.; Easton, A.J.; Andrew, P.W.; O’Callaghan, C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Lara-Hernandez, I.; Muñoz-Escalante, J.C.; Bernal-Silva, S.; Noyola, D.E.; Wong-Chew, R.M.; Comas-García, A.; Comas-Garcia, M. Ultrastructural and Functional Characterization of Mitochondrial Dynamics Induced by Human Respiratory Syncytial Virus Infection in HEp-2 Cells. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Sarrion, I.; Armengot, M.; Carda, C.; Martinez, I.; Melero, J.A.; Cortijo, J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS One 2012, 7, e48037. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Nie, Y.; Zhan, F.; Zhu, B. Oxidative stress and ROS-mediated cellular events in RSV infection: potential protective roles of antioxidants. Virology Journal 2023, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Pitt, T.; Taylor, G.; Watson, D.; MacDermot, J.; Sykes, D.; Roberts, D.; Cole, P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest 1987, 79, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Steinfort, C.; Wilson, R.; Mitchell, T.; Feldman, C.; Rutman, A.; Todd, H.; Sykes, D.; Walker, J.; Saunders, K.; Andrew, P.W.; et al. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun 1989, 57, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Workman, A.D.; Carey, R.M.; Chen, B.; Rosen, P.L.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Cohen, N.A. Fungal Aflatoxins Reduce Respiratory Mucosal Ciliary Function. Sci Rep 2016, 6, 33221. [Google Scholar] [CrossRef] [PubMed]
- Hessel, J.; Heldrich, J.; Fuller, J.; Staudt, M.R.; Radisch, S.; Hollmann, C.; Harvey, B.G.; Kaner, R.J.; Salit, J.; Yee-Levin, J.; et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One 2014, 9, e85453. [Google Scholar] [CrossRef]
- Leopold, P.L.; O’Mahony, M.J.; Lian, X.J.; Tilley, A.E.; Harvey, B.G.; Crystal, R.G. Smoking is associated with shortened airway cilia. PLoS One 2009, 4, e8157. [Google Scholar] [CrossRef]
- Fox, B.; Bull, T.B.; Oliver, T.N. The distribution and assessment of electron-microscopic abnormalities of human cilia. Eur J Respir Dis Suppl 1983, 127, 11–18. [Google Scholar] [PubMed]
- Lungarella, G.; Fonzi, L.; Ermini, G. Abnormalities of bronchial cilia in patients with chronic bronchitis. An ultrastructural and quantitative analysis. Lung 1983, 161, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wedes, S.H.; Wu, W.; Comhair, S.A.; McDowell, K.M.; DiDonato, J.A.; Erzurum, S.C.; Hazen, S.L. Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J Pediatr 2011, 159, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Verra, F.; Escudier, E.; Lebargy, F.; Bernaudin, J.F.; De Crémoux, H.; Bignon, J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med 1995, 151, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Pan, C.X.; Tang, C.L.; Cen, L.J.; Zhang, X.X.; Huang, Y.; Lin, Z.H.; Li, H.M.; Zhang, X.F.; Wang, L.; et al. Motile Ciliary Disorders of the Nasal Epithelium in Adults With Bronchiectasis. Chest 2023, 163, 1038–1050. [Google Scholar] [CrossRef]
- Zi, X.X.; Guan, W.J.; Peng, Y.; Tan, K.S.; Liu, J.; He, T.T.; Ong, Y.K.; Thong, M.; Shi, L.; Wang, D.Y. An Integrated Analysis of Radial Spoke Head and Outer Dynein Arm Protein Defects and Ciliogenesis Abnormality in Nasal Polyps. Front Genet 2019, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, M.; Bush, A.; Maggi, F.; Michelucci, A.; Ricci, V.; Conidi, M.E.; Cangiotti, A.M.; Bodini, A.; Simi, P.; Macchia, P.; Boner, A.L. Nasal nitric oxide and nitric oxide synthase expression in primary ciliary dyskinesia. Eur Respir J 2011, 37, 572–577. [Google Scholar] [CrossRef]
- Gray, D., Lissi, E. , and Heicklen, J. Reaction of hydrogen peroxide with nitrogen dioxide and nitric oxide. J Phys Chem 1972, 1919-1924.
- Haddad, I.Y.; Pataki, G.; Hu, P.; Galliani, C.; Beckman, J.S.; Matalon, S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 1994, 94, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.K.; Tan, W.S.D.; Peh, H.Y.; Wong, W.S.F. Aeroallergens Induce Reactive Oxygen Species Production and DNA Damage and Dampen Antioxidant Responses in Bronchial Epithelial Cells. The Journal of Immunology 2017, 199, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bonser, L.R.; Zlock, L.; Finkbeiner, W.; Erle, D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest 2016, 126, 2367–2371. [Google Scholar] [CrossRef]
- Stefanska, J.; Pawliczak, R. Apocynin: molecular aptitudes. Mediators Inflamm 2008, 2008, 106507. [Google Scholar] [CrossRef]
- Miles, A.M.; Bohle, D.S.; Glassbrenner, P.A.; Hansert, B.; Wink, D.A.; Grisham, M.B. Modulation of Superoxide-dependent Oxidation and Hydroxylation Reactions by Nitric Oxide (∗). Journal of Biological Chemistry 1996, 271, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Mendoza, C.C.; Ayala-Mata, F.; Cortés-Rojo, C.; García-Pérez, M.E.; Rodríguez-Orozco, A.R. [Antioxidant vitamins in asthma]. Rev Alerg Mex 2018, 65, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Bongiorno, A.; Pintaudi, A.M.; D’Anna, R.; D’Arpa, D.; Livrea, M.A. Synergistic interactions between vitamin A and vitamin E against lipid peroxidation in phosphatidylcholine liposomes. Arch Biochem Biophys 1996, 326, 57–63. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Mirra, V.; Caffarelli, C.; Maglione, M.; Valentino, R.; Perruolo, G.; Mazzarella, C.; Di Micco, L.L.; Montella, S.; Santamaria, F. Hypovitaminosis D: a novel finding in primary ciliary dyskinesia. Ital J Pediatr 2015, 41, 14. [Google Scholar] [CrossRef]
- Paff, T.; Omran, H.; Nielsen, K.G.; Haarman, E.G. Current and Future Treatments in Primary Ciliary Dyskinesia. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Miranda, L.; Guerrero, J. [Measurement of exhaled nitric oxide fraction in lung diseases]. Rev Med Chil 2021, 149, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Blue, E.; Louie, T.L.; Chong, J.X.; Hebbring, S.J.; Barnes, K.C.; Rafaels, N.M.; Knowles, M.R.; Gibson, R.L.; Bamshad, M.J.; Emond, M.J. Variation in Cilia Protein Genes and Progression of Lung Disease in Cystic Fibrosis. Ann Am Thorac Soc 2018, 15, 440–448. [Google Scholar] [CrossRef]
- Sivaprasad, U.; Gibson, A.M.; Wang, N.; Khurana Hershey, G.K. Expression of Cilia Structural Genes is Downregulated in Asthma and Single Nucleotide Polymorphisms in These Genes Correlate with Asthma. Journal of Allergy and Clinical Immunology 2009, 123, S81. [Google Scholar] [CrossRef]
- Koppelman, G.H.; Meyers, D.A.; Howard, T.D.; Zheng, S.L.; Hawkins, G.A.; Ampleford, E.J.; Xu, J.; Koning, H.; Bruinenberg, M.; Nolte, I.M.; et al. Identification of PCDH1 as a novel susceptibility gene for bronchial hyperresponsiveness. Am J Respir Crit Care Med 2009, 180, 929–935. [Google Scholar] [CrossRef] [PubMed]
| Study | Asthma drug withhold before the study | Maximum bronchodilator | Methacholine if failed bronchodilator | Prospective | Number | Percent of PCD patients with positive bronchodilatator response (BD) | Reference |
|---|---|---|---|---|---|---|---|
| Indiana | Yes | Yes | Yes | Yes | 9 | 81 | [30] |
| Israel | No | No | No | No | 46 | 56 | [26] |
| Toronto | No | No | No | No | 474 | 18 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





