Introduction
While advances in immunotherapy, particularly checkpoint inhibitors, have been promising for breast cancer patients, clinical trials have shown that breast cancer is largely resistant to these measures [1-3]. One major challenge of effective immunotherapy for breast and other immunologically “cold” cancers is the immunosuppressive tumor microenvironment (TME), which is a complex network containing multiple types of immunosuppressive cells, chemokines, cytokines, physical barriers (stromal cells and extracellular matrix), chemical factors, metabolites, etc [4, 5]. Various approaches and strategies have been proposed and studied to target these TME immunosuppressive components and improve treatment outcomes [6, 7].
In situ vaccination (ISV), a clinical observation 130 years ago, has attracted increasing attention as a potential paradigm shift for cancer immunotherapy [8, 9]. Unlike conventional vaccines or systemic immunotherapies, ISV can overcome the immunosuppressive TME by direct administration of drugs/adjuvants into the tumor. Current advances in tumor biology, immunology, technological innovations, and targeted therapies have improved immune outcomes of ISV approaches. Nano-pulse Treatment (NPT), also known as nano-pulse stimulation (NPS) or nanosecond pulsed electric fields or electric pulses/ (nsPEFs/nsEPs), is a pulsed-power technology that compresses electric energy and releases it in high powered (generally 100 megawatts) nanosecond (1~999 ns) duration electric pulses [10, 11]. NPT has been demonstrated to effectively ablate tumors in multiple animal models [12-16]. In our previous studies, NPT ablated localized tumors in poorly immunogenic cancer models including orthotopic 4T1-luc mouse triple negative breast [
12], N1S1 rat liver models and ectopic [
15] Pan02 mouse pancreatic cancer [
17]. Importantly, after the complete regression of primary tumors, NPT resulted in a 75-100% rejection of secondary live tumor challenges in these three cancer models, demonstrating its strong ISV effects. The 4T1 tumor model is a very aggressive and spontaneously metastatic malignancy with abundant immune suppressive cells in the TME, including regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) [18, 19]. The mechanisms behind the NPT-induced ISV effect are not well understood. Considering immunosuppressive prevalence in the TME, the immune system must overcome these roadblocks prior to the generation of antitumor immunity. To further clarify how NPT counteracts the immunosuppressive TME to elicit a strong ISV effect, we investigated in this study the dynamic changes of major immunosuppressive cells (Tregs, MDSCs, and TAMs), the Treg suppressive activity, and the acute and long-term T cell immune memory formation.
NPT is a novel type of drug-free ISV approach that can ablate primary tumors to generate strong vaccine protection against the second live tumor challenge. In the breast cancer model ISV effect correlates with an acute rise in tissue-resident memory T cells and the formation of long-term T cell memory. NPT also selectively induced activated Treg cell apoptosis and reduced their functional suppressive capacity while preserving cytotoxic T cells. NPT also eradicated TAMs by directly killing them and causing late diminishment of MDSCs without evidence of the induced cell death mechanism.
Materials and Methods
Animals and Cell Lines
Female BALB/c mice (8–10 weeks of age) were purchased from Jackson Laboratory. The mice were housed and maintained at the ODU AAALAC accredited animal facility. All animal procedures in this study were approved by IACUC at Old Dominion University.
4T1-luc cells were provided by Dr. G Gary Sahagian at Tufts University and maintained in high glucose DMEM (ATCC® 30-2002TM) supplemented 10% FBS, NEAA and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin). 4T1-luc cells with passage number 2-7 were thawed for expansion. Cells with passage number 9-20 were used in the described experiments. Cells were tested with MycoAlert® Mycoplasma Detection Kits (Lonza) to exclude mycoplasma contamination.
In Vivo Nano-Pulse Treatment and the Secondary Live Tumor Challenge
Tumors were initiated by injecting female Balb/c mice with 1 x 10
6 4T1-luc cells in 50 μL DPBS (Life Technologies) in the left posterior mammary fat pad. The control group received the tumor inoculation only. The remaining mice underwent NPT on Day 11 following tumor inoculation (Suppl.
Figure 1) when average tumor sizes were 6-8 mm or 60-100 mm
3. In all studies, the NPT parameters included a pulse duration 100 ns, electric field strengths 40-50 kV/cm, 1000 pulses, and 3 Hz. Nanosecond electric pulses were delivered to the tumor tissue using a two-plate pinch electrode with an 8 mm diameter. Prior to treatment, all hair was thoroughly removed, and the injection site was briefly treated with Nair. The tumor mass and electrodes were covered with ultrasound gel to maintain a good tumor-electrode contact and prevent air accumulation that creates electrical breakdowns.
Animals with tumor free over 7 weeks were challenged orthotopically in the right posterior mammary fat pad with 0.5 x 106 live 4T1-luc tumor cells. Tumor growth was monitored twice weekly by caliper measurements.
Tissue Harvesting and Processing for the Analysis of Immune Cells
The treated mice were euthanized 4 hours (4 hr), 8 hours (8 hr) (for local tissues only), and on Day 1 (D1), Day 3 (D3), and Day 7 (D7) post-NPT (Suppl.
Figure 2). Their tumor tissues, tumor-draining lymph nodes (dLN), blood and spleens were harvested. Control tissues were obtained from mice with untreated tumors. Single cell suspensions from each tissue were prepared to analyze immune cells including CD3, CD4, CD8 and tissue-resident marker CD103. To examine effect memory and central memory T cells, 3 months post-NPT treatment tumor-free animals were euthanized. Spleens and blood were harvested. Spleens/blood of tumor bearing mice were used as control. Single cell suspensions were prepared from spleens or blood then stained with CD3, CD4, CD8, CD44 and CD62L antibodies.
To quantify IFN-γ production and IFN-γ producing T cells, splenocytes (2 x 106/ml) 1 ml per well were incubated with media, tumor lysate (10 µg/mL) or plate bound low endotoxin/azide free LEAF anti-CD3 Ab (0.5 µg/mL in DPBS) in a 24-well plate. For intracellular cytokine staining, cells were incubated for 6 hrs and Monensin added for the final 4 hours. For IFN-g production, cells were incubated for 24 hr and supernatants were collected for ELISA assay (Biolegend). Preparation for single cell suspensions
The primary solid tumor, dLN, blood and spleen were collected and prepared into single cell suspensions for downstream analysis. The spleen and dLN were gently mashed through a 70 µm cell strainer into a conical tube. The dissociated spleen and the blood underwent RBC lysis to make cell suspensions for further analysis. For solid tumor processing, the fat and other surrounding tissues were removed. The tumor samples were washed with RPMI 1640 (Life Technologies) and cut into 1-5 mm3 pieces. The cut pieces were then dissociated with the Miltenyi Biotec tumor dissociation kit and the Gentlemacs Octo-dissociator (with heater) using the tough tumor dissociation protocol. The digest was then passed through a 70 µm cell strainer to remove clumps. Tumor single cell suspensions then underwent magnetic bead based CD45 TIL isolation (Miltenyi Biotec) to obtain CD45+ cells for downstream flow cytometric analysis.
Flow Cytometry To perform cell surface staining, single cell suspensions were washed with FACS buffer containing PBS with 2% FBS (Life Technologies) then incubated with anti-FcR (TruStain FcX™ PLUS, Biolegend) for 10 min on ice to block unspecific binding of antibodies. The following fluorochrome-labeled antibodies were used for Treg, MDSC and TAM labelling: CD4 FITC, CD8 APC/Cy7, CD3 BV510, CD25 APC, CTLA-4 PerCP/Cy5.5, PD-1 PE/Cy7, CCR4 BV421, 4-1BB APC, TGFβ BV421, CD45 Pacific Blue, CD11b PE, Gr-1 PE/Cy7, NKp46 APC, F4-80 FITC, and CD86 APC/Cy7. All cell surface antibodies were purchased from Biolegend.
For intracellular and intranuclear staining, single cell suspensions were first labelled with cell surface antibodies, followed by fixation and permeabilization using the Foxp3 transcription factor buffer set (Thermofisher Scientific). Permeabilized cells were then labelled with Foxp3 PE, Helios PerCP/Cy5.5, IL-17 PE/Cy7, RORγt PerCP-Cy5.5 and/or IFN-γ PerCP-Cy5.5 primary antibodies (Thermofisher Scientific). Cytokine staining using the above buffer and cytokine antibodies from Thermofisher Scientific was verified with the company’s in-house data as well as our own experimental data. This technique allowed us to co-stain for Foxp3, RORγt and IL-17 in the same panel to investigate for T cell reprogramming.
A sample Treg gating strategy is shown (Suppl.
Figure 3) whereby Tregs are identified as CD3
+CD8
-CD4
+CD25
+Foxp3
+ T cells. T effector and Tconv refer to non-Treg CD4 (CD3
+CD8
-CD4
+CD25
-Foxp3
-) and CD8 (CD3
+CD4
-CD8
+CD25
-Foxp3
-) T cells respectively.
For cell apoptosis studies, cells were labelled with Zombie NIR and Annexin V (Biolegend). Due to the sensitivity of the phosphatidylserine bond (for Annexin V binding) to the fixation/permeabilization process (for Foxp3 analysis), we did not incorporate Annexin V and Foxp3 co-staining in the same panel. To perform the cell apoptosis studies, freshly obtained single cell suspensions were first labelled with Zombie NIR using a serum-free PBS buffer. Cells were then washed with FACS buffer to perform cell surface staining. The labelled cells were then washed and resuspended in Annexin V binding buffer, stained with Annexin V, and immediately acquired by flow cytometry.
Stained cells were analyzed on a MACSQuant 10 Analyzer, BD FACS Calibur, or BD FACS Canto II at Old Dominion University and Eastern Virginia Medical School. The acquired data was analyzed using FlowJo software.
In Vitro Treg Suppression Assay
CD4+CD25+ Tregs were isolated from dLN of tumor-bearing mice and NPT-treated mice on post-treatment day 2. To obtain a sufficient quantity of Tregs, dLN from 2-3 mice were pooled together for each group. Spleen-derived CD8 responder (Tresp) cells were isolated from naïve mice by negative selection using magnetic beads (Stemcell Technologies). Purified responder cells were labelled with 5 µM CFSE (ThermoFisher Scientific) and plated in a 96-well round-bottom plate at a density of 4 × 104 responder cells per well. CD4+CD25+ Tregs were co-incubated with CFSE-labelled responder cells at the Treg:Tresp ratios 1:1, 1:2 and 1:4. The plates were incubated at 37°C with 5% CO2 for 60 hours in the presence of CD3/CD28 activation beads (ThermoFisher Scientific). Responder cell proliferation was quantified by flow cytometry based on the dilution of the CFSE dye. Treg suppression was calculated in the following manner: %Suppression = [1-(%proliferating Tresp at Treg: Tresp ratio / %proliferating Tresp-only cells)] x100.
Discussion
In situ vaccination is a 130-year-old concept that has had some efficacy but with initial controversies. The original approach came from reports by Dr. William Coley in the 1890s using microorganisms, a mixture of
Streptococcus pyogenes and
Serratia marcescens, to treat unresectable sarcoma. Although Coley developed a variety of strategies, the approach was not widely accepted because of minimal quality control of the microbial reagent caused a lack of reproducibility. In 1957 a study [
21] showed that tumors could be recognized by the immune system beginning a slow increase in tumor immunotherapy research. In 1959, Bacilus Calmette-Guerin (BCG) was first reported as an ISV approach to treat mouse fibrosarcoma [
22]. Later, clinical studies demonstrated its efficacy and safety in bladder cancer, which lead to the FDA approval of the first cancer immunotherapy in history. The BCG approach presented no cancer antigens but activated the immune system.
In addition to BCG, toll-like receptor (TLR) agonists, oncolytic viruses, cytokines, radiation and hypothermia [
8], emerging novel drugs/technologies including checkpoint inhibitors, nanoparticles, electrochemotherapy (ECT) [23, 24], gene electro-transfer (GET) [25, 26] and NPT [12, 17, 27] can serve as ISV approaches as well. The most documented phenomenon but rare occurrence is the radiation oncologists’ term of an abscopal effect. In these rare cases irradiation shrinks one localized tumor then shrinks tumors outside the irradiated zone. Radiation damage to tumors can therefore stimulate systemic antitumor immunity leading to the regression of metastatic cancer. Like radiation, moderate hyperthermia with iron oxide nanoparticles and an alternating magnetic field induces local hyperthermia to damage tumors, demonstrating a promising potential of local hyperthermia treatment to induce anti-tumor immune responses [
28]. A combination of ECT with peritumoral IL-12 GET enhanced successful tumor treatment as ISV [
23]. It was also shown that the administration of three treatments of intratumoral plasmid encoding IL-12 GET to B16 melanoma cells had a therapeutic effect on primary tumors as well as distant lung nodes and exhibited resistance to challenges with live B16 cells [
26].
Mechanistically there are two aspects for optimal ISV, both of which are satisfied by NPT, while the other strategies require two different treatments to embrace each attribute. The first feature is the conversion of the TME from immunosuppressive to immunostimulatory. The studies reported the regression of Tregs and TAMs by apoptosis and MDSC by another cell death mechanism. This event provided an environment for the simultaneous rise in CD8
+CD103
+ tissue-resident memory T cells and TAM M1 polarization. The second independent mechanism is the production of immunogenic cell death (ICD) molecules. NPT induced the release of ATP, calreticulin, and HMGB [
12]. Our observations support NPT as a novel type of ISV approach. However, whether NPT can modify the TME, especially whether it can directly impact immune cells and their function, has not been explored. Our research discoveries here provide further insight into NPT-induced antitumor immunity of which NPT has a profound impact on immunosuppressive cells in the TME in this 4T1-luc model.
NPT appears to collapse all three major immunosuppressive cell populations in the TME. Interestingly, the distinctive cell frequency and death marker changes among each group hints at the involvement of distinctive population-specific intrinsic mechanisms. A rapid drop of cell counts concomitant with the presence of apoptotic death markers as early as 4 hours post-NPT occurs in both Tregs and TAMs. These results indicate NPT likely induces cell death or directly kills these suppressive cells. However, a delayed decrease of cell counts takes place in MDSCs at day-3 post-NPT. This result together with a reduction in apoptotic markers 4 hours post-treatment suggests an alternative mechanism behind how NPT diminishes MDSCs. Considering the increase of dendritic cells in the TME previously reported by our group in both 4T1 breast [
12] and Pan02 pancreatic [
13] cancer models, we suspect that NPT may shrink the MDSC population not by direct induction of cell death but rather by promotion of MDSC differentiation into dendritic cells (DCs). The differentiated DCs can further participate in antigen-presentation and promote antitumor T cell response. This postulate needs further investigation.
A significant discovery in this study is the selective cytotoxicity of NPT to Tregs while effector CD4
+ and CD8
+ T cells are spared. Treg predominance suggests worse outcomes for many cancer types, including breast, while a high CD8
+ or CD8
+/Treg ratio is associated with better prognosis [29-32]. A significant knockdown of Tregs and increase of effector CD4
+ and CD8
+/Treg ratios would favor the induction of antitumor immunity. Prior studies by Plaza-Sirvent et al. may help clarify the above selective cytotoxicity observation. The group demonstrated that Tregs had an increased sensitivity to apoptosis compared to Tconv [
33]. This sensitivity was correlated with a lower expression of c-Flip
L, a member of the extrinsic apoptosis family, among Tregs when compared to Tconv. Among Tregs, the increased sensitivity to apoptosis was mostly restricted to activated CD44
+CD62L
- Tregs when compared to naïve CD44
-CD62L
+ Tregs [
33]. Mirandola et al. reported that both resting and activated CD8
+ T cells showed a high expression of c-FLIPs as well [
34]. These studies are consistent with our findings that the ratios of activated Tregs (CD44
+CD62L
-) to naïve Tregs (CD44
-CD62L
+) and Tregs to CD8
+ T cells significantly decreased on days 1 and 3 after NPT. The lower c-Flip
L expression in activated Tregs may explain why they are more vulnerable than their Tconv/CD8
+ counterparts to apoptotic changes resulting from NPT. Our results also support a proposal by Drs. Overacre-Delgoffe and Vignali who suggested that Treg fragility may be a valuable target for effective antitumor immunity [
35].
Another important finding is the ability of NPT to impair the Treg suppressive function, likely via the selective removal of the activated Treg (CD44+CD62L-) phenotype discussed above. The analysis of functional markers, including 4-1BB and TGFβ expression, further supports this concept. The expressions of both 4-1BB and TGFβ are dramatically diminished at 1-day post-NPT and remain at low levels for at least one week. These findings show a distinct contrast to results from radiotherapy, which has been studied as an ISV or enhancement for immune outcomes. Radiotherapy has been demonstrated to not only increase the frequency of Tregs by promoting their proliferation but also amplify their functional capacity [36-39]. Noticeably, the dynamics of Tregs are very different between radiotherapy and NPT. NPT rapidly shrinks Tregs as early as at 4 hours post treatment and sustains the low level for at least one week whereas radiotherapy expands Tregs 3 days after treatment then maintains the high level for 9-13 days [37, 39]. The mechanisms behind these distinct Treg responses between NPT and radiotherapy warrant further exploration.
We also note that 4-1BB is exclusively expressed among Foxp3
+ CD4 Tregs in tumor-bearing mice, absent among other T cell subsets, and entirely absent in naïve mice T cells. 4-1BB expression on Tregs is also downregulated post-treatment, making it an excellent marker to track the impact of NPT on the local and systemic Treg dynamics. Additionally, studies suggest that 4-1BB is a valuable therapeutic target to enhance antitumor immunity [40, 41]. 4-1BB targeting therapy can greatly improve the efficacy of immunotherapy via the depletion of Tregs [
40]. The depletion of 4-1BB
+ Tregs can inhibit tumor growth, and Tregs lacking 4-1BB exhibit impaired suppressive function [
41]. Although no systemic antibody is administered in our study, the depletion of 4-1BB
+ Tregs can still be achieved by NPT. This non-drug approach to selectively deplete active Tregs has great potential for cancer immunotherapy because it should minimize immune-related adverse events commonly associated with systemic immunotherapy.
As the TME immunosuppressive prevalence is being removed, the initiation of antitumor immune responses is evidenced with rapid DC activation, TAM-M1 polarization and CD8+ Trm elevation. Thus, NPT encourages coordinate host changes in both the lymphoid and myeloid immune systems to shift from an immunosuppressive to an immunostimulatory performance as part of NPT-induced immune responses. Consequently, the dramatic increase of long-term effector and central memory T cells and their cytotoxic function is consistent with a strong ISV effect whereby the complete rejection (or delayed tumor growth) of the secondary tumor challenge is achieved following successful treatment of the primary tumor.
While this study did not examine other immunosuppressive cells such as tumor-associated fibroblasts, tumor-associated neutrophils, regulatory B cells, etc., the reversal of an immunosuppressive to an immunostimulatory TME supports these findings of ISV and a significant landscape for immunity in this model. The characterization of other immunosuppressive cells would be appropriate in the future study. Whether this reversed immunosuppression and induced ISV is NPT-parameter dependent is another topic that needs further investigation. So far, our available data implies that NPT with short durations, fast rise times, and high electric field strengths seem better than those with long pulse durations with low electric field strengths. NPT with 100 ns and 40-50 kV/cm was reported to result in 75-100% ISV protect in Pan02 pancreatic [
17], 4T1 breast [
12] and N1S1 liver cancer [
27] models whereas NPT with 200 ns and 25-30 kV/cm only leads to 33.3% and 0% ISV protection in B16 melanoma [
42] and Pan02 pancreatic cancer [
13] models, respectively. Nevertheless, whether the NPT-induced ISV is tumor- intrinsic or NPT-parameter dependent can be determined by utilizing the same cancer model treated with various sets of NPT parameters. Our group is also investigating the molecular mechanisms of NPT-selective toxicity to Tregs over effector T cells and how NPT diminishes MDSCs without the induction of extra cell death.
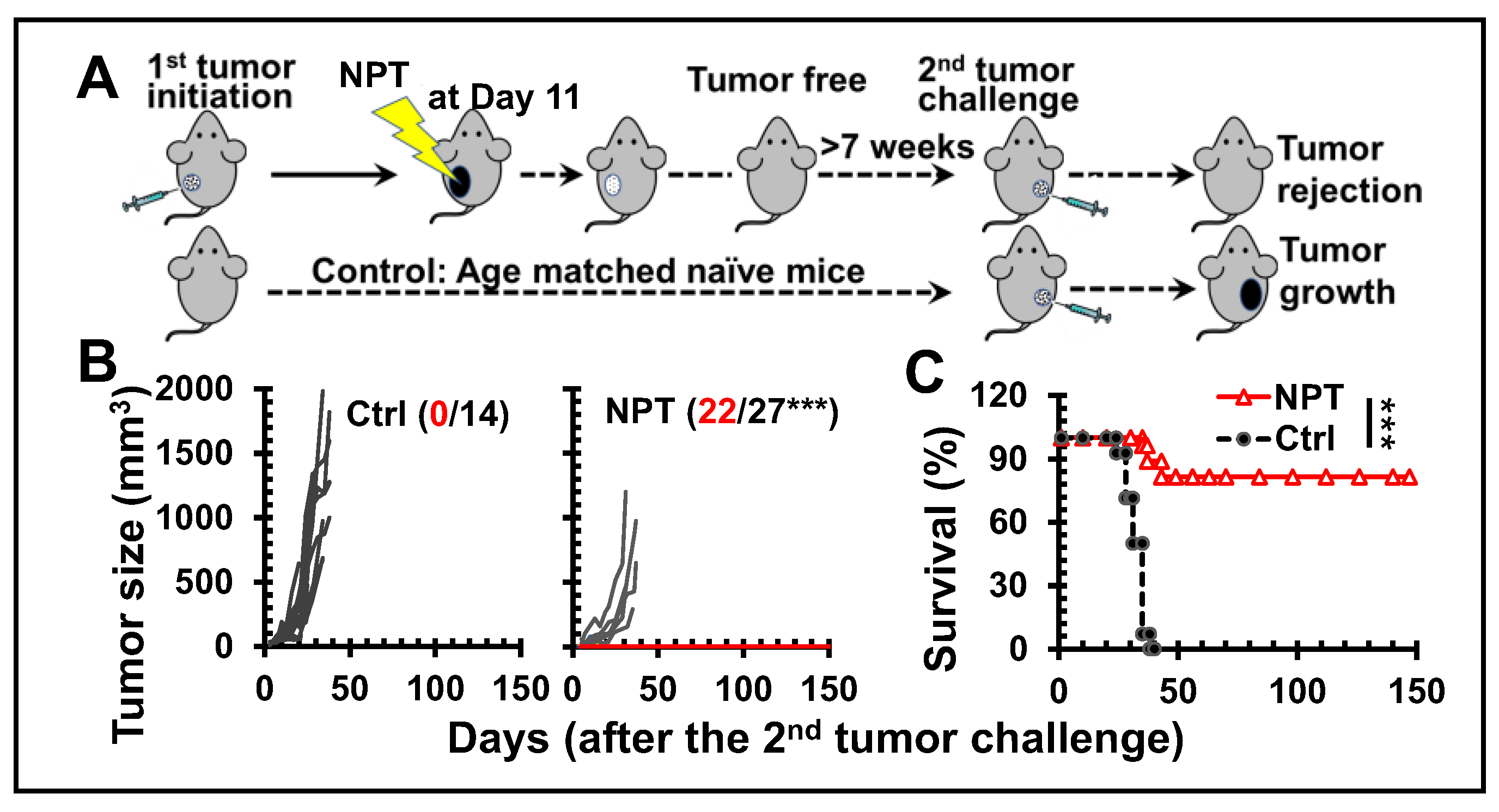
Figure 1.
NPT tumor ablation results in in situ vaccination protection. A: NPT and tumor rechallenging
scheme. Female BALB/c mice with orthotopic tumors (6-8 mm) are treated with NPT.
Animals tumor free over 7 weeks are challenged orthotopically (4T1-luc) with 0.5 x 106 live tumor
cells. Ctrl: age-matched naïve mice without prior NPT. NPT: mice tumor free over 7 weeks after NPT
(100ns, 40-50 kV/cm, 3 Hz and 1000 pulses) treatment are rechallenged with live tumor cells. B and C:
4T1 tumor growth and survival curves of animals following a second live tumor challenge: The
number of tumor free vs total mice are indicated. ***: p<0.001.
Figure 1.
NPT tumor ablation results in in situ vaccination protection. A: NPT and tumor rechallenging
scheme. Female BALB/c mice with orthotopic tumors (6-8 mm) are treated with NPT.
Animals tumor free over 7 weeks are challenged orthotopically (4T1-luc) with 0.5 x 106 live tumor
cells. Ctrl: age-matched naïve mice without prior NPT. NPT: mice tumor free over 7 weeks after NPT
(100ns, 40-50 kV/cm, 3 Hz and 1000 pulses) treatment are rechallenged with live tumor cells. B and C:
4T1 tumor growth and survival curves of animals following a second live tumor challenge: The
number of tumor free vs total mice are indicated. ***: p<0.001.
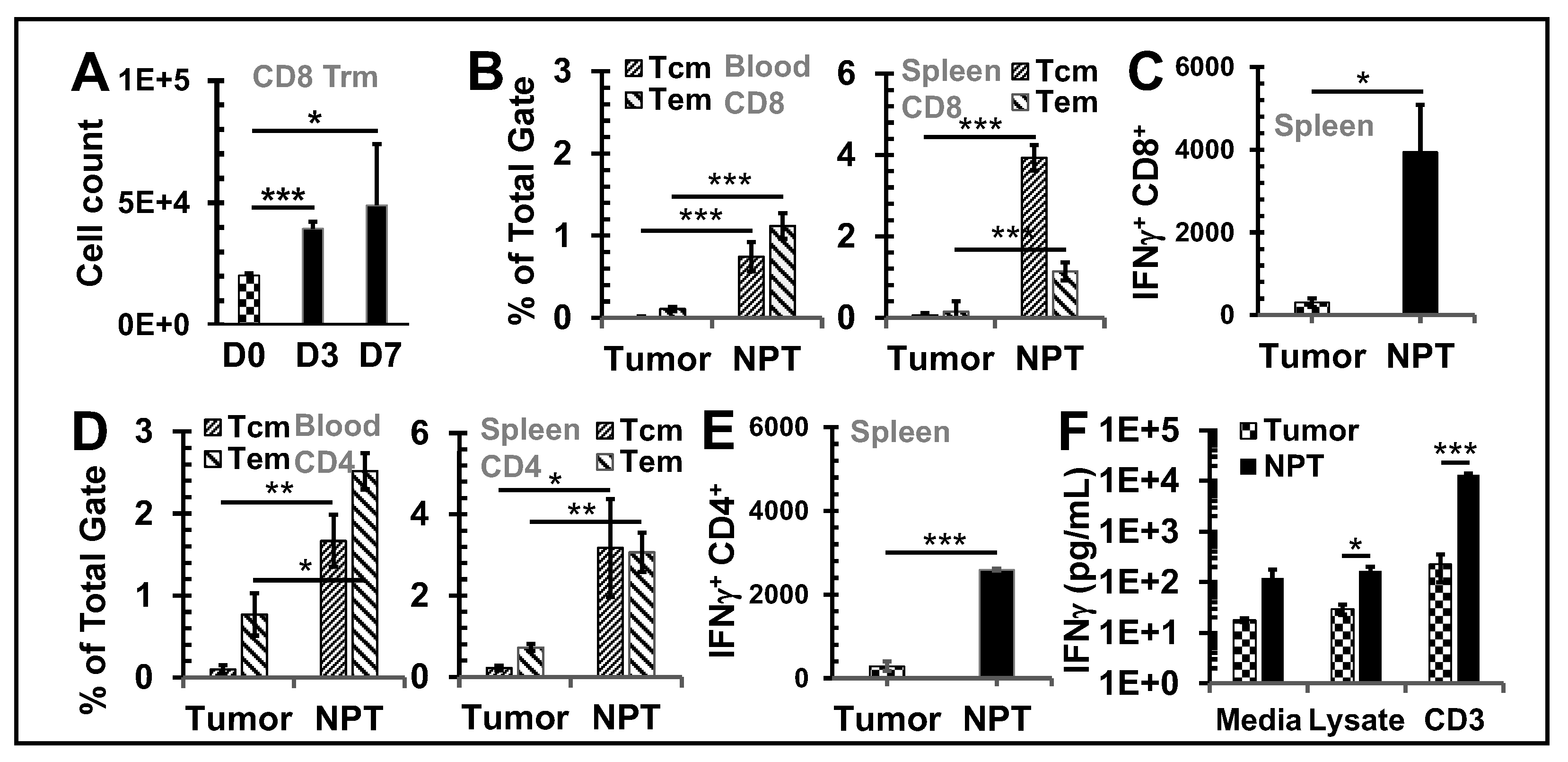
Figure 2.
NPT elicits antitumor memory response. A: Cell counts of CD8+ Trms in per million CD45+
cells in dLNs. D0, D3 and D7: Day-0, -3, and -7. B, D: CD8+ (B) and CD4+ (D) memory T cells in the
blood and spleens of mice. Tcm: CD44+CD62L+ T cells; Tem: CD44+CD62L- T cells. C, E: IFN-γ+ CD8+
(C) and CD4+ (E) T cells from splenocytes after 6-hour incubations with plate bound anti-CD3. F: IFN-
γ product of splenocytes after 24-hour incubations with tumor lysate. Groups: Tumor: untreated
tumor-bearing mice and NPT: NPT treated mice. Note: In B-F, tumor mice were at the end point for
euthanasia (age 15-16 weeks) while NPT-mice were euthanized post-treatment 3 months (age 21-22
weeks). N=3-5. Error bars: SD. *: p<0.05, **: p<0.01, and ***: p<0.001 (One-way ANOVA or t-test).
Figure 2.
NPT elicits antitumor memory response. A: Cell counts of CD8+ Trms in per million CD45+
cells in dLNs. D0, D3 and D7: Day-0, -3, and -7. B, D: CD8+ (B) and CD4+ (D) memory T cells in the
blood and spleens of mice. Tcm: CD44+CD62L+ T cells; Tem: CD44+CD62L- T cells. C, E: IFN-γ+ CD8+
(C) and CD4+ (E) T cells from splenocytes after 6-hour incubations with plate bound anti-CD3. F: IFN-
γ product of splenocytes after 24-hour incubations with tumor lysate. Groups: Tumor: untreated
tumor-bearing mice and NPT: NPT treated mice. Note: In B-F, tumor mice were at the end point for
euthanasia (age 15-16 weeks) while NPT-mice were euthanized post-treatment 3 months (age 21-22
weeks). N=3-5. Error bars: SD. *: p<0.05, **: p<0.01, and ***: p<0.001 (One-way ANOVA or t-test).
Figure 3.
NPT reverses the Treg dominance locally and systemically Breast tumors were established
with an injection of 1x106 4T1-luc cells into the posterior part of the mammary fat pad. The control
group (n=4) received the tumor inoculation only. The remaining mice underwent NPT (100 ns pulses,
50 kV/cm, 3 Hz, 1000 pulses) on Day 11 following tumor inoculation. The treated mice were
euthanized 4 hours (4hr), 8 hours (8hr), and on Day 1 (D1), Day 3 (D3), and Day 7 (D7) post-NPT.
Their tumor tissues, tumor-draining lymph nodes (dLN), blood and spleens were harvested. Control
tissues were obtained from mice with untreated tumors. A: Summary flow plots represent Foxp3+
Tregs and Foxp3- Tconv among the total CD4+ T cell population in the blood. B-D: Quantitative bar
graphs depict the percentage of Tregs among the CD4+ T cell population in blood (B), spleens (C) or
dLNs (D). E: The Tconv/Treg ratios dLNs. F: TIL Tregs are represented in quantitative bar graphs as
the percentage of Tregs among CD4+ TILs. G: A standardized CD4 Treg vs CD4 Tconv cell count. N=4
per group. Error bars, SD. **: p < 0.01 and *: p < 0.05 (one-way ANOVA).
Figure 3.
NPT reverses the Treg dominance locally and systemically Breast tumors were established
with an injection of 1x106 4T1-luc cells into the posterior part of the mammary fat pad. The control
group (n=4) received the tumor inoculation only. The remaining mice underwent NPT (100 ns pulses,
50 kV/cm, 3 Hz, 1000 pulses) on Day 11 following tumor inoculation. The treated mice were
euthanized 4 hours (4hr), 8 hours (8hr), and on Day 1 (D1), Day 3 (D3), and Day 7 (D7) post-NPT.
Their tumor tissues, tumor-draining lymph nodes (dLN), blood and spleens were harvested. Control
tissues were obtained from mice with untreated tumors. A: Summary flow plots represent Foxp3+
Tregs and Foxp3- Tconv among the total CD4+ T cell population in the blood. B-D: Quantitative bar
graphs depict the percentage of Tregs among the CD4+ T cell population in blood (B), spleens (C) or
dLNs (D). E: The Tconv/Treg ratios dLNs. F: TIL Tregs are represented in quantitative bar graphs as
the percentage of Tregs among CD4+ TILs. G: A standardized CD4 Treg vs CD4 Tconv cell count. N=4
per group. Error bars, SD. **: p < 0.01 and *: p < 0.05 (one-way ANOVA).
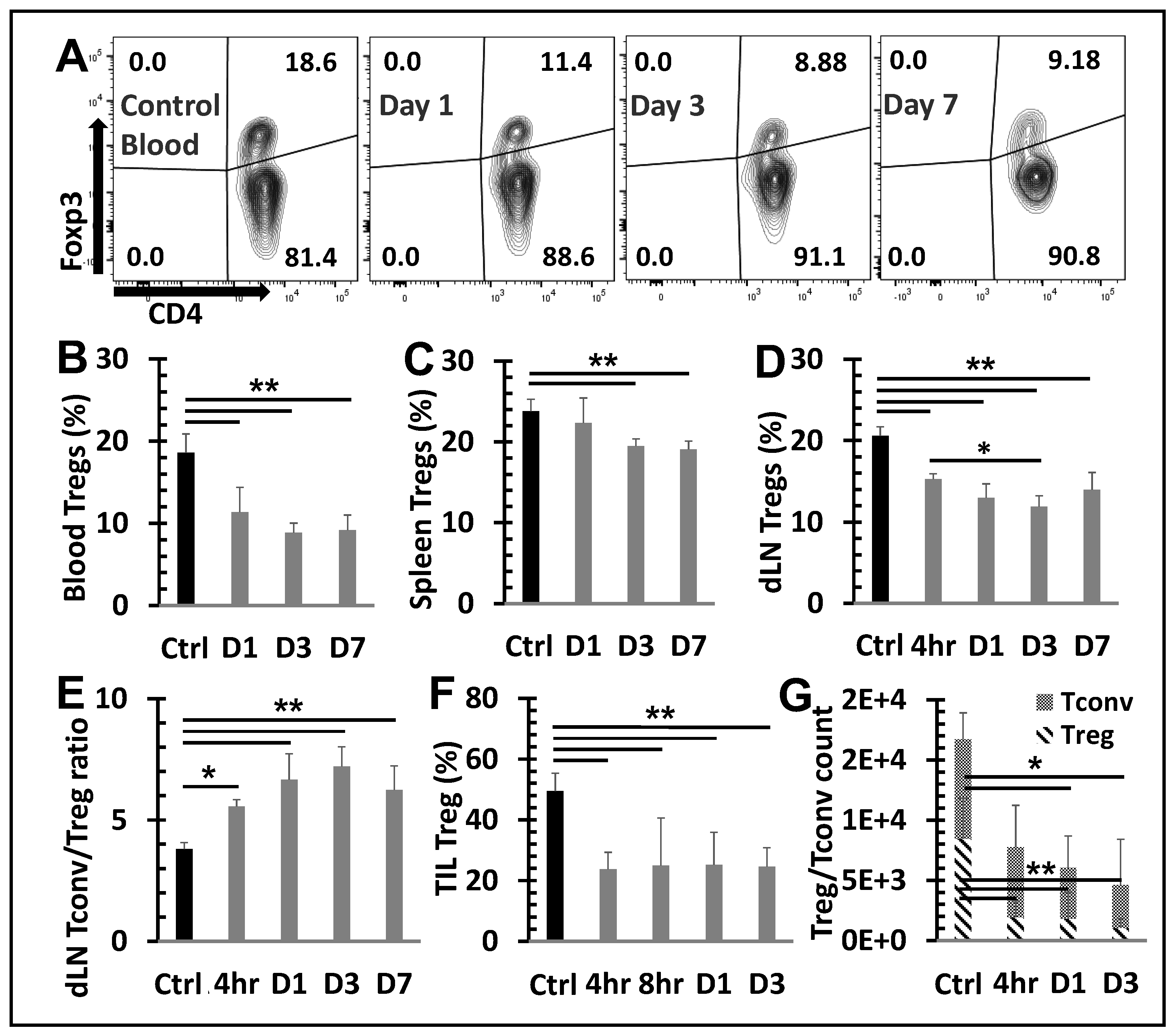
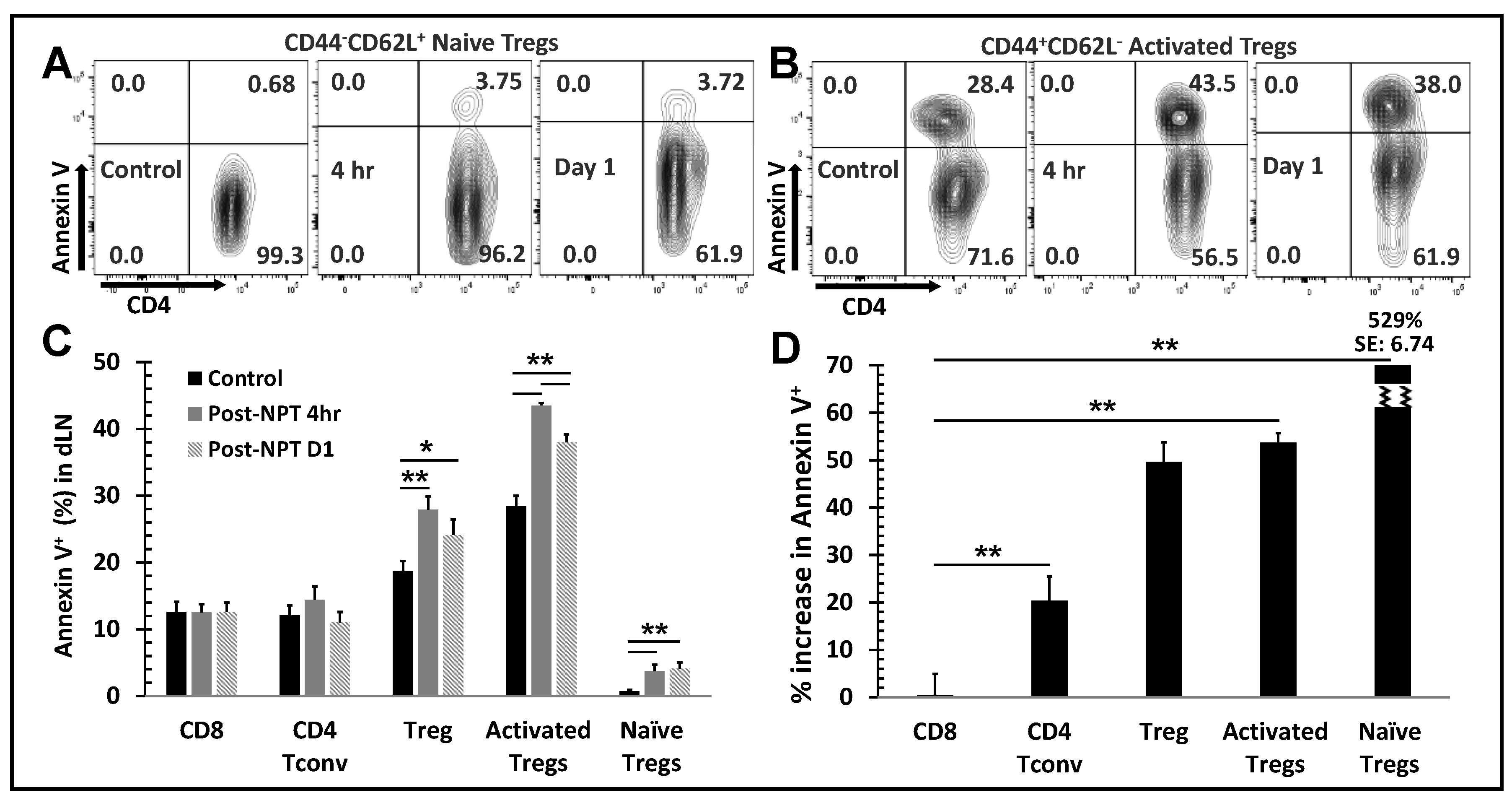
Figure 4.
Changes in apoptosis among T cells subsets following NPT.A and B, Summary flow plots
represent Annexin V expression among activated and naïve Treg subsets in the dLN at 4-hour and 1-
day post-NPT. C, Quantitative graph shows Annexin V expression among CD8, CD4 Tconv, CD4
total Treg, activated Treg and naïve Treg subsets in the dLN at 4-hour and 1-day post-NPT. N=4 per
group. Error bars, SD. D, Quantitative graph shows the percentage of Annexin V expression increase,
among the above subsets, from the untreated Control to 4-hour post-treatment. Error bars, SE. *: p <
0.05 and **: p < 0.01 (One-way ANOVA).
Figure 4.
Changes in apoptosis among T cells subsets following NPT.A and B, Summary flow plots
represent Annexin V expression among activated and naïve Treg subsets in the dLN at 4-hour and 1-
day post-NPT. C, Quantitative graph shows Annexin V expression among CD8, CD4 Tconv, CD4
total Treg, activated Treg and naïve Treg subsets in the dLN at 4-hour and 1-day post-NPT. N=4 per
group. Error bars, SD. D, Quantitative graph shows the percentage of Annexin V expression increase,
among the above subsets, from the untreated Control to 4-hour post-treatment. Error bars, SE. *: p <
0.05 and **: p < 0.01 (One-way ANOVA).
Figure 5.
NPT eradicates activated Tregs and impairs Treg function. A:In vitro suppression assay
showed a reduced functional suppression capacity of Tregs isolated from the NPS-treated mice. Tregs
isolated from dLNs of tumor-bearing (Ctrl) or NPT-treated (NPT) mice were incubated with CFSElabelled
responder cells at the Treg:Tresponder ratios 1:1, 1:2 and 1:4 for 60 hours in the presence of
CD3/CD28 activation beads. Responder cell proliferation was analyzed based on the dilution of the
CFSE dye. The quantitative plots represent the percentage suppression at each Treg:Tresponder ratio
in the control and treatment groups. B &D: Changes in activated and naïve Treg distribution in the
dLN are represented in the summary flow plots (B) and quantitative bar graphs (D). C, E&F:
Phenotypic changes in the 4-1BB activation marker expression among Foxp3+ Tregs are represented
in the summary flow plots (C) and quantitative bar graphs (E and F) in the dLN (C and E) and spleen
(F). G: Changes in the TGFβ expression among Tregs in the dLN is represented in the quantitative
bar graph. N=4 per group. Error bars, SD. *: p < 0.05 and **: p < 0.01 (One-way ANOVA).
Figure 5.
NPT eradicates activated Tregs and impairs Treg function. A:In vitro suppression assay
showed a reduced functional suppression capacity of Tregs isolated from the NPS-treated mice. Tregs
isolated from dLNs of tumor-bearing (Ctrl) or NPT-treated (NPT) mice were incubated with CFSElabelled
responder cells at the Treg:Tresponder ratios 1:1, 1:2 and 1:4 for 60 hours in the presence of
CD3/CD28 activation beads. Responder cell proliferation was analyzed based on the dilution of the
CFSE dye. The quantitative plots represent the percentage suppression at each Treg:Tresponder ratio
in the control and treatment groups. B &D: Changes in activated and naïve Treg distribution in the
dLN are represented in the summary flow plots (B) and quantitative bar graphs (D). C, E&F:
Phenotypic changes in the 4-1BB activation marker expression among Foxp3+ Tregs are represented
in the summary flow plots (C) and quantitative bar graphs (E and F) in the dLN (C and E) and spleen
(F). G: Changes in the TGFβ expression among Tregs in the dLN is represented in the quantitative
bar graph. N=4 per group. Error bars, SD. *: p < 0.05 and **: p < 0.01 (One-way ANOVA).
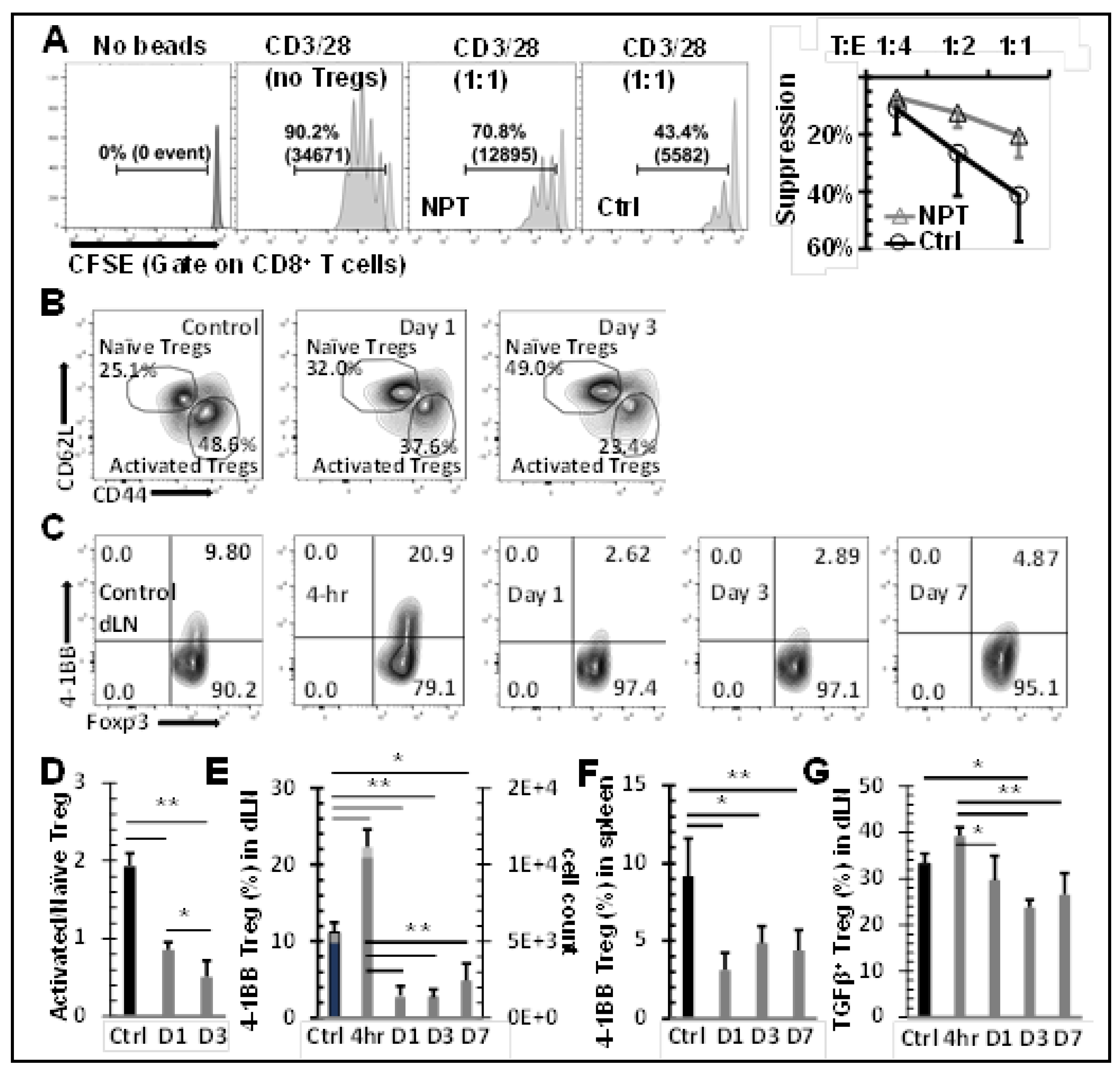
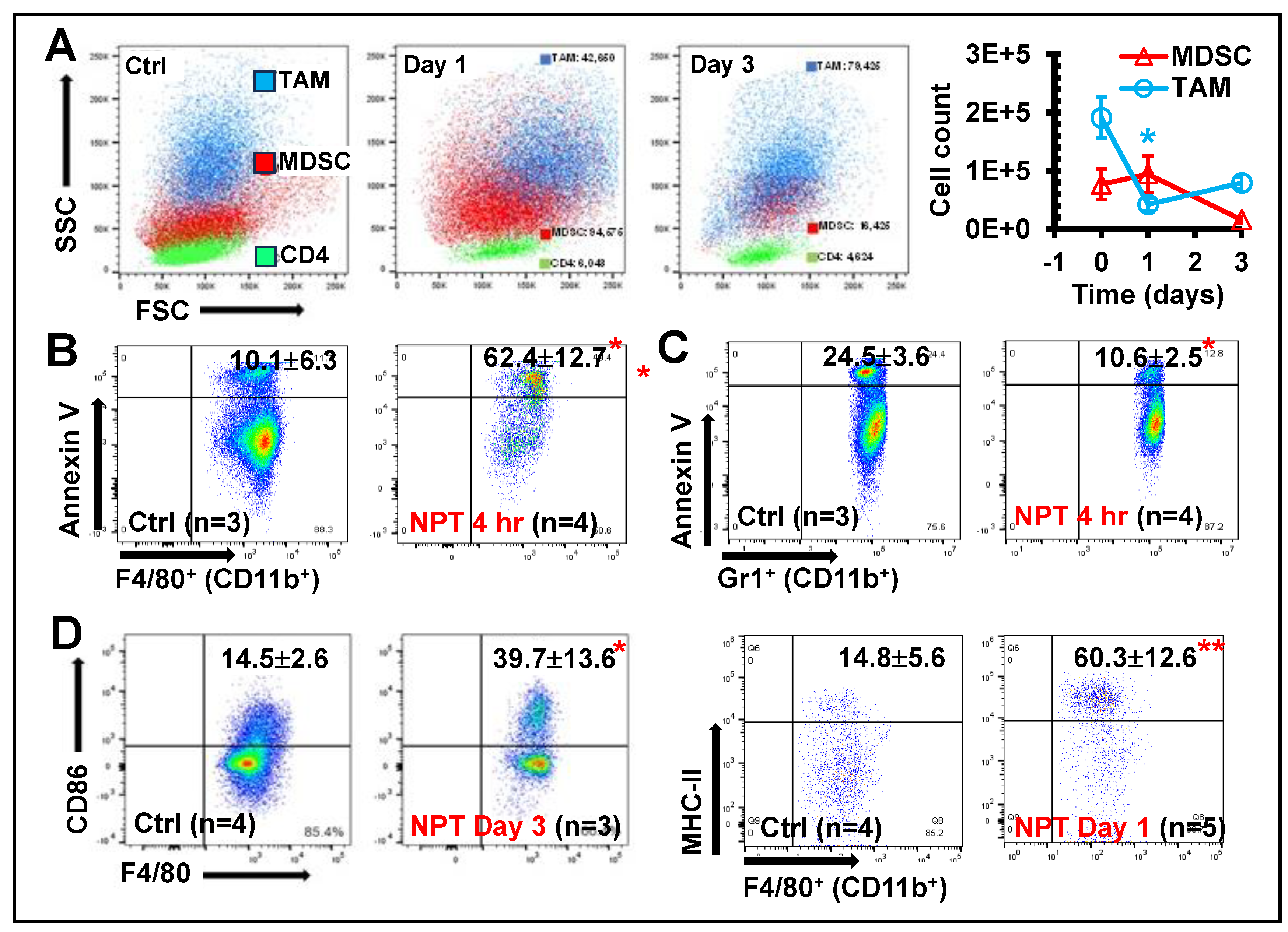
Figure 6.
NPT diminishes intratumoral TAMs and MDSCs with differential characteristics. A: Changes in intratumoral TAMs and MDSCs distribution at Day 1 and Day 3 post-treatment are shown
in a representative flow plot and quantitative graph. B &C: Intratumoral TAM (B) & MDSC (C)
apoptosis representative flow plot indicated with mean ± SD are shown at 4 hours post-NPT. D:
Changes in the CD86 and MHC-II costimulatory marker expression among TAMs were examined on
the day-1 or day-3 post-treatment. A representative flow plot with mean ± SD are shown. N =3-5 per
group. Error bars, SD. *: p < 0.05 and **: p < 0.01 (One-way ANOVA).
Figure 6.
NPT diminishes intratumoral TAMs and MDSCs with differential characteristics. A: Changes in intratumoral TAMs and MDSCs distribution at Day 1 and Day 3 post-treatment are shown
in a representative flow plot and quantitative graph. B &C: Intratumoral TAM (B) & MDSC (C)
apoptosis representative flow plot indicated with mean ± SD are shown at 4 hours post-NPT. D:
Changes in the CD86 and MHC-II costimulatory marker expression among TAMs were examined on
the day-1 or day-3 post-treatment. A representative flow plot with mean ± SD are shown. N =3-5 per
group. Error bars, SD. *: p < 0.05 and **: p < 0.01 (One-way ANOVA).