1. Introduction
According to the WHO prostate cancer is the third most common cancer worldwide, accounting for approximately 10% of all cancer cases and is the second leading cause of cancer-related deaths [
1]. If there was worldwide screening for prostate cancer, it would be probably the most common cancer of humanity, surpassing breast and lung cancer, since in men over the age of 65 years the histological incidence rate is nearly 60% [
2]. Differences in incidence rates are almost 160-fold between the populations at the highest rate (France, Guadeloupe, 157.5), and the populations with the lowest rate (Bhutan, 1.1) reflecting risk factors and screening practices [
3]. Prostate cancer, as breast cancer, is an elusive neoplasm regarding its etiology. If in other tumor types, risk factors such as smoking, infections (HPV, HBV, HCV, EBV, Helicobacter pylori etc.) and pollutants could easily explain most of cases, in prostate cancer diet-related conditions, race and ethnicity and a Western-lifestyle are the only major risk factors [
4]. Newer, hypothetical etiologic factors are anaerobic bacteria, found through genetic studies and identification from urine and semen culture [
5].
Prostate cancer is one of the most curable cancers, if diagnosed early. Still, a certain percentage of cases will relapse despite the most meticulous staging and prompt treatment. It is still difficult to triage patients in the more and less aggressive groups. D’Amico risk groups are most frequently employed, which include T stage, Gleason score and initial PSA (iPSA), as originally described by D’Amico et al. [
6] Including additional variables in risk stratification tools often enhances granularity, but it also introduces greater complexity and can hinder usability. This trade-off is evident in more recent risk scores and nomograms [
7].
One of the most extensive analysis and validation of different prognostic tools has been performed by Zelic et al. [
8] The study team has analyzed 9 different prognostic systems: D’Amico, National Institute for Health and Care Excellence (NICE), European Association of Urology (EAU), Genito-Urinary Radiation Oncologists of Canada (GUROC), American Urological Association (AUA), National Comprehensive Cancer Network (NCCN), and Cambridge Prognostic Groups (CPG) risk group systems; the Cancer of the Prostate Risk Assessment (CAPRA) score; and the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram. These tools were used in predicting prostate cancer death by estimating the concordance index (C-index) in a very large population of prostate cancer patients, of almost 140 000 individuals treated in Sweden. The results showed that the MSKCC nomogram, CAPRA score, and CPG risk grouping system performed better in predicting death from prostate cancer than the D’Amico and derived systems (NICE, GUROC, EAU, AUA, and NCCN). These, more complex systems included age, number or percentage of positivity of biopsy cores, dominant Gleason subtype and more detailed clinical stage. Of note, that none of the tools included serum calcium levels, alkaline phosphatase or lactate-dehydrogenase.
There are newer, readily available prognostic classifiers, such as multiparametric prostate magnetic resonance imaging with fusion-targeted biopsy, cribriform growth in Gleason score 7 prostate cancer, third type of histologic core, neutrophil-to-lymphocyte ratio and PSA density. DNA tests promise the ultimate prognostication [
9].
Is further study of prognostic tools a necessity? We believe that the answer is yes, until virtually all low-risk labelled prostate cancers can be cured. But what if new classifiers show a higher risk of relapse and/or death? And here is our second task to fulfill: readily address micrometastases with new, efficient treatment modalities.
The aim of this study, based on a previous milestone publication [
10], was to analyze if the pretreatment calcium (Ca) bears any prognostic significance. The main difference between our study and that of Skinner et al, is that they have analyzed prediagnostic Ca, at median of almost 10 years before the diagnosis of a fatal prostate cancer. We have included in our investigation Ca levels at diagnosis.
Total calcium levels are measured in serum or plasma. Serum levels are normally between 8.8 to 10.2-10.4 mg/dL (2.2 to 2.6 mmol/L) in healthy subjects [
11].
The role of calcium in prostate cancer cell homeostasis has been summarized by Ardura et al. [
12].
2. Materials and Methods
Our retrospective observational study was performed at a tertiary academic cancer center and included consecutive patients with prostate cancer diagnosed between June 2005 and March 2011, who underwent curative treatment with radiotherapy (adjuvant or primary; external beam, brachytherapy, or both) with or without neoadjuvant and adjuvant hormone blockade.
Inclusion criteria:
Primary confirmed prostate adenocarcinoma
Clinical or pathological stage I, II or III (cT1-4, N0)
Curative treatment
At least 10 years of follow-up
Proper TNM staging and proper follow-up procedures
Exclusion criteria:
The database used for this study was selected by browsing through 207 files concerning patients curatively treated with prostate cancer from our institution to identify those that fulfill the inclusion criteria. As a result, only 84 patients were selected in this period of almost 6 years.
We collected available Ca values measured during the pretreatment window of one month. We have also noted a surrogate marker for bone metastasis, alkaline phosphatase (AlPh) values, when available. The institutional protocol did not require either measurement of Ca levels or of that of AlPh. This was the main reason why we excluded more than half of our patients treated in this time interval.
Serum Ca was measured with a colorimetric assay in our laboratory. AlPh was measured through spectrophotometry.
All patients underwent proper staging with either MRI of the pelvis or endorectal ultrasound (or both) and CT scan of the pelvis, abdomen, and thorax. If initial (iPSA) was higher than 20 ng/mL or the patient presented bone pain, the also underwent bone scintigraphy.
Restaging was performed according to AJCC Cancer Staging Manual, Eighth Edition, from 2017 [
13].
All data were collected in a FileMaker 10 database and analysis was performed through Excel Microsoft Office 2007.
The overall survival and disease-specific survival (DSS) were determined through the Kaplan-Meier method. Survival differences were evaluated through the log-rank test. To analyze the influence of initial Ca on DSS, we chose the cut-off value 9.65 mg/dL with the aid of a ROC curve and chi-squared test (using Yate's correction). The cut-off value was chosen by the minimum distance of ROC curve to point (0,1). The odds ratio was calculated with the chi square test.
The confidence intervals were estimated at 95% confidence level. Statistical significance was defined by a value of p ≤ 0.05.
3. Results
Patients’ ages ranged between 48 and 77 years and the median age was 67 years. The median follow-up was 14.1 years or 169.3 months (range 10.3 - 22.4 years). All patients (pts) had the most common type of prostate adenocarcinoma, the acinar subtype. All patients were cN0 or pN0. Patient characteristics are shown in
Table 1.
Most of our patients were treated with radiotherapy, and only 9 patients (10.7%) underwent surgery (prostatectomy and one cystoprostatectomy) and adjuvant or salvage radiotherapy. Six of our patients underwent surgical castration.
A total of additional 63 patients underwent medical testosterone blockade with complete blockade (LHRH agonist-antagonist plus bicalutamide, 14 patients) or only partial blockade with LHRH agonist-antagonist (49 patients). Since most of our patients were high risk, the length of the hormone blockade was intended to last 2-3 years, unless contraindication or side-effects; at the end of analysis we observed, that only a total of 22 patients received hormone-therapy for more than 1 year, additional to the 6 patients who underwent surgical castration. Hormone therapy was started 2-3 months before radical treatment to aid local procedures. Although 80 of our patients had high-risk disease, only 28 of these (35%) were prescribed or could maintain long term hormone blockade, although short course induction hormone therapy was more common, to aid local treatment.
For patients receiving external beam radiotherapy (EBRT) as the only local treatment procedure (52 patients, comprising 61.9% of the total group), the radiation dose ranged from 70 Gy to 78 Gy, with 2 Gy/day, 5 days a week. The vast majority, 43/52, i.e. 82.7%, received o dose of either 74Gy/37 fractions (16 patients), or 76Gy/38 fractions (27 patients), under current guidelines at the time of treatment, with respecting doses at organs at risk (OARs). Adjuvant or salvage EBRT after prostatectomy was administered in a dose of 50 Gy/25 fr in one patient, 66 Gy/33 fr in 3 pts, 70 Gy/35 fr in 3 pts and 76 Gy/38 fr in 2 pts.
Almost 30% of our patients received a combined EBRT-BT (brachytherapy) treatment in order to boost the primary tumor and decrease local toxicity. The BT consisted of a permanent I125 implant of 110 Gy, followed at 1-2 months by EBRT to cover possible or proven extraprostatic extension and lymph node areas, when needed. BT was performed by an expert radiation oncologist (G.K.) with extensive previous experience in Nice, France. Only EBRT was reimbursed by state insurance, not BT.
For the EBRT-only patients we have employed a 3D technique (dual energy Varian and Siemens Primus LINACs of 6 and 15-16 MV) without fiducial markers, the only type of treatment available at that time at our institution (before the introduction of intensity modulated radiotherapy-IMRT and stereotactic radiotherapy). Treatment was applied with a bladder-filling protocol and an empty rectum. For patients with BT implants we could identify these on portal imaging to guide positioning,
Unfortunately, we do not have data about prostate volume for patients not receiving BT as part of their treatment, thus we have no data about PSA-density for the entire patient group. Another issue is that perineural and lymphatic invasion was poorly documented on the biopsy reports.
In the our long follow-up period 32/84 of patients, i.e. 38.1%, presented relapse of prostate cancer. Two patients presented biochemical only failure (a PSA rise of 2 ng/ml or more above post-EBRT nadir). One patient presented lymphatic-only relapse, in a right obturatory lymph node, successfully salvaged with surgery. Three patients presented local relapse only. Most patients presented bone metastases as a relapse, 26/32 (81.3%). In two of these patients there were accompanying metastases to the peritoneum or lymph nodes plus parenchymatous organs (liver and lung). The relapse of prostate cancer occurred at a median of 7.8 years (range 0.3-11.9).
The two patients presenting biochemical-only failure passed away due to second cancers (metastatic colon cancer and advanced urothelial bladder cancer) at 6 years from the diagnosis of prostate cancer. These were the only deaths from a secondary malignancy in our group, although two more patients presented a second malignancy, i.e. transverse-colon adenocarcinoma and germ-cell testicular cancer, which were cured.
At the end of the follow-up period of 14 years, more than half of the patients have passed away (44/84, i.e. 52.4%), although only 12 (14.3%) due to prostate cancer. Most deaths were caused by cardiovascular and infectious disease (30/84, 35.7%); 2/30 of these patients had stable relapsed disease at death from other cause. Ten patients were still alive with relapsed prostate cancer. The contribution of hormone therapy to cardiovascular deaths could not be determined in this retrospective study.
The most important long-term side effect was urethral stricture (15/84, 17.9%), followed by urinary incontinence in 3 patients and G3 rectal bleeding in 2 patients (treated with LASER coagulation).
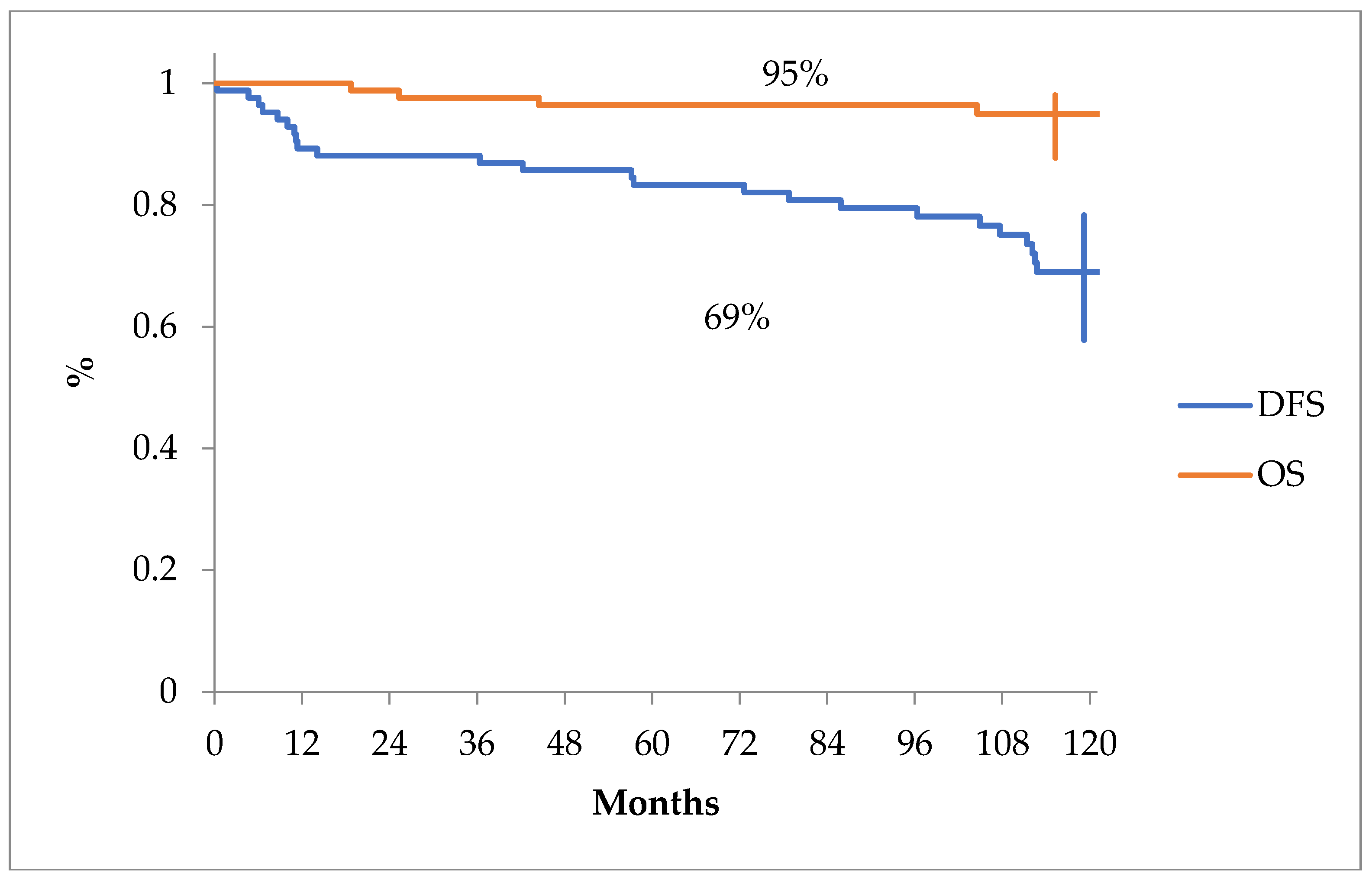
The overall survival and disease free survival curves can be seen in
Figure 1.
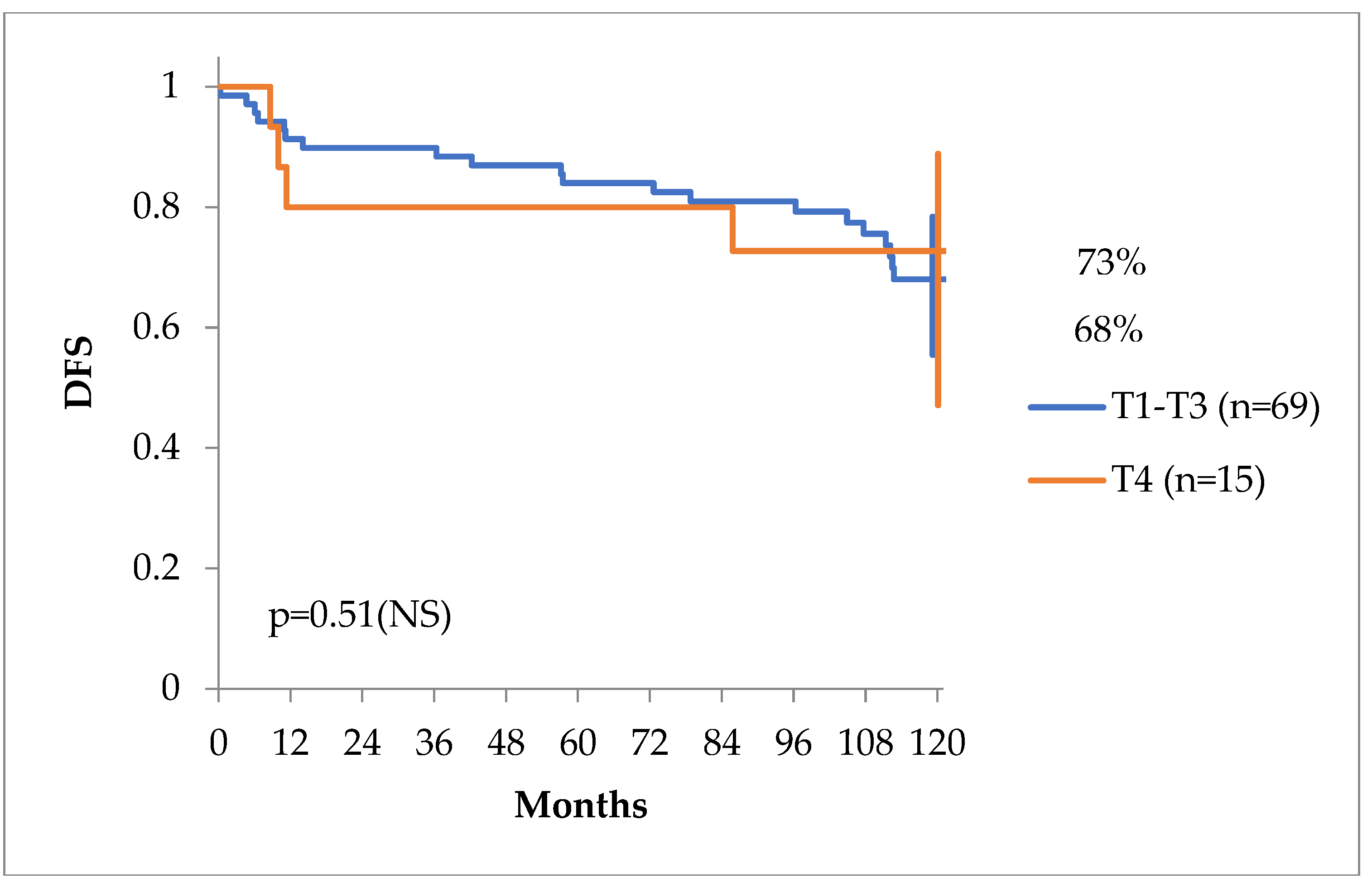
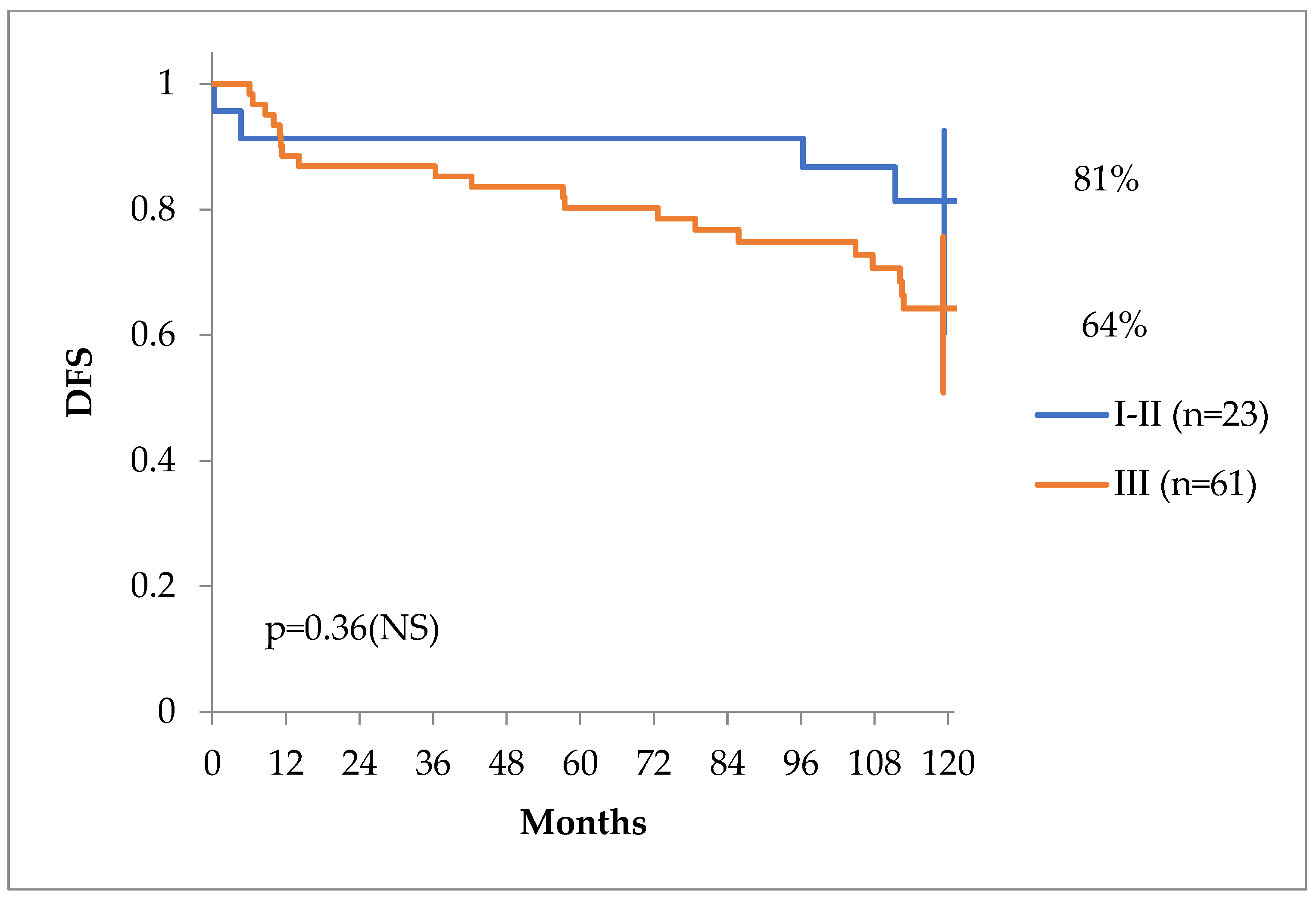
The clinical stage of the primary tumor (cT or pT stage) was not of any predictive value, as seen in
Figure 2, but clinical stages including TNM, Gleason score and PSA behaved differently in regards of DFS with a tendency for statistical significance, as seen in
Figure 3.
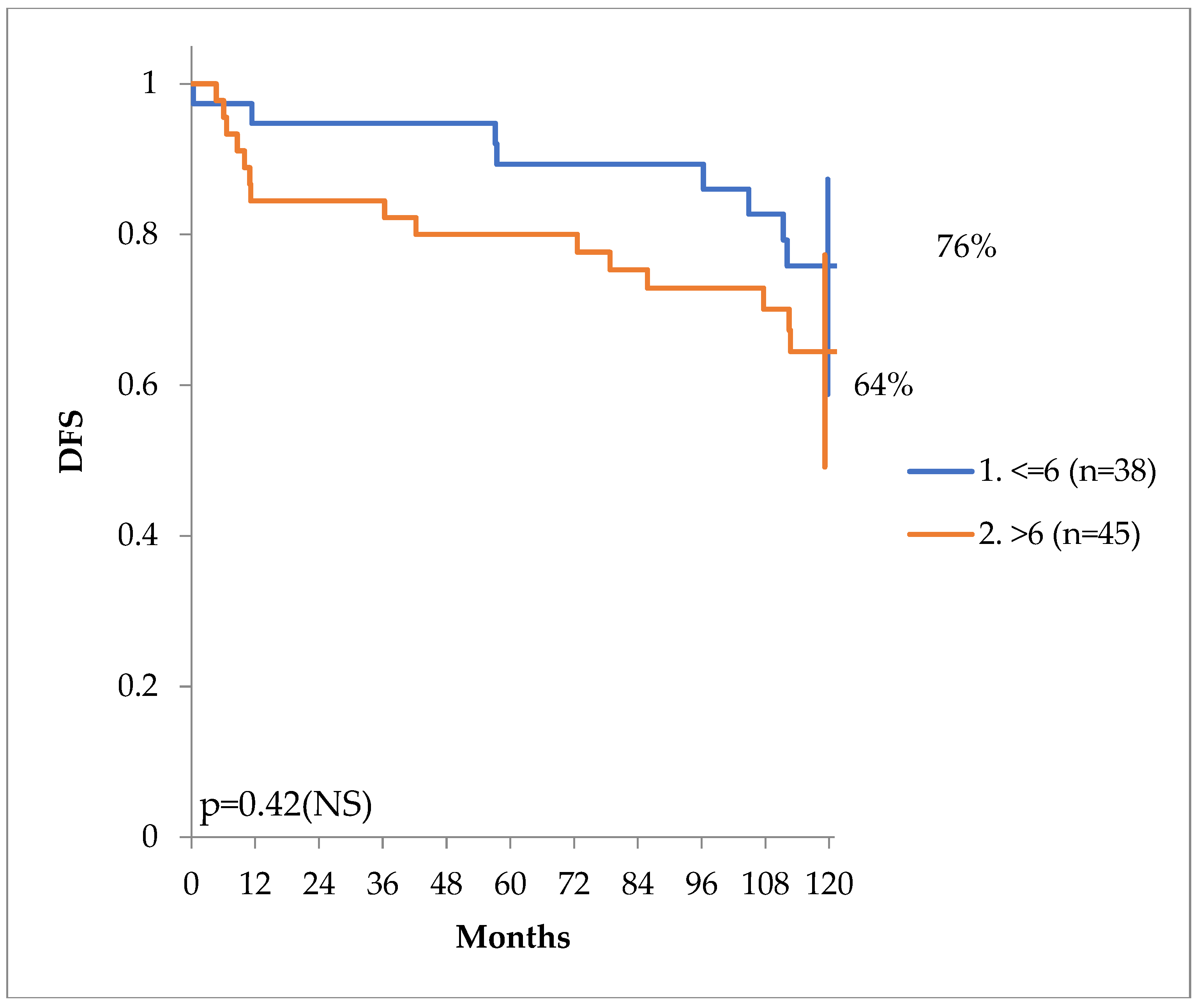
There was also a tendency for higher disease-free survival for patients with a Gleason score of less or equal to 6 (prognostic group 1), versus those with a score of more than 6 (prognostic groups 2 through 5), as seen in
Figure 4.
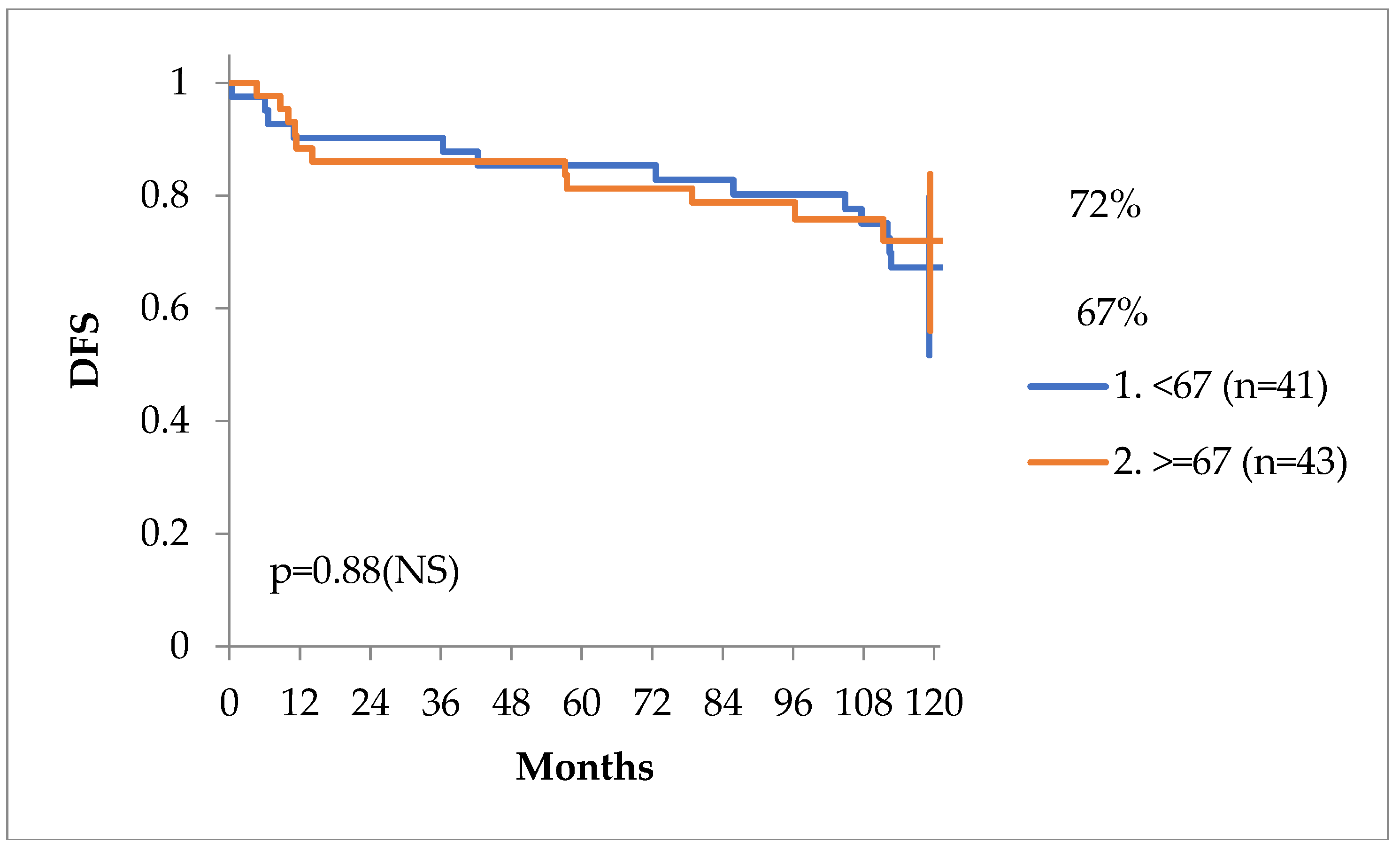
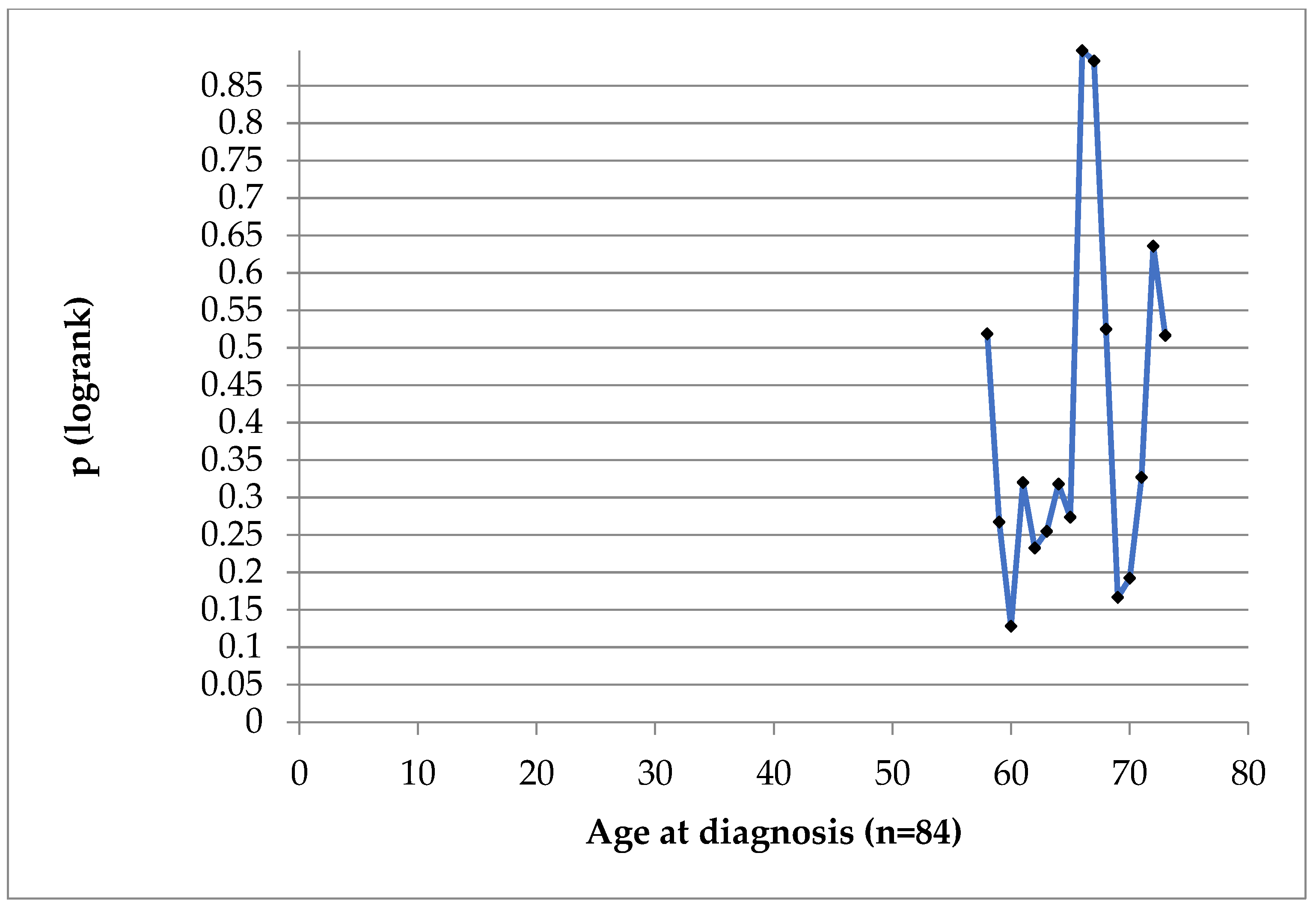
Patients ‘age at diagnosis was not of prognostic value in our case series, as seen in
Figure 5 and
Figure 6.
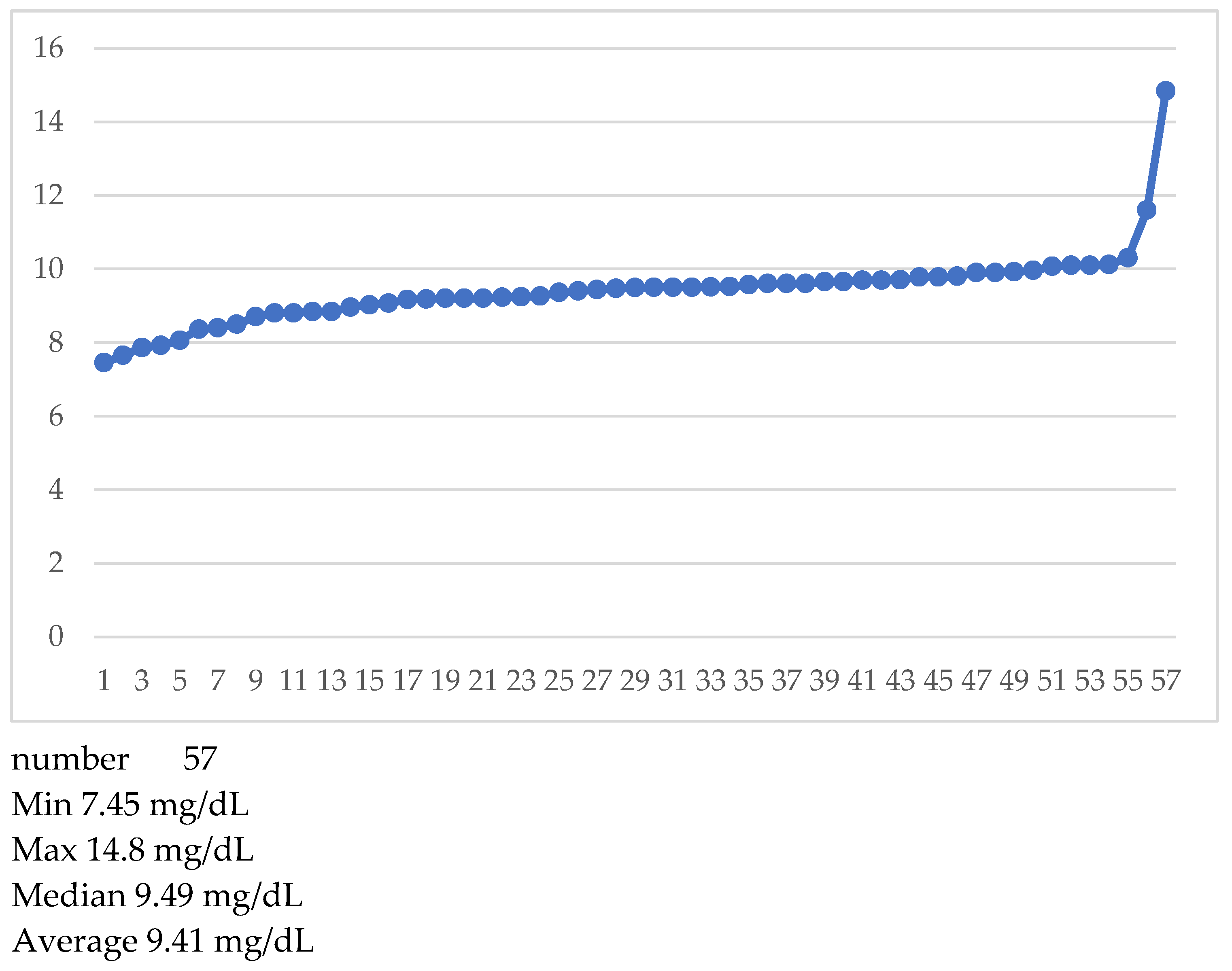
After searching for classical prognostic factors, we looked at serum Calcium and alkaline phosphatase at diagnosis. Calcium was available only for 57 patients (
Table 2), alkaline phosphatase for 69.
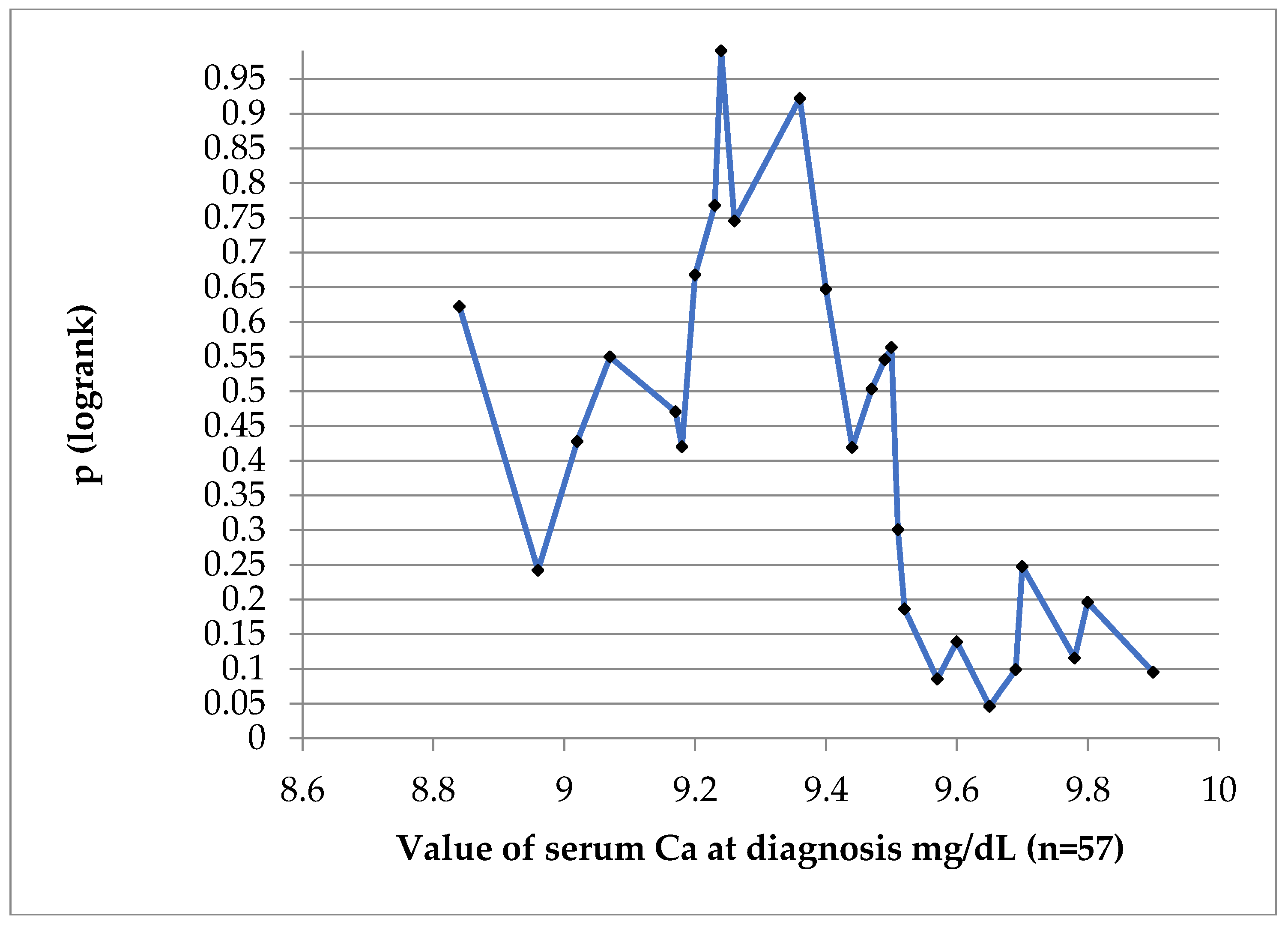
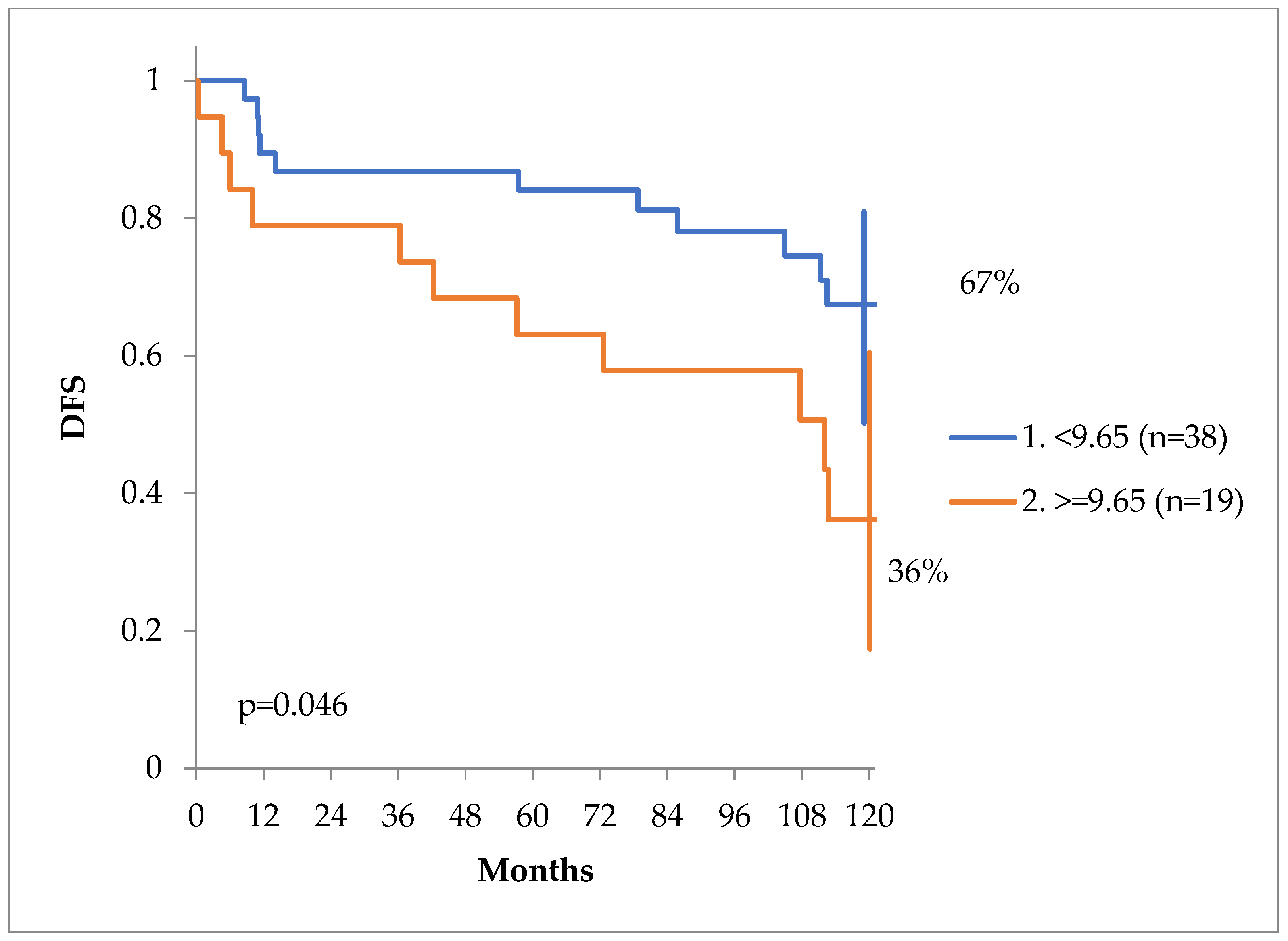
In order to find a value of Ca which might be prognostic for DFS at 10 years, we have performed a log-rank test, showed on
Figure 7. The value of 9.65 mg/dL was of prognostic significance. DFS curves can be seen on
Figure 8. At 10 years the DFS of patients with Calcium values less than 9.65 mg/dL was 67% versus only 36% for patients with a value of equal or more than 9.65 mg/dL, p = 0.046.
In
Figure 9 we have listed individual Calcium values.
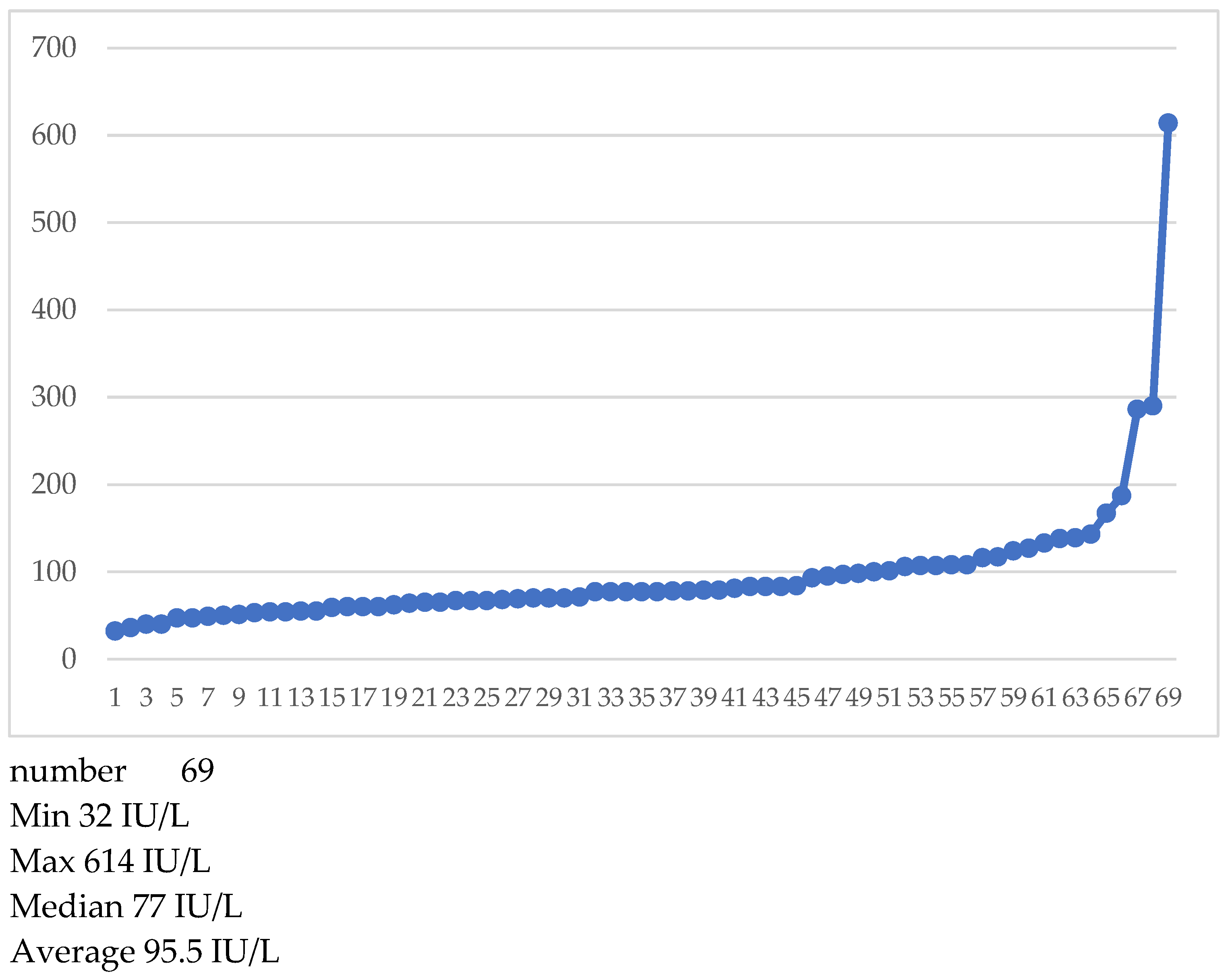
Alkaline phosphatase was available for 69 patients. At our laboratory normal values were considered those between 44 and 129 international units IU/L. The distribution of alkaline phosphatase values can be observed in
Table 3 and
Figure 10.
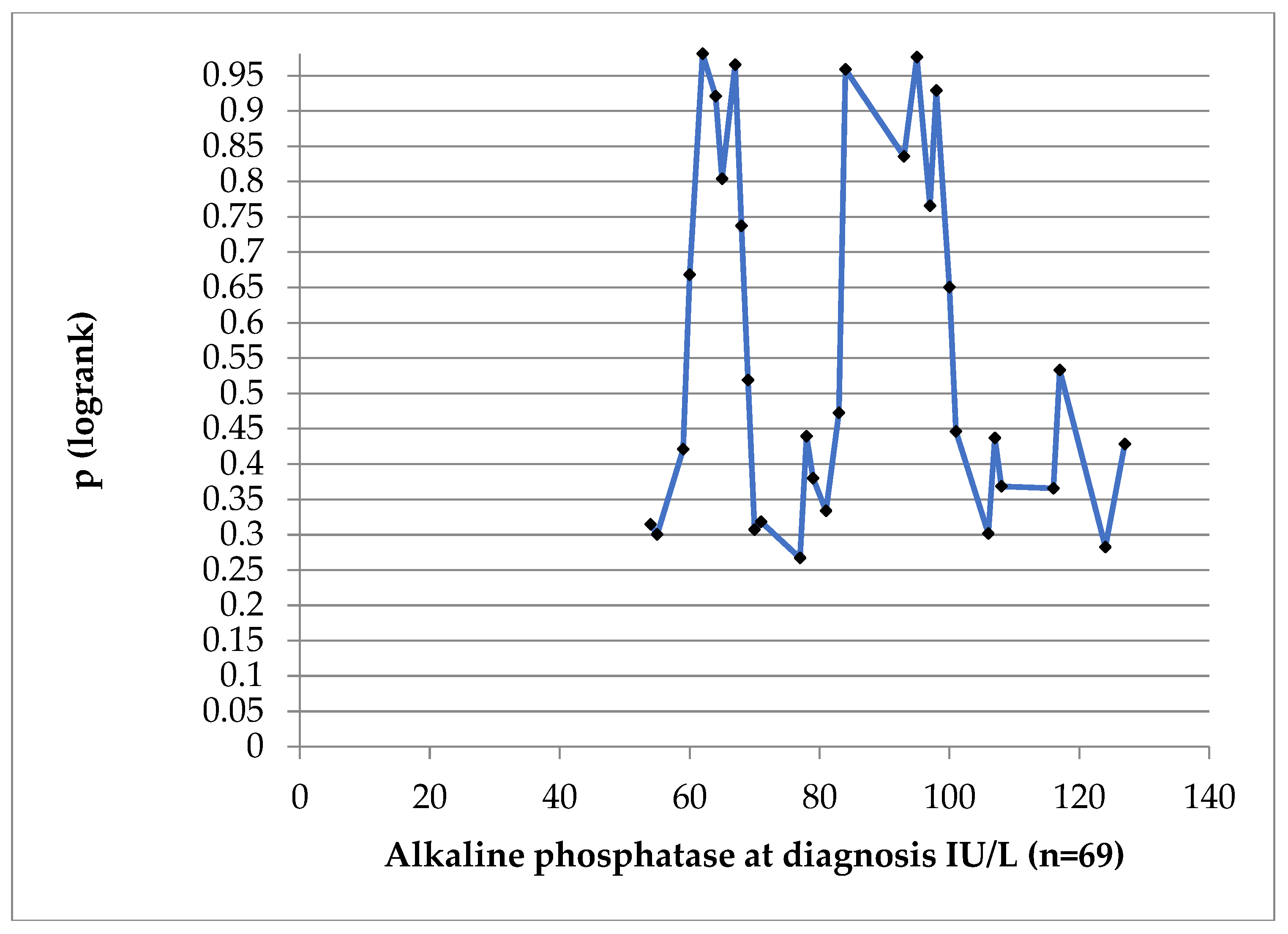
At no cut-off value was AlkPh statistically significant for DFS (log-rank test in
Figure 11).
4. Discussion
The disease-free survival was significantly influenced by the value of the serum Calcium at diagnosis, at a cut-off value of 9.65 mg/dL. Several studies have demonstrated the pre-diagnosis value of Calcium in predicting death from prostate cancer, but no research groups, as of our knowledge, looked at the relationship of serum Calcium at diagnosis and relapse of prostate cancer.
In the publication of Skinner et al. [
11] the study team examined the association between serum Ca levels and prostate cancer using a prospective cohort, the National Health and Nutrition Examination Survey (NHANES). Comparing men in the top tertile with men in the bottom tertile of serum Ca, the multivariable-adjusted relative hazard ratio (HR) for fatal prostate cancer was 2.68 (95% confidence interval, 1.02-6.99; p = 0.04). Of note, that not hypercalcemia, but Ca in the upper tertile was the factor determining an increased risk of death, since only very few patients had hypercalcemia, similar to our study (only 3 patients in our patient group). Serum calcium was determined an average of 9.9 years before the diagnosis of prostate cancer. Their finding of a >2.5-fold increase in the risk of death from prostate cancer for men in the highest tertile of serum calcium was comparable in magnitude with the increased risk associated with family history (2.5).
Skinner et al confirmed their results on another cohort as well [
14].
The concentration of serum ionized Ca (the biologically active fraction total Ca, representing about 50% of the total amount) is tightly regulated by parathyroid hormone (PTH) and usually does not deviate by more than 2% from its stabilized value. In our study the values of total Ca have not been adjusted to the albumin level, since all patients had normal levels of serum albumin, and in non-malnourished patients, total serum Calcium has a good correlation to PTH [
15]. Indeed, recently, Kim et al [
16] has proven that in prostate cancer the levels of PTH are higher than the levels in matched controls.
A PTH increase and thus a parallel Ca rise, is in some way connected to or induced by prostate cancer.
In the study of Kim et al. the average PTH level was 41.67 pg/ml in PC patients, compared to only 27.06 pg/ml in the matched benign hyperplasia (BPH) group, p < 0.001, irrespective of PSA levels (≤20 or >20 ng/mL), Gleason score (≤7 or ≥8), or stage (≤T3 or ≥ T4). The average postoperative PTH level (26.93 pg/ml) was significantly lower than the preoperative PTH level (36.71 pg/ml) in the same patients who underwent radical prostatectomy.
Yarmolinsky et al proved that not hypercalcemia
per se is a risk factor for prostate cancer [
17], thus there is probably another mechanism, which induces both PTH and Ca rise and prostate cancer.
Datta et al [
18] has proven that higher serum Ca levels are correlated with more advanced melanoma stage: the OR of higher stage increased 60% for each 1.0 mg/dL increase in albumin-corrected calcium.
PTH or a similar hormone might be induced by prostate and other types tumor cells. Parathyroid hormone-related protein (PTHRP) is a 139- to 173-amino-acid protein with N-terminal homology to parathyroid hormone (PTH) [
19]. Normally, PTHRP produced by osteoblasts is a regulator of bone formation [
20]. In the measurement of PTH other oligopeptide fragments, such PTH-like peptides and PTHRP might interfere with the determination of PTH [
21,
22]. Thus “high PTH” might reflect high PTHRP. In fact, in a diverse population of cancer patients, plasma PTHRP levels were elevated in 76% of patients with hypercalcemia due to malignancy [
23]. PTHRP is expressed by prostate cancer and it increases cancer cell growth and enhances the osteolytic effects of prostate cancer cells. PTHRP gene expression is upregulated via a transcriptional mechanism by epidermal growth factor (EGF), which is secreted by prostate cancer cells [
24].
Peterson et al [
25] had a similar approach, as ours, but their study was negative: a pretreatment Ca value did not predict biochemical relapse. Their study differed from ours, since their patient cohort consisted of only salvage radiotherapy patients, where the Ca levels might have been influenced by radical prostatectomy.
A large majority our patients (92.9%) had stage T3 disease, reflecting the lack of screening in Romania. Initial PSA was high as well (with a median value of 15.9 ng/mL, with almost 40% of patients having values higher than 20 ng/mL), but there were no clinical metastases and 60/84 (71.4%) of our patients did not relapse. Moreover, more than 95% of our patients have been high-risk patients. Our results regarding the value of Ca and the apparent uselessness of PSA must be interpreted in the setting of more advanced and high-risk prostate cancer, but still N0. A multivariate analysis was not possible due to a limited number of subjects in each risk category.
Skinner et al also proposed that serum PTH stimulates prostate growth in men without clinical prostate cancer [
26].
PTH and PTH-like proteins are seemingly both a predictive (or even inducing) factor for prostate cancer and an aggressivity inducing feature, when prostate cancer is already present.
The quest to identify new circulating, spermatic and biopsy-identified biomarkers in prostate cancer is still open for early detection, prediction, and management [
27,
28].
5. Conclusions
Total serum Calcium level at the time of diagnosis of non-metastatic prostate cancer had a prognostic significance for disease-free survival in our cohort comprised mostly of high-risk, high-PSA patients. A cut-off value of 9.65 mg/dL was found to be significant. Based on our understanding and search of the literature we are the first to publish this concept with a positive association study.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, including the use of clinical data for scientific analysis.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy.
Acknowledgments
The authors thank the personnel of the Institute of Oncology “Prof dr. Ion Chiricuta“, Cluj-Napoca for their continuous efforts in fighting cancer.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Prostate cancer. www.who.int. 2023. Available online: https://platform.who.int/mortality/themes/theme-details/topics/indicator-groups/indicator-group-details/MDB/prostate-cancer (accessed on 2 April 2024).
- SEER Cancer Statistics Review, 1975–2013. National Cancer Institue, Bethesda, MD. 2016. Available online: https://seer.cancer.gov/csr/1975_2015/ (accessed on 2 April 2024).
- Cancer Today. gco.iarc.who.int. Available online: https://gco.iarc.who.int/today/en/dataviz/maps-heatmap?cancers=27&types=1&sexes=0&palette=Reds&mode=population (accessed on 2 April 2024).
- Rawla, P. Epidemiology of Prostate Cancer. World J Oncol 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, M.; Choi, A.; Yoo, S. Microbiome and Prostate Cancer: Emerging Diagnostic and Therapeutic Opportunities. Pharmaceuticals 2024, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- D'Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Clinckaert, A.; Devos, G.; Roussel, E.; Joniau, S. Risk stratification tools in prostate cancer, where do we stand? Transl Androl Urol 2021, 10, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zelic, R.; Garmo, H.; Zugna, D.; Stattin, P.; Richiardi, L.; Akre, O.; Pettersson, A. Predicting Prostate Cancer Death with Different Pretreatment Risk Stratification Tools: A Head-to-head Comparison in a Nationwide Cohort Study. Eur Urol 2020, 77, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, A. Pretreatment Risk Stratification Tools for Prostate Cancer-Moving from Good to Better, Toward the Best. Eur Urol 2020, 77, 189–190. [Google Scholar] [CrossRef]
- Skinner, H.G.; Schwartz, G.G. Serum calcium and incident and fatal prostate cancer in the National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev 2008, 17, 2302–2305. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements (ODS). (n.d.). Calcium—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/#en1 (accessed on 12 April 2024).
- Ardura, J.A.; Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Alonso, V. Role of Calcium Signaling in Prostate Cancer Progression: Effects on Cancer Hallmarks and Bone Metastatic Mechanisms. Cancers 2020, 12, 1071. [Google Scholar] [CrossRef]
- AJCC Cancer Staging Manual. link.springer.com. Available online: https://link.springer.com/book/9783319406176 (accessed on 20 April 2024).
- Skinner, H.G.; Schwartz, G.G. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 2009, 18, 575–578. [Google Scholar] [CrossRef]
- Nordin, B.E.C. Calcium, phosphate, and magnesium metabolism: clinical physiology and diagnostic procedures. Edinburgh; New York New York: Churchill Livingstone; distributed in the U.S. by Longman, 1976.
- Kim, W.T.; Bang, W.J.; Seo, S.P.; Kang, H.W.; Byun, Y.J.; Piao, X.M.; Jeong, P.; Shin, K.S.; Choi, S.Y.; Lee, O.J.; et al. Parathyroid hormone is associated with prostate cancer. Prostate Int 2020, 8, 116–120. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Berryman, K.; Langdon, R.; Bonilla, C.; PRACTICAL consortium; Davey Smith, G.; Martin, R.M.; Lewis, S.J. Mendelian randomization does not support serum calcium in prostate cancer risk. Cancer Causes Control 2018, 29, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Savage, P.; Lovato, J.; Schwartz, G.G. Serum calcium, albumin and tumor stage in cutaneous malignant melanoma. Future Oncol 2016, 12, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Burtis, W.J. Parathyroid hormone-related protein: structure, function, and measurement. Clin Chem 1992, 38, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest 2005, 115, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Plebani, M.; Delanaye, P.; Souberbielle, J.C. Considerations in parathyroid hormone testing. Clin Chem Lab Med 2015, 53, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Mu, D.; Wang, D.; Qiu, L.; Cheng, X. Preanalytical considerations in parathyroid hormone measurement. Clinica Chimica Acta 2023, 539, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Chuang, M.J.; Lu, C.C.; Hao, L.J.; Yang, C.Y.; Han, T.M.; Lam, H.C. Parathyroid hormone and parathyroid hormone related protein assays in the investigation of hypercalcemic patients in hospital in a Chinese population. J Endocrinol Invest 1997, 20, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Tovar Sepulveda, V.A.; Falzon, M. Regulation of PTH-related protein gene expression by vitamin D in PC-3 prostate cancer cells. Mol Cell Endocrinol 2002, 190, 115–124. [Google Scholar] [CrossRef]
- Peterson, J.L.; Buskirk, S.J.; Heckman, M.G.; Parker, A.S.; Diehl, N.N.; Tzou, K.S.; Paryani, N.N.; Ko, S.J.; Daugherty, L.C.; Vallow, L.A.; et al. Evaluation of Serum Calcium as a Predictor of Biochemical Recurrence following Salvage Radiation Therapy for Prostate Cancer. ISRN Oncol 2013, 2013, 239241. [Google Scholar] [CrossRef]
- Skinner, H.G.; Schwartz, G.G. The relation of serum parathyroid hormone and serum calcium to serum levels of prostate-specific antigen: a population-based study. Cancer Epidemiol Biomarkers Prev 2009, 11, 2869–2873. [Google Scholar] [CrossRef]
- Batra, J.S.; Girdhani, S.; Hlatky, L. A Quest to Identify Prostate Cancer Circulating Biomarkers with a Bench-to-Bedside Potential. J Biomark 2014, 2014, 321680. [Google Scholar] [CrossRef] [PubMed]
- Sumanasuriya, S.; Omlin, A.; Armstrong, A.; Attard, G.; Chi, K.N.; Bevan, C.L.; Shibakawa, A.; IJzerman, M.J.; De Laere, B.; Lolkema, M.; et al. Consensus Statement on Circulating Biomarkers for Advanced Prostate Cancer. European Urology Oncology 2018, 1, 151–159. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).