Background
Fontan procedure is the final stage of three-stage palliation in patients with univentricular heart whether Hypoplastic Left Heart Syndrome (HLHS) or other types of Univentricular heart. Fontan physiology results in diminished cardiac output and increased systemic venous pressure. Venous congestion can be complicated by Protein Losing enteropathy (PLE) [
1].
Directing systemic venous return from the lower part of the body to the pulmonary arteries, without intervening pumping chamber, is non-physiologic and results in inevitable systemic congestion [
2].
This systemic congestion is reflected gradually in intestinal lymphatics leading to progressive intestinal lymphangiectasia and severe protein-losing enteropathy. Secondary lymphangiectasia does not only cause repeated admissions with edema and the need for albumin infusion, but it also causes impaired humoral and cellular immunity due to the loss of immunoglobulins and lymphocytes into the intestinal lumen [
3].
Mechanical circulatory support for left ventricular failure using pulsatile and, more recently, axial flow pumps is established. Axial flow pumps, however, are not well-versed in low-pressure circuits, such as those supporting the right ventricle or even a TCPC circulation. Patients with failing Fontan circulation may be able to reverse poor hemodynamics and subsequent organ failure with pump support [
4].
This article aims to explore all the in-vivo and in-vitro trials of different types of Fontan assist devices.
Methodology
A literature review was conducted on the relevant databases: Pubmed, Scopus, and Web of Science.
Keywords and Inclusion Criteria
The following keywords and inclusion criteria were used:
-Fontan and/or vena-cava assist devices.
-In vitro (animal or human) and/or in vivo
Outcome Parameters
-Device design
-Device settings: Pump rate or compression rate
-Flow rate achieved by each device setting
-IVC mean pressure
-Upstream pressure in the SVC
-The presence of any evidence of hemolysis or thrombosis
Statistical Analysis
Data were analyzed using MedCalc statistical software (trial version). Numerical data were tested for normality using the Shapiro-Wilk test and expressed as mean ± SD when normally distributed and as median and IQR when not normally distributed. Categorical data were expressed as numbers and percentages. Numerical data across the three groups were compared using the ANOVA test, and between two groups using the independent T-test, categorical data were compared using the chi-square test.
An SVC pressure exceeding 10 mmHg was set as a classification variable to perform a receiver-operating characteristic analysis, to determine the cut-off flow rate predicting adverse outcome (in the form of upstream elevation of pressure) in the connecting chamber and the intravascular pump designs.
Results
The literature search identified three main designs for the Fontan assist device; the connecting chamber, which serves as a hydraulic chamber to receive the anastomosis between the two peripheral pulmonary arteries, the superior and inferior vena cavae, the intravascular propeller or pump and finally the compression device.
A total of 10 trials [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14] were identified which fulfilled the inclusion criteria as well as the needed outcome parameters; while another 10 trials were excluded due to unmet outcomes [
15,
16,
17,
18,
19,
20,
21,
22]. Some of the included trials involved different settings in terms of pump speed or terms of device design, a total of 23 sets of adjustments were finally analyzed in terms of their impact on IVC and SVC pressure and resultant flow rate. (
Figure 1 shows the PRISMA flow chart for selection of the studies)
Out of the 23 settings, 11 employed design 1, 9 employed design 2, and only 3 were applying design 3.
The intravascular pump design was the most frequently tried in vivo accounting for 33% of all sets of adjustments within this category, while 100% and 91% of the trials for the connecting chamber and the compression device were performed in vitro, respectively.
We analyzed the outcome parameters across the three designs (
Table 1), the flow rate was significantly lower in the trials implemented using the compression device.
The connecting chamber needed significantly fewer rotations per minute than the intravascular pump to achieve almost an equal flow rate. (4000 (4000–4775) vs. 12000 (9750–21750), p < 0.01)
There were no significant differences achieved in SVC or IVC pressures across the three study designs.
By visually examining the data we were able, to identify a certain pattern differentiating intravascular pumps and connecting chambers.
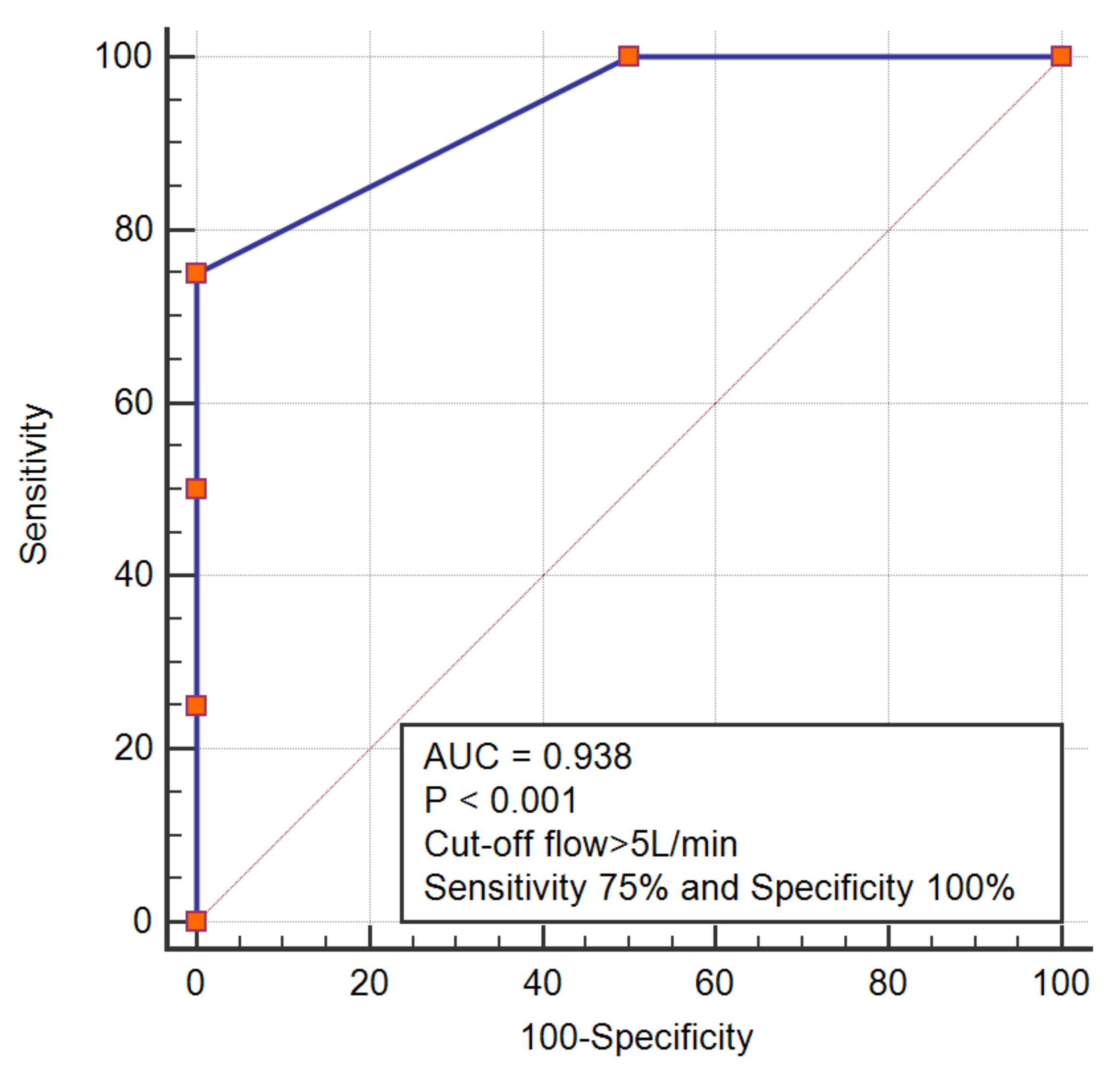
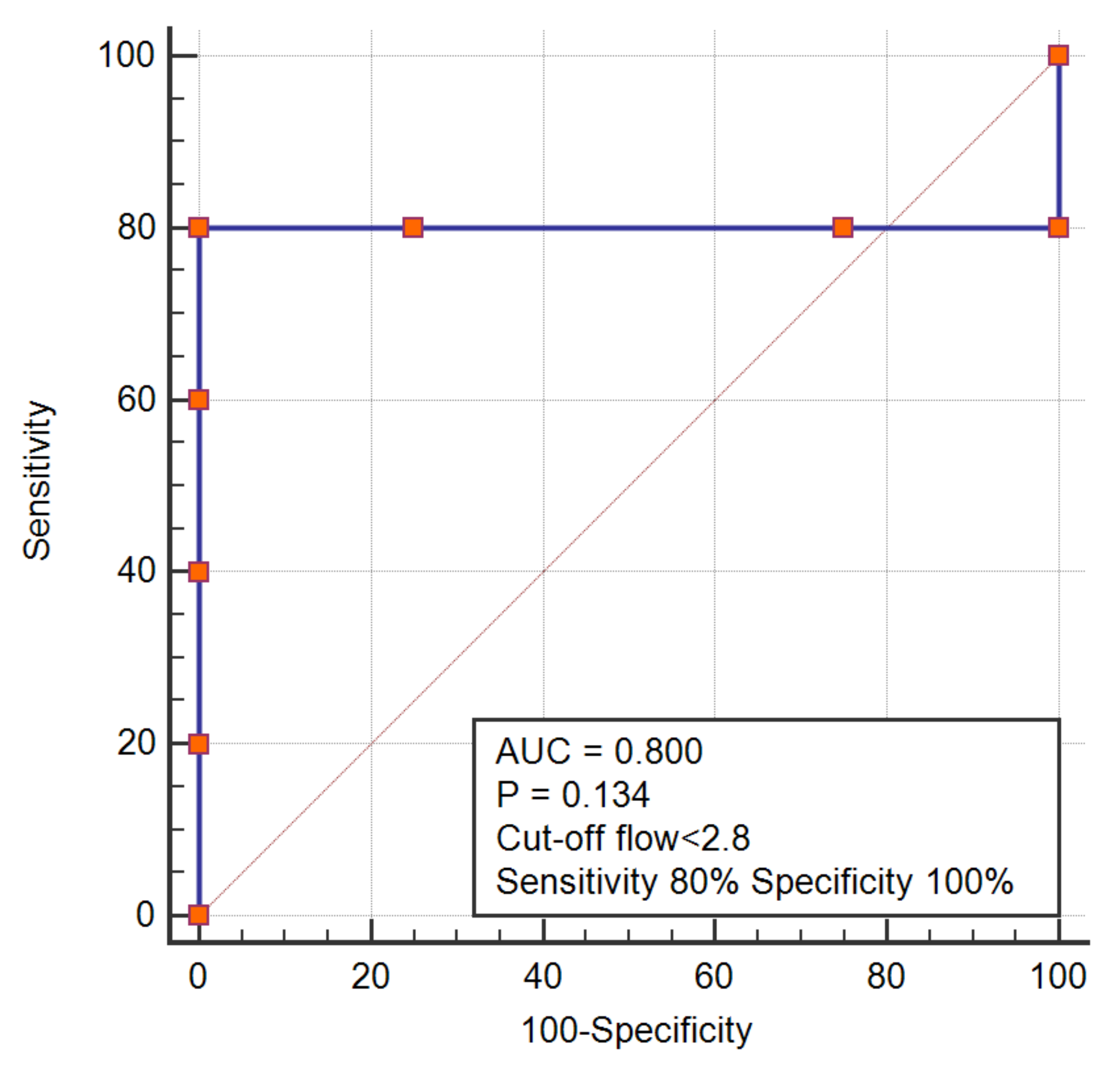
We noticed that SVC pressure might be positively correlated with the flow rate in the connecting chamber design, but negatively correlated with the flow rate in the intravascular pump design. To confirm this pattern, we performed a receiver operating characteristic analysis, which demonstrated that a flow rate > 5 in connecting chamber design could predict SVC pressure > 10, while a flow rate < 2.8 in an intravascular pump was associated with an SVC pressure > 10 (
Figure 2 and
Figure 3)
Only Good et reported the occurrence of thrombosis and hemolytic reaction.
Discussion
It is well established that Fontan surgery, a palliative surgical procedure performed on patients with a single functional ventricle, helps alleviate symptoms and improve the quality of life for these patients. The surgery redirects blood flow so that oxygen-poor blood from the body bypasses the heart and goes directly to the lungs, improving oxygenation. Although Fontan significantly increases survival rates for patients with a single left ventricle, it eventually leads to declining hemodynamics, resulting in Fontan failure [
23].
Efforts to treat a failing Fontan have been focused primarily on heart transplants, whenever possible. Over the recent years, however, cavo-pulmonary assist devices (CPAD) have been developed and tested in vivo and in vitro, in efforts to reduce the strain on the single functional ventricle. These devices come in designs that uphold or substitute the conventional total Cavo pulmonary connection. In this systemic review, we discuss the findings of the different trials conducted on the types of Fontan assist devices. Three main Cavo pulmonary assist devices were studied in vitro and in vivo which are the connecting chamber, the compression device, and the impeller (the intravascular pump) [
8].
Comparing the feasibility of each of these designs, it is evident that the intravascular pump is more feasible regarding its mode of insertion. The connecting chamber and the compression device require a surgical approach for their insertion, while intravascular pump designs are inserted via a transcatheter route. This has been reflected in the number of in-vivo trials across the three designs, which clearly shows that most of the trials (67%) employed in the intravascular design were in-vivo compared to 0% of compression devices’ trials and 9% of the connecting chamber designs [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14].
Another factor worth examining is the power consumption of each device. The power consumption was not mentioned in the examined papers, but it largely depends on the pump speed. It was concluded that the impeller, or the intravascular pump, functions at the highest pump speed rendering its power supply under question [
5,
6,
7,
8,
9,
11,
12,
13,
14].
In addition to the power consumption, to comprehensively grasp the hemodynamics of the pumps, it is crucial to assess their hemolytic potential as the pumps are subjected to high pressure of the blood flowing through the devices.
The clinical trials have shown that fluid shear stresses within the assist devices consistently stayed below typical thresholds associated with red blood cell damage. Nevertheless, according to, Good et al. (2022), the risk of hemolysis was not observed with the compression device and was seen most frequently in the impeller, or the intravascular device [
9].
Given that the purpose of the assist devices is to help failing Fontan by decreasing the systemic intravenous pressure, unloading Fontan congestion, and preventing subsequent complications, it is critical to study the effect of the devices on the superior vena cava pressures, and not only on the inferior vena cava pressure. Theoretically, a higher flow rate will relieve initially the IVC congestion but will elevate the upstream vascular resistance leading ultimately to an elevation of the downstream pressures. The latter vicious circle means that there should be a limiting flow rate above which, pressures in the SVC (reflecting PA pressures) will start to rise leading to the ultimate failure of the system. When reviewing the literature, it has been noted that the correlation between the superior vena cava pressure and the flow rate of the pump was not studied previously. Therefore, we aimed to inspect the possibility of a relationship between the flow rate of the pump and SVC pressure. Since a pre-Fontan SVC pressure below 12–14 mmHg [
24], is often regarded as ideal for completion of Fontan, we placed an SVC pre-set pressure value of 10 mmHg as a classifying variable that determines whether the assist device has started this vicious circle of failure or not. For this purpose, we performed a receiver-operating characteristic analysis, where we tried to determine the cut-off flow rate above or below which, SVC pressures will exceed an optimal value of 10 mmHg. Having said that, a close examination of the trials published so far revealed that for the connecting chamber design, a flow rate higher than 5, will, lead to an elevation of SVC pressure > 10 mmHg. As opposed to the intravascular pump, which in comparison, showed that lower flow rates were associated with higher SVC pressures. At this point, we had to re-explore the mechanical facts behind pump designs; two pump categories exist, the centrifugal and positive displacement pumps. Both pumps employed in Fontan assist device, employ the centrifugal techniques. Centrifugal pumps are also alternatively termed continuous or fixed flow pumps, and it is certain that centrifugal pumps encounter the mentioned limiting factor of higher upstream pressure with higher flow rates. Unlike a centrifugal pump which produces pressure, a positive displacement pump does not produce pressure—it is the system itself that develops pressure from the pressure drop which then creates a back pressure which largely depends on the flow rate through the system, and they do require higher flow rates. It is therefore, very intriguing, that the intravascular pumps used for the Fontan assist, are designed as centrifugal devices, but functionally they behave as a positive displacement pump [
25,
26].
Therefore, the insertion mode, and the absence of limiting flow-rate might favour the use of intravascular pump design, however trials need to go a step beyond, toward extensive human experimentation, to determine their feasibility and success.
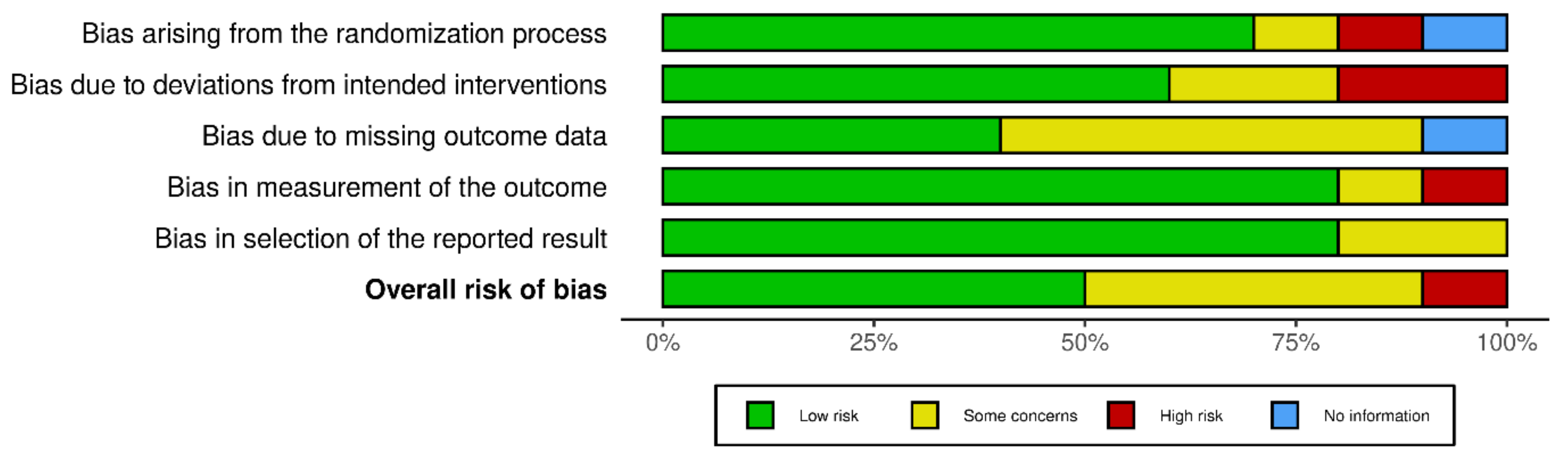
Risk of bias assessment of the included trials is described in
Figure 4.
Conclusions
After carefully reviewing and analysing the literature regarding pumps used in Fontan failure, a conclusion can be made that studies regarding this topic are limited. The shortage of studies could be explained by the fact that the Fontan population is a minority. Moreover, the number of patients with a failing Fontan who are eligible for pump insertion is even less. This review calls for more studies to be done on Fontan failure pumps to better understand the suitability of each pump to a given patient to achieve a patient-centered treatment.
List of Abbreviations
CPAD: Cavo-pulmonary assist device
HLHS: Hypoplastic left heart syndrome.
PA: Pulmonary artery
SVC/IVC: Superior/Inferior vena cava.
Author Contributions
Conceptualization, AA; Methodology, AA, ZA, MT, RG, AF, OM, LK; software, AA, ZA, MT, RG, AF, OM, LK; investigation, AA, ZA, MT, RG, AF, OM, LK; resources, AA, ZA, MT, RG, AF, OM, LK; data curation, AA, ZA, MT, RG, AF, OM, LK; writing—original draft preparation, AA, ZA, MT, RG, AF, OM, LK; writing—review and editing, AA, ZA, MT, RG, AF, OM, LK; supervision, AA project administration, AA; funding acquisition, (none). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable as this study is a systematic review of the reported cases.
Informed Consent Statement
Not applicable as this study is a systematic review of the reported cases.
Data Availability Statement
All data is made available within the manuscript.
Acknowledgments
I wanted, as a first author, to dedicate this work to anyone who is considered as a “black sheep” in his workplace, college or school environment, just because he is beautifully different. We all, through different stages of our life, have gone through similar, difficult times, where we felt alone and non-appreciated, but you should know that your personal worth is unrelated to others’ perception.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hassan A, Chegondi M, Porayette P. Five decades of Fontan palliation: What have we learned? What should we expect? J Int Med Res [Internet]. 2023 Oct 1;51(10). Available online: http://journals.sagepub.com/doi/10.1177/03000605231209156. [CrossRef]
- Heying R, D’Udekem Y, Gewillig M, Rychik J. Editorial: The fontan circulation: Problems and solutions. Front Pediatr [Internet]. 2022 Dec 1;10. Available online: https://www.frontiersin.org/articles/10.3389/fped.2022.1087739/full.
- Schamroth Pravda N, Richter I, Blieden L, Dadashev A, Vig S, Yehuda D, et al. Long-Term Outcomes and Characteristics Associated With Mortality of Adult Patients Post Fontan Surgery: 27-Year Single-Center Experience. Am J Cardiol [Internet]. 2023 Nov;207:392–8. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0002914923009566.
- Ferrari MR, Di Maria M V., Jacot JG. Review on Mechanical Support and Cell-Based Therapies for the Prevention and Recovery of the Failed Fontan-Kreutzer Circulation. Front Pediatr. 2021;8(January).
- Riemer RK, Amir G, Reichenbach SH, Reinhartz O. Mechanical support of total cavopulmonary connection with an axial flow pump. J Thorac Cardiovasc Surg. 2005;130(2):351–4.
- Haggerty CM, Fynn-Thompson F, McElhinney DB, Valente AM, Saikrishnan N, Del Nido PJ, et al. Experimental and numeric investigation of Impella pumps as cavopulmonary assistance for a failing Fontan. J Thorac Cardiovasc Surg [Internet]. 2012;144(3):563–9. [CrossRef]
- Cysyk J, Clark JB, Newswanger R, Jhun CS, Izer J, Finicle H, et al. Chronic In Vivo Test of a Right Heart Replacement Blood Pump for Failed Fontan Circulation. ASAIO J. 2019;65(6):593–600.
- Rodefeld MD, Boyd JH, Myers CD, LaLone BJ, Bezruczko AJ, Potter AW, et al. Cavopulmonary Assist: Circulatory Support for the Univentricular Fontan Circulation. Ann Thorac Surg. 2003;76(6):1911–6.
- Good BC, Ponnaluri S V., Weiss WJ, Manning KB. Computational Modeling of the Penn State Fontan Circulation Assist Device. ASAIO J. 2022;68(12):1513–22.
- Valdovinos J, Shkolyar E, Carman GP, Levi DS. In vitro evaluation of an external compression device for fontan mechanical assistance. Artif Organs. 2014;38(3):199–207.
- Cysyk JP, Lukic B, Joseph Brian C, Newswanger R, Jhun CS, Izer J, et al. Miniaturized Fontan Circulation Assist Device: Chronic in Vivo Evaluation. ASAIO J. 2021;67(11):1240–9.
- Derk G, Laks H, Biniwale R, Patel S, De Lacruz K, Mazor E, et al. Novel techniques of mechanical circulatory support for the right heart and Fontan circulation. Int J Cardiol [Internet]. 2014;176(3):828–32. [CrossRef]
- Rodefeld MD, Marsden A, Figliola R, Jonas T, Neary M, Giridharan GA. Cavopulmonary assist: Long-term reversal of the Fontan paradox. J Thorac Cardiovasc Surg [Internet]. 2019;158(6):1627–36. [CrossRef]
- Lacour-Gayet FG, Lanning CJ, Stoica S, Wang R, Rech BA, Goldberg S, et al. An Artificial Right Ventricle for Failing Fontan: In Vitro and Computational Study. Ann Thorac Surg [Internet]. 2009 Jul;88(1):170–6. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0003497509006043.
- Granegger M, Thamsen B, Hubmann EJ, Choi Y, Beck D, Valsangiacomo Buechel E, et al. A long-term mechanical cavopulmonary support device for patients with Fontan circulation. Med Eng Phys [Internet]. 2019 Aug;70:9–18. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1350453319301110.
- Escher A, Strauch C, Hubmann EJ, Hübler M, Bortis D, Thamsen B, et al. A Cavopulmonary Assist Device for Long-Term Therapy of Fontan Patients. Semin Thorac Cardiovasc Surg. 2022;34(1):238–48.
- Trusty PM, Tree M, Maher K, Slesnick TC, Kanter KR, Yoganathan AP, et al. An in vitro analysis of the PediMag and CentriMag for right-sided failing Fontan support. J Thorac Cardiovasc Surg [Internet]. 2019;158(5):1413–21. [CrossRef]
- Farahmand M, Kavarana MN, Trusty PM, Kung EO. Target Flow-Pressure Operating Range for Designing a Failing Fontan Cavopulmonary Support Device. IEEE Trans Biomed Eng. 2020;67(10):2925–33.
- Lin WCP, Doyle MG, Roche SL, Honjo O, Forbes TL, Amon CH. Computational fluid dynamic simulations of a cavopulmonary assist device for failing Fontan circulation. J Thorac Cardiovasc Surg [Internet]. 2019;158(5):1424-1433.e5. [CrossRef]
- Rodefeld MD, Coats B, Fisher T, Giridharan GA, Chen J, Brown JW, et al. Cavopulmonary assist for the univentricular Fontan circulation: Von Kármán viscous impeller pump. J Thorac Cardiovasc Surg [Internet]. 2010 Sep;140(3):529–36. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0022522310005520.
- Michel SG, Menon AK, Haas NA, Hörer J. Cavopulmonary support with a modified cannulation technique in a failing Fontan patient. Interact Cardiovasc Thorac Surg. 2022;35(2):2–4.
- Ponnaluri S V., Christensen EJ, Good BC, Kubicki CJ, Deutsch S, Cysyk JP, et al. Experimental Hemodynamics Within the Penn State Fontan Circulatory Assist Device. J Biomech Eng. 2022;144(7):1–10.
- Schumacher KR, Cedars A, Allen K, Goldberg D, Batazzi A, Reichle G, et al. Achieving consensus, severity-graded definitions of Fontan-associated complications to characterize Fontan circulatory failure. J Card Fail [Internet]. 2024 Mar. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1071916424000757.
- AbdelMassih A, Kiraly L, El Badaoui H, Khan M, Hetharsi B, Till J, et al. Predictors of protein losing enteropathy after Fontan completion: An 8-year retrospective study at Sheikh Khalifa Medical City. Glob Cardiol Sci Pract [Internet]. 2023 Jun 27;2023(3). Available online: https://globalcardiologyscienceandpractice.com/index.php/gcsp/article/view/607.
- Nasir A, Dribssa E, Girma M. The pump as a turbine: A review on performance prediction, performance improvement, and economic analysis. Heliyon [Internet]. 2024 Feb;10(4):e26084. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2405844024021157.
- Li P, Mei X, Ge W, Wu T, Zhong M, Huan N, et al. A comprehensive comparison of the in vitro hemocompatibility of extracorporeal centrifugal blood pumps. Front Physiol [Internet]. 2023 May 9;14. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2023.1136545/full.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).