1. Introduction
Interferon (IFN)-γ, a type II IFN (IFN-II), is essential for the host’s defense against infection with intracellular pathogens [
1,
2]. Although the exact mechanisms behind the autoantibodies formation against IFN-γ (anti-IFN-γ autoAbs) remain unclear, several studies have shown that these autoAbs have a suppressive effect on IFN-γ signal transduction [
3,
4]. Therefore, neutralizing anti-IFN-γ autoAbs are associated with increased risks of opportunistic infections (OIs), including varicella-zoster virus (VZV) infection [
3,
4,
5,
6]. Hong et al. revealed that anti-IFN-γ autoAb titers were closely related to the severity of infections, reflecting the biological activity of anti-IFN-γ autoAbs [
7]. Anti-IFN-γ autoAbs have also been observed in autoimmune inflammatory diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [
8,
9], despite the unclear clinical impact.
RA, an inflammatory disease, is characterized by persistent synovitis, bone destruction, and poor life quality [
10]. Epidemiological studies reveal that RA patients have an elevated risk of HZ compared to the general population [
11,
13]. Targeting the complex pathogenesis of RA, emerging new agents are available for the treatment of this disease, including biologic disease-modifying anti-rheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) such as Janus kinase inhibitors (JAKi) [
14]. Although their therapeutic efficacies are comparable to bDMARDs, JAKi are associated with a significantly increased incidence of HZ in clinical trials and long-term extension studies [
15,
16]. Involvement of the JAK/signal transducer and activator of transcription (STAT1)-pathway in the anti-viral actions of IFN-γ [
17,
18] may explain an increased HZ risk associated with JAKi therapy. However, the associations between anti-IFN-γ autoAbs and the risk of new-onset HZ has yet to be explored in RA patients treated with JAKi.
This pilot study aimed to compare the difference in serum titers of anti-IFN-γ IgG between RA patients with and without new-onset HZ during the therapeutic period of JAKi. We also determined plasma levels of IFN-γ, monocyte chemoattractant protein-1 (MCP-1), and IFN-γ inducible protein-10 (IP-10), which were related to the IFN-γ signaling-mediated STAT1 transactivation. Finally, the associations of anti-IFN-γ IgG with RA activity parameters and proinflammatory cytokines/chemokines were examined in JAKi-treated patients.
2. Materials and Methods
2.1. Patients and Study Design
In this prospective and single-center study, we enrolled twenty-four patients who had RA according to the American College of Rheumatology/European League Against Rheumatism criteria [
19] and developed new-onset HZ after starting JAKi therapy. Forty-two RA patients without the development of new-onset HZ during the JAKi therapeutic period were included as the control group. All RA patients were in active disease status and received JAKi due to responding inadequately to csDMARDs or bDMARDs. The JAKi used included tofacitinib (11mg once daily, n=50), baricitinib (4mg once daily, n=4), and upadacitinib (15 mg once daily, n=12), either as monotherapy or in combination with methotrexate (MTX), leflunomide, or hydroxychloroquine. RA disease activity was assessed using the 28-joint disease activity score-erythrocyte sedimentation rate (DAS28-ESR) [
20], with active disease defined as a DAS28 score of 3.2 or higher. The exclusion criteria were current infection, malignancy, or severe hepatic impairment before starting JAKi therapy. Blood samples were obtained before the emergence of new-onset HZ in RA patients receiving JAKi therapy. This study was approved by the Institutional Review Board of Chinese Medicine University Hospital (CMUH110-REC1-086), and informed consent was obtained from each participant according to the Declaration of Helsinki.
2.2. Diagnosis and Severity of HZ
HZ was diagnosed based on rheumatologists’ clinical assessment and the use of anti-viral therapy, according to the medical records. This methodology has been validated in previous large studies [
21], demonstrating a positive predictive value of 97.5% in identifying HZ cases [
21]. The history of HZ before starting JAKi treatment was extracted from patients' medical records and was confirmed by phone calls. Disseminated HZ (a diffuse rash spanning more than six dermatomes), HZ with multi-dermatomal involvement (2 non-adjacent or 3-6 adjacent dermatomes), central nervous system involvement such as encephalitis, or involvement of non-skin organs were considered to be the severe HZ.
2.3. The Clinical Phenotypes
The clinical data collected from RA patients included demographic data, disease duration, disease activity, the positivity for rheumatoid factor (RF) or anti-citrullinated peptide antibodies (ACPA), history of HZ prior to initiating JAKi therapy, the concomitant use of corticosteroids or csDMARDs, and smoking status. Seropositive RA was defined as the presence of rheumatoid factor (RF) and/or anti-citrullinated peptide antibodies (ACPA), and patients negative for both RF and ACPA were considered seronegative.
2.4. Determination of RF and ACPA
Serum levels of RF-immunoglobulin (Ig)M were determined using the IMMAGE® Immunochemistry Systems and Calibrator 5 Plus (Beckman Coulter Ireland Inc., Mervue Business Park, Mervue, Galway, Ireland). Levels <20 IU/mL were considered negative. The ACPA-IgG levels were determined using the EliA™ technique (POhadia 250; Thermo Fisher Scientific, Uppsala, Sweden). Levels were considered negative if <7 U/mL, equivocal if 7–10 U/mL, and positive if >10 U/mL).
2.5. Determination of Serum Titers of Anti-IFN-γ IgG with ELISA
Each ten-milliliter whole blood sample was collected in a tube containing EDTA (BD Biosciences, San Jose, CA, USA) and centrifuged at 2,000 rpm for 10 min. According to the manufacturer’s instructions, serum titers of IgG autoantibodies to IFN-γ were determined with ELISA (cat. abx585481, Abbexa Ltd, Cambridge, UK).
2.6. Determination of Plasma Levels of IFN-γ, MCP-1 and IP-10
We used commercial ELISA kits to determine plasma levels of IFN-γ (DY285B, R&D Systems, Minneapolis, MN, USA), MCP-1 (DY279, R&D Systems, Minneapolis, MN, USA), and IP-10 (DY266, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
2.7. Statistical Analysis
The results were presented as numbers (percent), mean ± standard deviation (SD), or median (interquartile range [IQR]). The p-values were calculated using the chi-squared test for between-group differences in categorical variables. The Mann-Whitney U and Fisher exact t-test were used for between-group comparison of numerical variables. The correlation coefficient was obtained through the nonparametric Spearman’s rank correlation test. The missing values were excluded from the statistical analysis. A two-sided probability of less than 0.05 was considered significant.
3. Results
3.1. The Comparison of Clinical Characteristics, Laboratory Data, and HZ-Related Status between RA Patients with and without New-Onset HZ
Among these 66 RA patients who received JAKi therapy during the mean follow-up of 4.3 years, twenty-four (36.4%) had new-onset HZ. Of the 24 patients with HZ after starting JAKi therapy, severe HZ was observed in three patients who had multi-dermatomal involvement. RA patients with new-onset HZ had significantly longer disease duration, higher doses of concomitant corticosteroids, and a higher proportion of high-dose corticosteroids compared to those without HZ after starting JAKi therapy. There were no significant differences in age at study entry, the proportion of females, the proportion of prior HZ history, baseline disease activity, the positive rate of RF or ACPA, the proportion of JAKi monotherapy, he concomitant csDMARDs, different JAKi, or smoking status between RA patients with and without new-onset HZ (
Table 1).
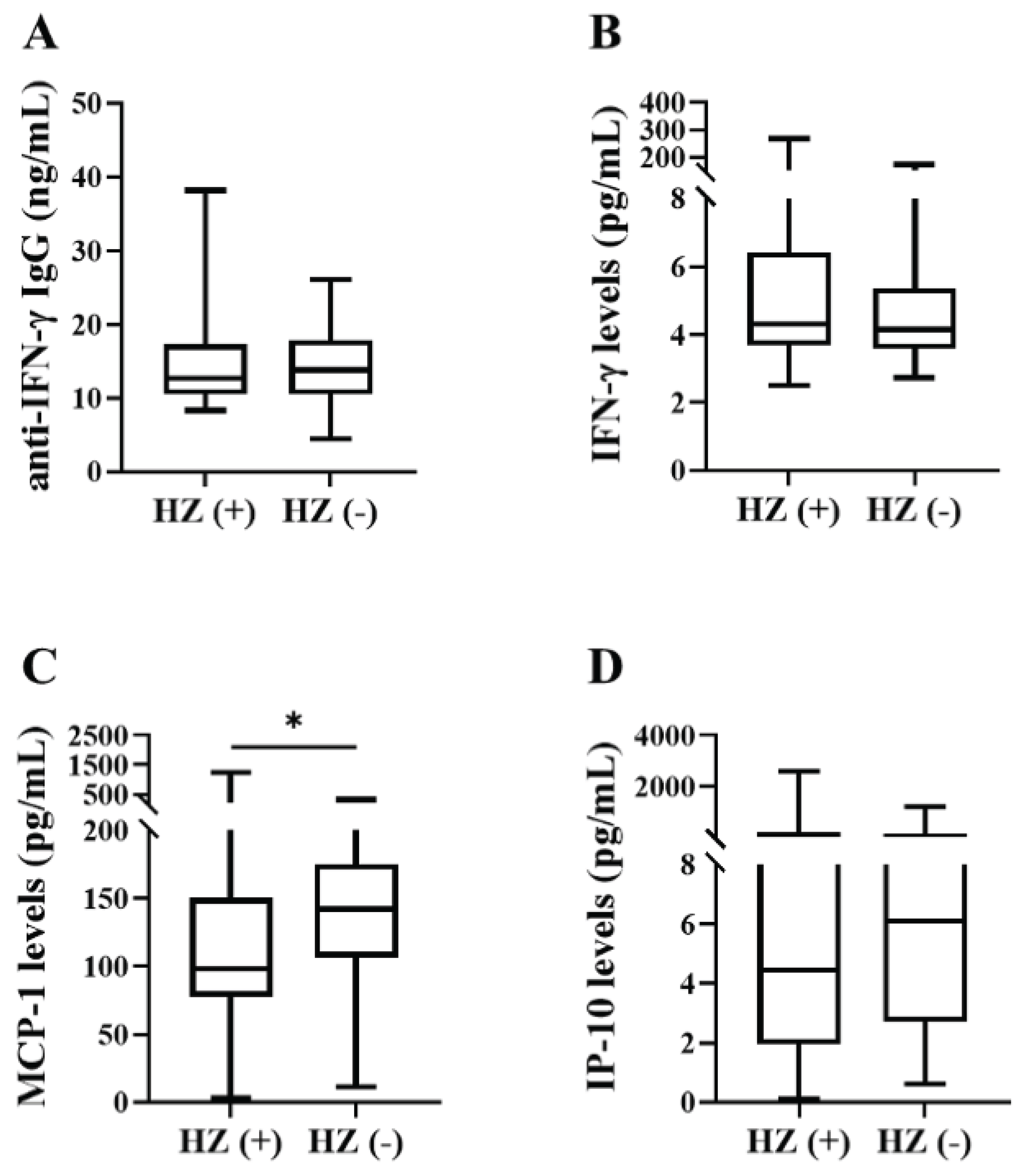
3.2. Comparison of the Titers of anti-IFN-γ IgG, Plasma Levels of IFN-γ, MCP-1, and IP-10 between RA Patients with and without HZ.
As illustrated
Figure 1C, significantly lower levels of MCP-1 were observed in RA patients with new-onset HZ compared to those without HZ (median, 98.21pg/ml, interquartile range (IQR) 77.63-150.30pg/ml versus median 142.3pg/ml, IQR 106.7-175.6pg/ml, p<0.05). There was a trend of lower IP-10 levels (
Figure 1D) observed in RA patients with HZ compared to those without HZ (mean, 4.45pg/mL versus 6.10pg/mL, p=0.48,
Figure 1D). However, there was no significant difference in the titers of anti-IFN-γ IgG (median 12.69ng/ml, IQR 10.46-17.35ng/ml versus median 13.83ng/ml, IQR 10.46-17.96ng/ml,
Figure 1A), or plasma levels of IFN-γ (median, 4.32pg/ml, IQR 3.69-6.42pg/ml versus 4.15pg/ml, IQR 3.58-5.36pg/ml, p=0.56,
Figure 1B) between RA patients with and without new-onset HZ.
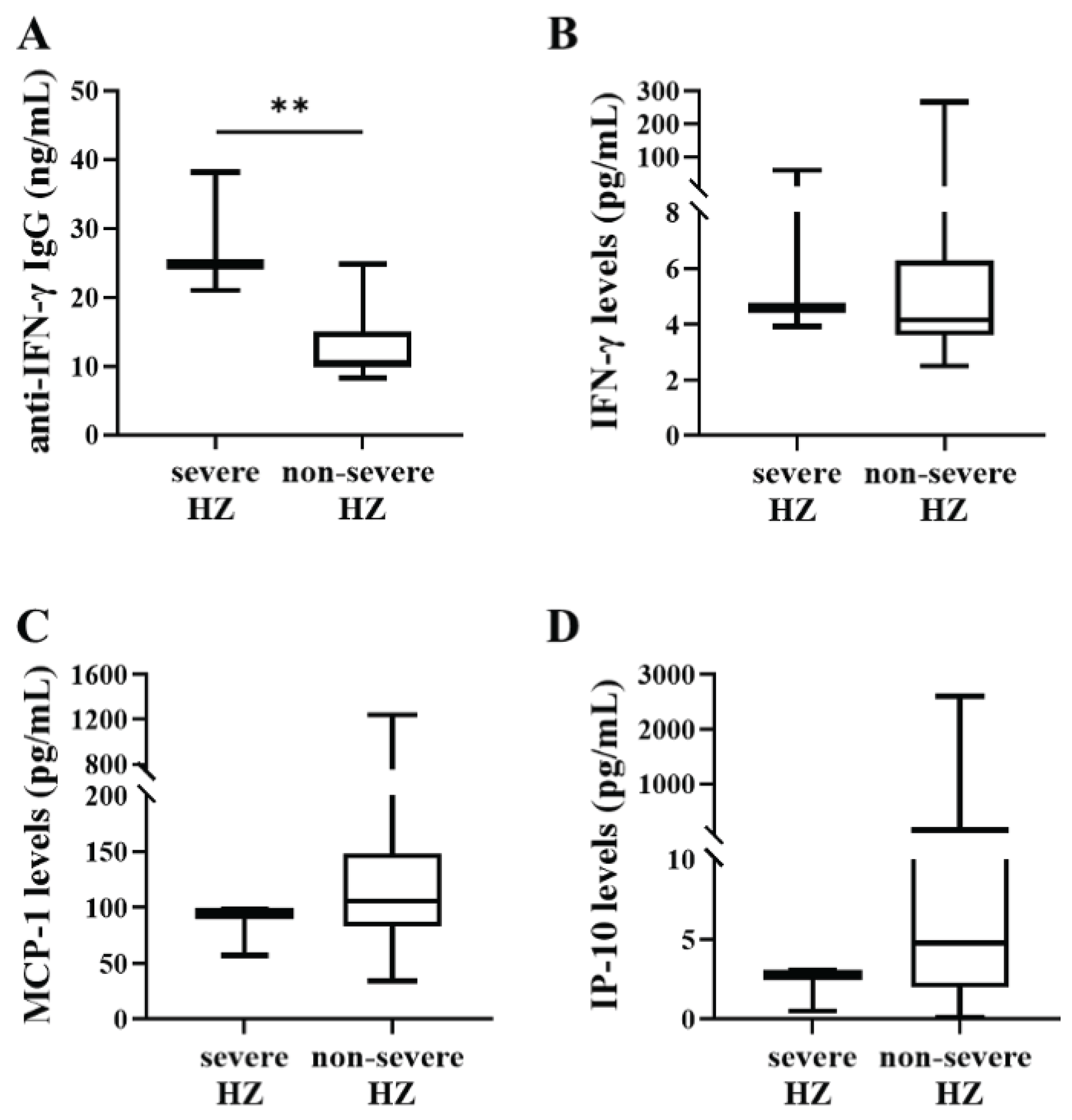
3.3. Comparison of the Titers of Anti-IFN-γ IgG, Plasma Levels of IFN-γ, MCP-1, and IP-10 between RA Patients with Severe and Non-Severe HZ.
Among 24 patients with new-onset HZ after starting JAKi therapy, three patients had severe HZ presenting with multi-dermatomal involvement. As illustrated in
Figure 2A, significantly higher titers of anti-IFN-γ IgG (median 24.8ng/ml, IQR 21.0-38.2ng/ml versus median 10.5ng/ml, IQR 9.9-15.0ng/ml, p<0.005). However, there was no significant difference in plasma levels of IFN-γ (median, 4.59pg/ml, IQR 3.93-59.03pg/ml versus 4.15pg/ml, IQR 3.61-6.29pg/ml, p=0.50,
Figure 2B), MCP-1 (median, 94.68pg/ml, IQR 57.03-98.21pg/ml versus median 105.80pg/ml, IQR 82.91-148.50pg/ml,
Figure 2C), or IP-10 (median 2.80ng/ml, IQR 0.50-3.10ng/ml versus median 4.80ng/ml, IQR 2.00-204.60ng/ml,
Figure 2D) between RA patients with severe and non-severe HZ.
Among 24 patients with new-onset HZ, three patients had severe HZ presenting with multi-dermatomal involvement. * Data are presented as box-plot diagrams, with the box encompassing the 25th percentile (lower bar) to the 75th percentile (upper bar). The horizontal line within the box indicates median value for each group. **p<0.005, determined by the Mann-Whitney U test. Anti-IFN-γ IgG, anti-interferon-γ IgG; IFN-γ, interferon-γ; MCP-1, monocyte chemoattractant protein-1; IP-10, IFN-γ inducible protein-10; RA: Rheumatoid arthritis.; HZ, herpes zoster.
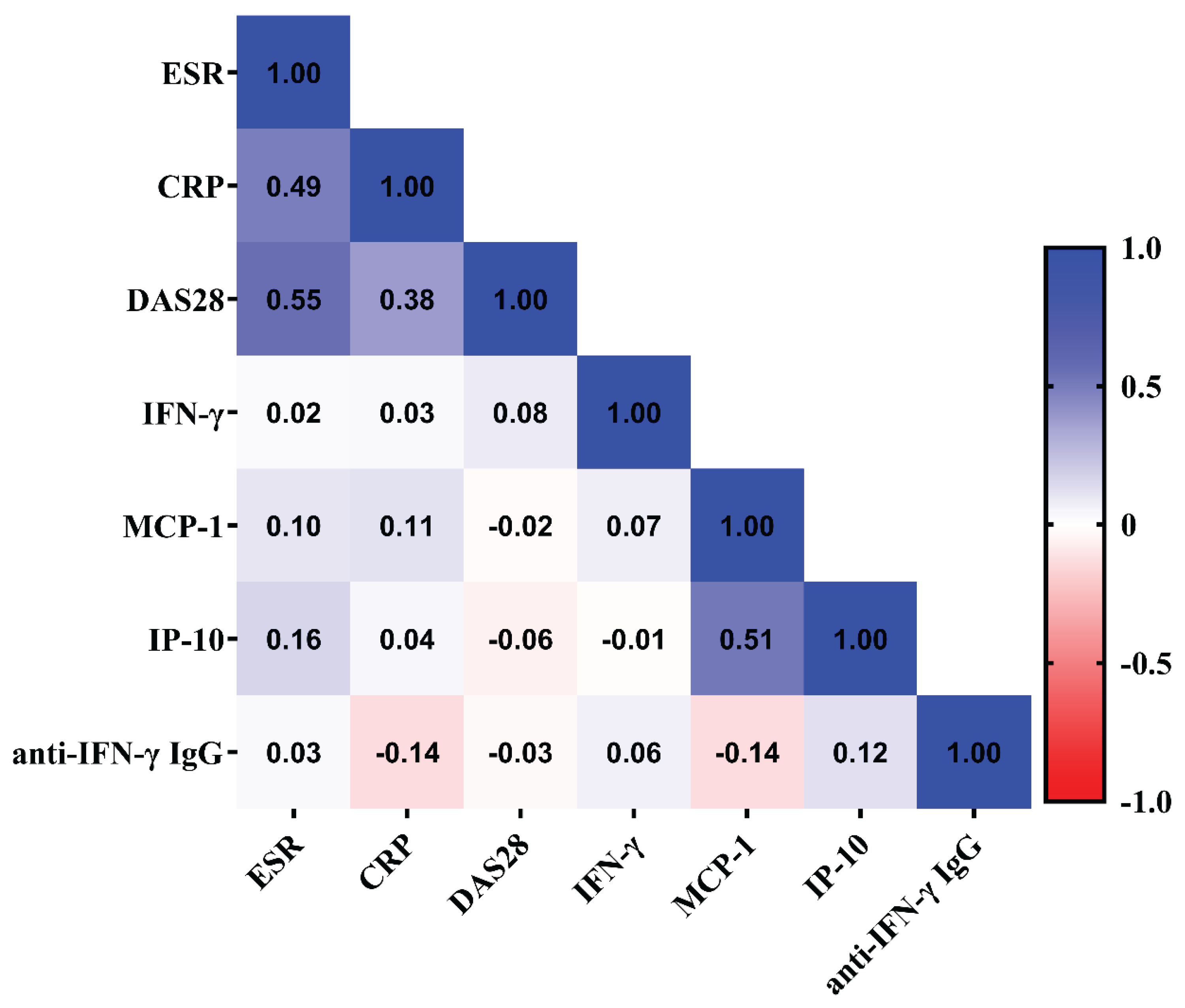
3.4. Association of Serum Titers of anti-IFN-γ IgG with RA Disease Activity and Inflammatory Parameters
As shown in
Figure 3, there was a trend of inverse correlation between anti-IFN-γ IgG titers and plasma levels of MCP-1. However, there was no significant correlation between anti-IFN-γ IgG titers and RA disease activity, reflected by DAS28 scores, and inflammatory parameters in RA patients.
4. Discussion
Increasing evidence suggests a close link between anti-IFN-γ autoAbs or chemokines and OIs [
3,
4,
5,
6,
7,
22,
23], yet their relationship in RA patients with new-onset HZ has not been explored. Compared to RA patients without HZ development, our JAKi-treated patients with new-onset HZ have a longer disease duration, higher doses of corticosteroids, and a higher proportion of high-dose corticosteroids used. We also reveal significantly lower levels of MCP-1, but not anti-IFN-γ IgG, in RA patients with VZV reactivation after starting JAKi therapy. It is worth mentioning that significantly higher titer of anti-IFN-γ IgG in three patients with severe HZ presenting with multi-dermatomal involvement than those with non-severe HZ. These findings suggest that low-level MCP-1 is associated with HZ development, and high-titer anti-IFN-γ IgG is probably related to severe HZ in RA patients after initiation of JAKi therapy. Further validation in studies with a larger sample size is needed.
Although anti-cytokine autoAbs have been observed in immune-mediated inflammatory diseases [
8,
22,
23], whether anti-IFN-γ autoAbs in these diseases contribute to the susceptibility to infections remains unclear. Recently, Chen et al. revealed significantly higher titers of anti-IFN-γ IgG in SLE patients with severe infections compared to those without infections [
23]. They also identified anti-IFN-γ IgG as a significant predictor for developing severe infections in SLE patients [
23]. Anti-IFN-γ IgG-positive SLE patients were also more susceptible to mycobacterial and fungal infections compared to those without anti-IFN-γ IgG [
23]. Although we demonstrated no significant difference in serum titers of anti-IFN-γ IgG between RA patients with and without new-onset HZ, significantly higher titers of anti-IFN-γ IgG were observed in RA patients with severe HZ than in those with non-severe HZ. These observations support the findings reported by Hong et al. that anti-IFN-γ IgG titers were strongly associated with the severity of infections [
7].
In the biological role of IFN-γ signaling against infection with intracellular pathogens [
1,
2], IFN-γ could activate the transcription of genes of proinflammatory cytokines or chemokines after STAT1 phosphorylation. Given that the neutralizing effects of anti-IFN-γ autoAbs on the IFN-γ signaling pathway are mediated by targeting STAT1 transactivation and chemokines production. Krisnawati et al. revealed that treatment with non-tuberculous mycobacterial infection (NTM) patients’ sera significantly blocked the IFN-γ-induced production of IFN-γ, MCP-1, and IP-10 [
24]. Resonated with these findings, we revealed significantly lower levels of plasma MCP-1 in RA patients with new-onset HZ compared to those without HZ. Given that MCP-1 could contribute to anti-microbial inflammatory response by attracting monocytes and T lymphocytes [
25], the low level of MCP-1 may increase infection with intracellular pathogens such as varicella-zoster virus. Gathering the evidence from other studies [
22,
23,
24,
25,
26] and ours, high-titer anti-IFN-γ IgG may reduce antimicrobial activity at least partly by counteracting the IFN-γ-mediated production of chemokines. Although anti-IFN-γ IgG could neutralize IFN-γ, the non-significant difference in plasma IFN-γ levels between RA patients with and without HZ was probably due to the small sample size in our study.
It has been proposed that the development of anti-cytokine antibodies may be part of an immune regulatory response to inflammation or due to abundant cytokine exposure [
27,
28]. Gupta et al. demonstrated an association of anti-IFN-γ autoAbs with SLE disease activity, rather than with opportunistic infections [
8]. Chen et al. also revealed a positive correlation between anti-IFN-γ IgG titers and SLE disease activity [
23]. However, we revealed no significant correlation between anti-IFN-γ IgG titers and disease activity or inflammatory parameters in RA group. The discrepancy may be the differences in the enrolled diseases and patients’ characteristics, the methods for detecting anti-IFN-γ IgG, and the medications used.
Despite the novel findings, there are some limitations of this study. The lack of a significant difference in anti-IFN-γ IgG titers between RA patients with and without new-onset HZ might be due to the insufficient number of patients with new-onset HZ. Given the higher dose of corticosteroids prescribed in RA patients with HZ than in those without HZ (
Table 1), the titers of anti-IFN-γ IgG might also be influenced by the therapeutic agents in the present study. The non-significant correlations between anti-IFN-γ IgG titers and RA activity or the downstream cytokines /chemokines due to the small sample size of our RA cohort. Finally, HZ development may result from the inherited mutations of IFN-γ-signaling-related genes, which were not evaluated in our RA patients. Therefore, a large-scale study with sufficient statistical power is needed to validate this finding and support its clinical implication.
5. Conclusions
We are the first to examine the associations between anti-IFN-γ autoAbs and HZ development in RA patients treated with JAKi. The results revealed significantly lower MCP-1 levels in RA patients with HZ than those without, and significantly higher titer of anti-IFN-γ IgG in patients with severe HZ compared to those with non-severe HZ. Nevertheless, these associations were observed in a small cohort of our RA patients and needed further validation.
Author Contributions
P-KC conceived and designed the study, acquired the laboratory data, performed the data analysis, and drafted the manuscript. Y-MC and H-HC acquired the clinical data and performed the data analysis. T-LL acquired the laboratory data and performed data analysis. S-HC, K-JY, and P-HH acquired the clinical data. D-YC conceived and designed the study, acquired the clinical data, performed data analysis, and revised the manuscript. All authors have read and approved the final manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Shiow-Jiuan Wey, MD, of the Chung Shan Medical University Hospital, Taiwan, for manuscript editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schoenborn, J.R.; Wilson, C. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007, 96, 41–101. [Google Scholar]
- Shakya, A.K.; O’Callaghan, D.J.; Kim, S.K. Interferon gamma inhibits Varicella-Zoster virus replication in a cell line-dependent manner. J Virology 2019, 93, e00257–19. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.Y.; Ding, L.; Brown, M.R.; Lantz, L.; Gay, T.; Cohen, S.; Martyak, L.A.; Kubak, B.; Holland, S.M. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 2005, 175, 4769–4776. [Google Scholar] [CrossRef] [PubMed]
- Wipasa, J.; Chaiwarith, R.; Chawansuntati, K.; Praparattanapan, J.; Rattanathammethee. K.; Supparatpinyo, K. Characterization of anti-interferon-γ antibodies in HIV-negative immunodeficient patients infected with unusual intracellular microorganisms. Experimental Biology and Medicine 2018, 243, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, PA.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012, 36, 7725–734. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.Y.; Chu, C.C.; Liu, J.P.; Lin, C.H.; Ho, M.W.; Lo, W.J.; Lin, P.C.; Chen, H.J.; Chou, C.H.; Feng, J.Y.; et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*1602 and HLA-DQB1*0502 and the reactivation of latent varicella-zoster virus infection. Blood 2013, 121, 1357–1366. [Google Scholar] [CrossRef]
- Hong, G.H.; Ortega-Villa, A.M.; Hunsberger, S.; Chetchotisakd, P.; Anunnatsiri, S.; Mootsikapun, P.; Rosen, L.B.; Zerbe, C.S.; Holland, S.M. Natural history and evolution of anti-interferon-γ autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis 2020, 71, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tatouli, I.P.; Rosen, L.B.; Hasni, S.; Alevizos, I.; Manna, Z.G.; Rivera, J.; Jiang, C.; Siegel, R.M.; Holland, S.M.; et al. Distinct functions of anti-interferon autoantibodies in systemic lupus erythematosus: a comprehensive analysis of anticytokine antibodies in common rheumatologic diseases. Arthritis Rheumatol. 2016, 68, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Wu, U.I.; Wang, J.T.; Sheng, W.H.; Sun, H.Y.; Cheng, A.; Hsu, L.Y.; Chang, S.C.; Chen, Y.C. Incorrect diagnoses in patients with neutralizing anti-interferon-gamma autoantibodies. Clin Microbiol Infection 2020, 26, 1684.e1–1684.e6. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Smitten, A.L.; Choi, H.K.; Hochberg, M.C.; Suissa, S.; Simon, T.A.; Testa, M.A.; Chan, K.A. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007, 57, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.R.; Zeringue, A.L.; Caplan, L.; Ranganathan, P.; Xian, H.; Burroughs, T.E.; Fraser, V.J.; Cunningham, F.; Eisen, S.A. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009, 48, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.L.; Chen, Y.M.; Liu, H., J.; Chen, D.Y. Risk and severity of herpes zoster in patients with rheumatoid arthritis receiving different immunosuppressive medications: a case-control study in Asia. BMJ Open 2017, 7, e0140327. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: an overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60, vi12–vi20. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Tanaka, Y.; Mariette, X.; Curtis, J.R.; Lee, E.B.; Nash, P.; Winthrop, K.L.; Charles-Schoeman, C.; Thirunavukkarasu, K.; DeMasi, R.; et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5years: integrated analysis of data from the global clinical trials. Ann Rheum 2017, 76, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Curtis, J.R.; Charles-Schoeman, C.; Mysler, E.; Yamaoka, K.; Richez, C.; Palac, H.; Dilley, D.; Liu, J.; Strengholt, S.; et al. Safety profile of Upadacitinib in patients at risk of cardiovascular disease: integrated post hoc analysis of the SELECT phase III rheumatoid arthritis clinical programme. Ann Rheum Dis 2023, 82, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct Target Ther 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Raftery, N.; Stevenson, N.J. Advances in anti-viral immune defense: revealing the importance of the IFN JAK/STAT pathway. Cell Mol Life Sci 2017, 74, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O. 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van 't Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995, 38, 44–8. [Google Scholar] [CrossRef]
- Baxter, R.; Bartlett, J.; Fireman, B.; Marks, M.; Hansen, J.; Lewis, E.; Aukes, L.; Chen, Y.; Klein, N.P.; Saddier, P. Long-Term Effectiveness of the Live Zoster Vaccine in Preventing Shingles: A Cohort Study. Am J Epidemiol 2018, 187, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.K.; Liao, T.L.; Chang, S.H.; Yeo, K.J.; Chou, C.H.; Chen, D.Y. Anti-interferon-γ autoantibodies in patients with adult-onset Still’s diseases: association with opportunistic infections by inhibiting IFN-γ signaling chemokines. Front Med (Lausanne) 2023, 9, 1097514. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chi, H.; Teng, J.; Meng, J.; Zhang, H.; Su, Y.; Liu, H.; Ye, J.; Shi, H.; Hu, Q.; et al. Neutralizing anti-IFN-γ IgG was increased in patients with systemic lupus erythematosus and associated with susceptibility to infection. Clin Rheumatol 2024, 43, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Krisnawati, D.I.; Liu, Y.C.; Lee, Y.J.; Wang, Y.T.; Chen, C.L.; Tseng, P.C.; Lin, C.F. Functional neutralization of anti-IFN-gamma autoantibody in patients with non-tuberculous mycobacteria infection. Sci. Rep 2019, 9, 5682. [Google Scholar] [CrossRef]
- Lin, YG.; Gong, J.; Zhang, M.; Xue, W.; Barnes, P.F. Production of monocyte chemoattractant protein-1 in tuberculosis patients. Infect Immun 1998, 66, 2319–22. [Google Scholar] [CrossRef] [PubMed]
- Krisnawati, D.I.; Liu, Y.C.; Lee, Y.J.; Wang, Y.T.; Chen, C.L.; Tseng, P.C.; Shen, T.J.; Lin, C.F. Blockade effects of anti-interferon-(IFN-)γ autoantibody on IFN-γ-regulated antimicrobial immunity. J Immunol Res 2019, 2019, 162925. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Turano, A. Natural antibodies to interferon-γ. Biotherapy 1997, 10, 29–37. [Google Scholar] [CrossRef]
- Karin, N. Induction of protective therapy for autoimmune diseases by targeted DNA vaccines encoding pro-inflammatory cytokines and chemokines. Curr Opin Mol Ther 2004, 6, 27–33. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).