Submitted:
28 March 2024
Posted:
28 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
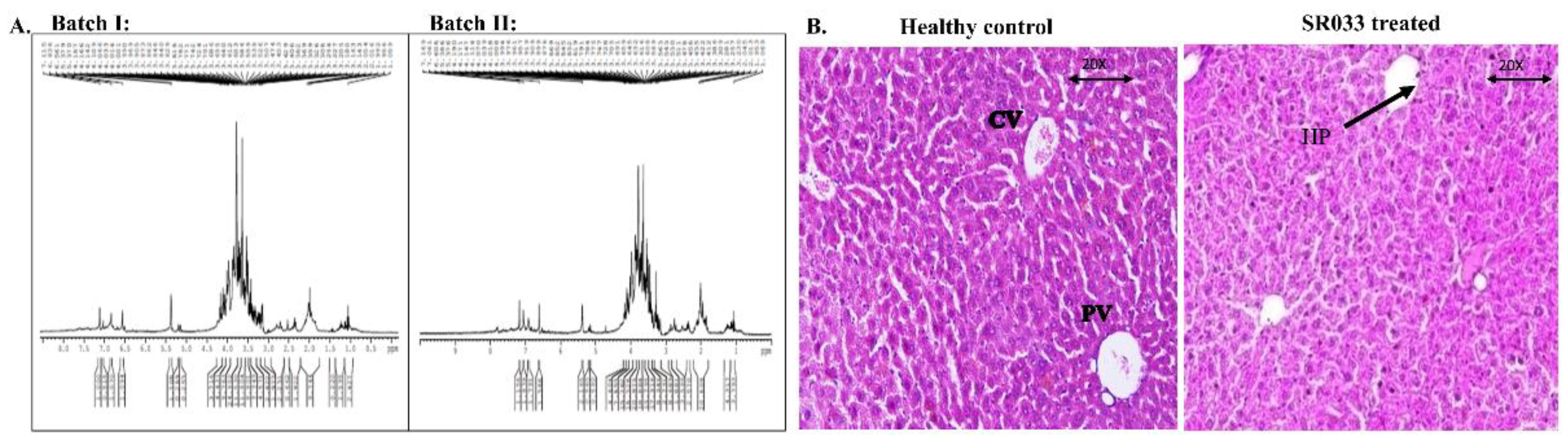
2.1. Quality Assessment and Safety of SR033
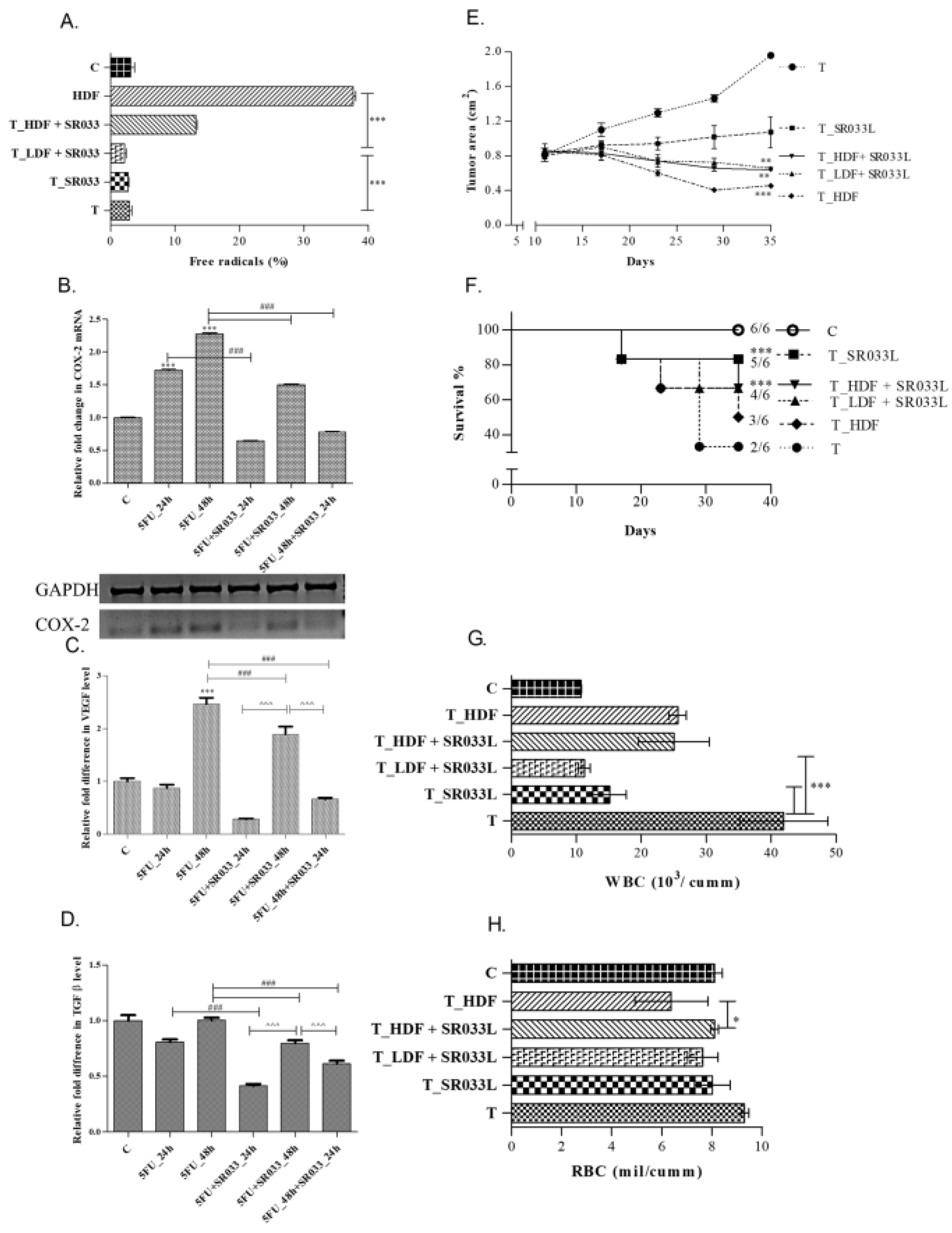
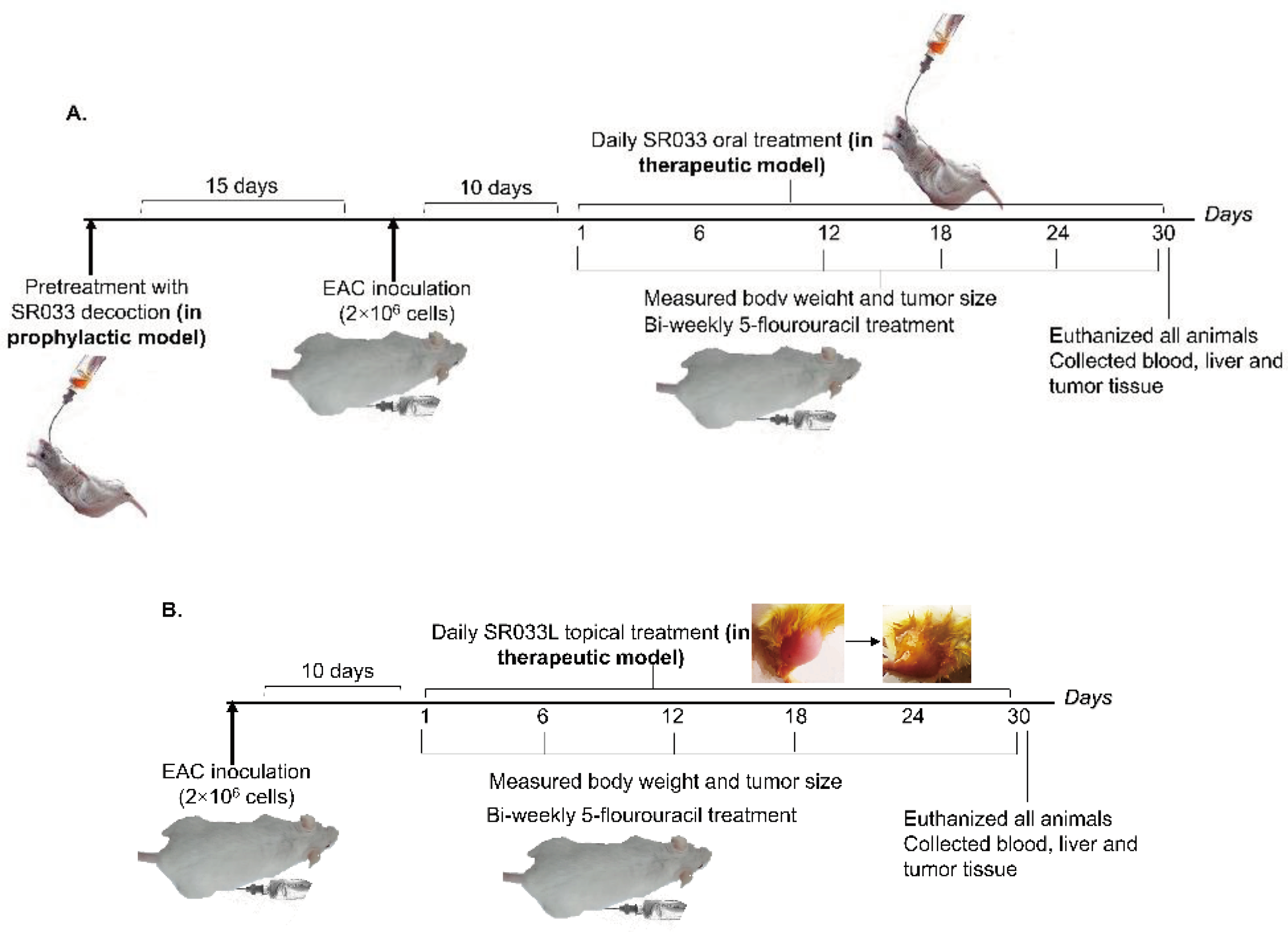
2.2. SR033 Prevents and Reduces Tumor Formation, and Improves Life Span in 5FU Treated EAC Tumor Mice Models by Maintaining Homeostasis
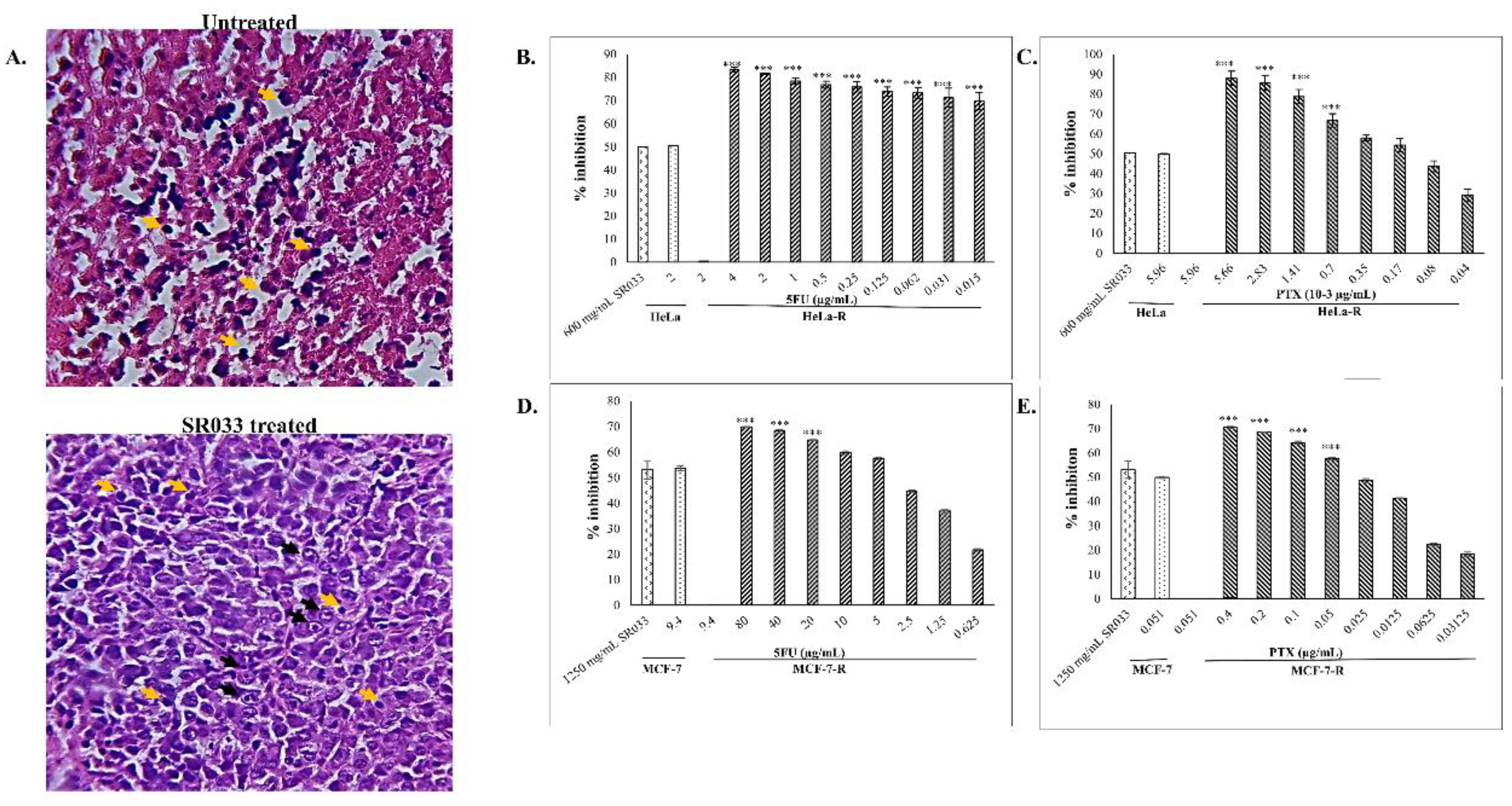
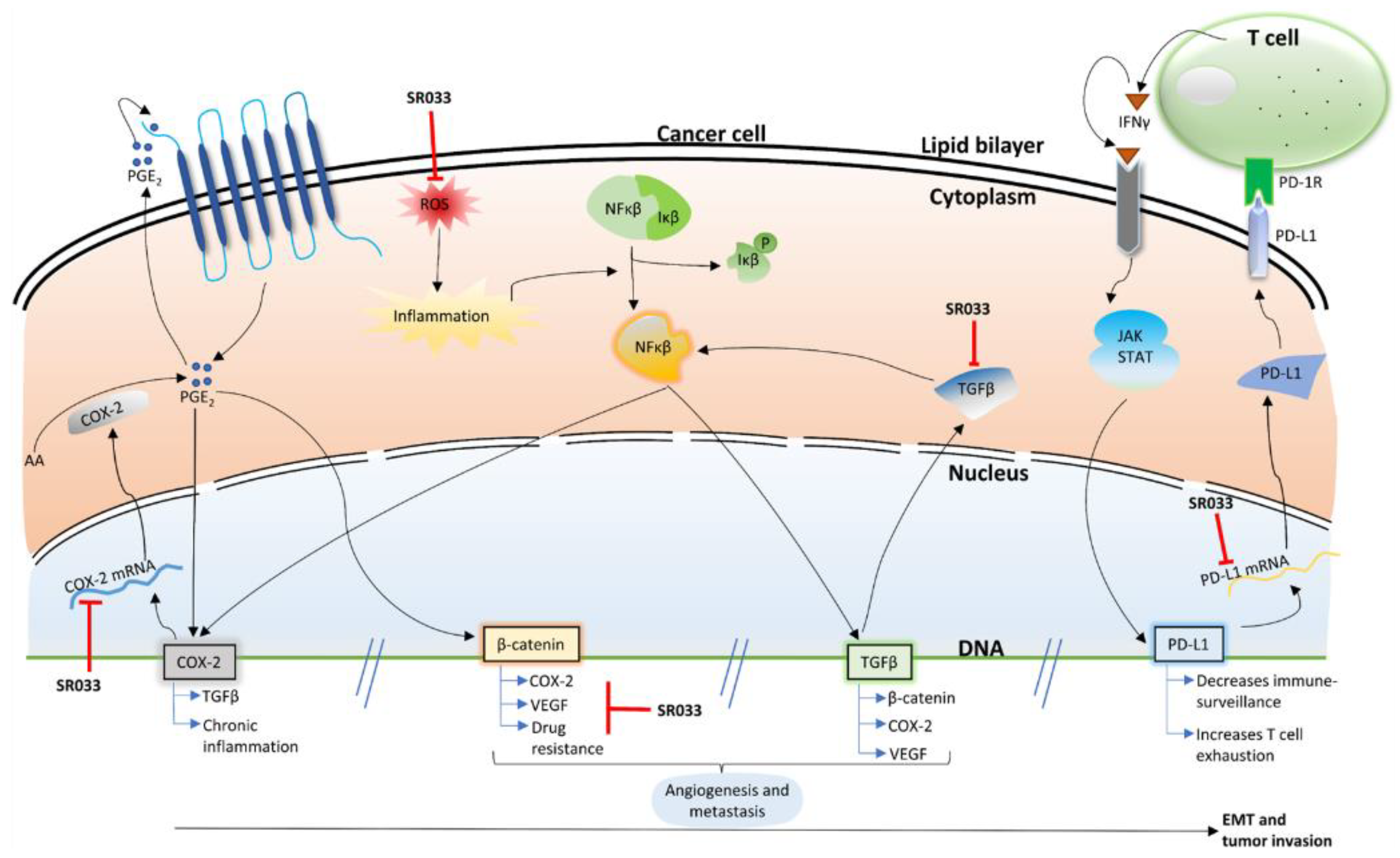
2.3. Integrative Approach: SR033 with Chemo-Drugs Prevents Chemo-Induced Inflammation, Acts Synergistically, Improves Survival, and Overcomes Drug Resistance
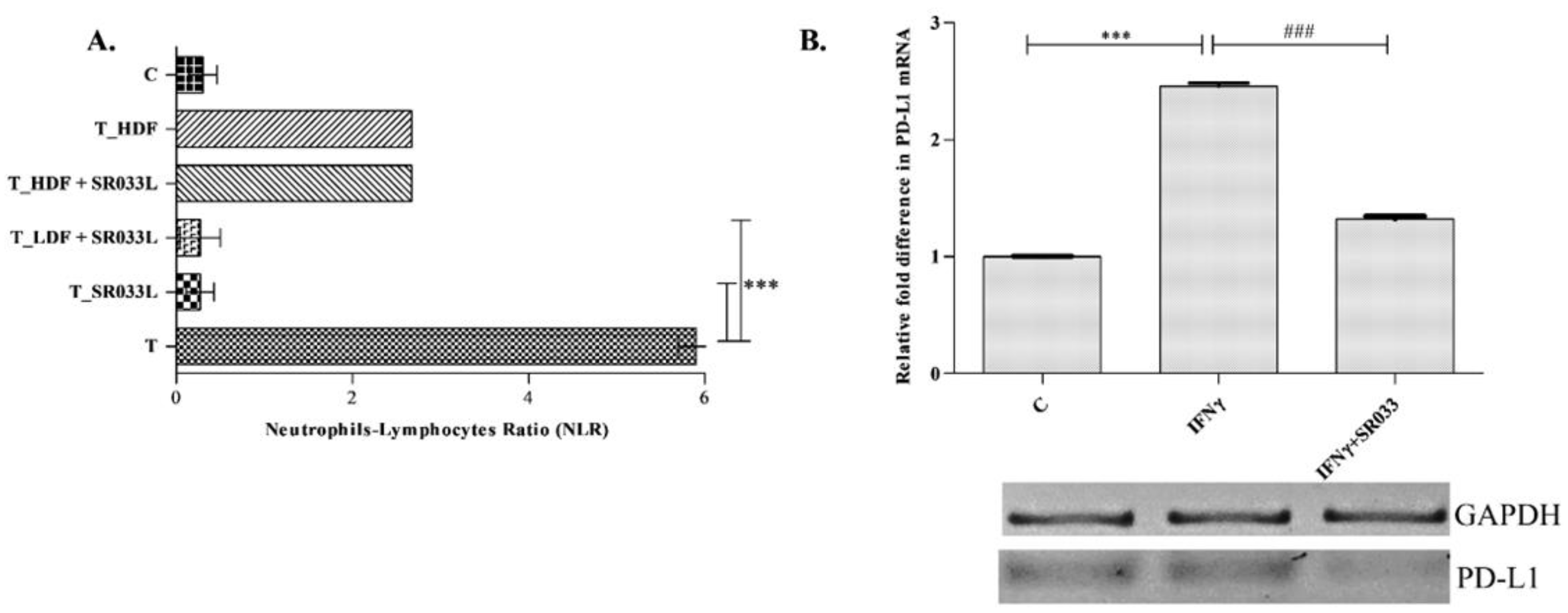
2.4. SR033 Restores Immune Status during Chemotherapy
3. Discussion
4. Materials and Methods
2.1. Herbal Formulation
2.2.1. H-NMR and HPLC
2.3. Cell Lines
2.3.1. Development of Resistant Cell Lines
2.3.2. In-Vitro Groups
2.3.3. RNA Semi-Quantitative RT-PCR
2.3.4. ELISA
2.3.5. Survival Assay
2.4. Animals
2.4.1. Acute Toxicity
2.4.2. Tumor Implantation
2.4.3. Oral Administration
2.4.4. Topical Application
2.4.5. Superoxide Dismutase (SOD) Assay
2.4.6. Histopathology
2.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bieniek, J.; Childress, C.; Swatski, M.D.; Yang, W. COX-2 inhibitors arrest prostate cancer cell cycle progression by down-regulation of kinetochore/centromere proteins. The Prostate 2014, 74, 999-1011. [CrossRef]
- Dutta, J.; Fan, Y.; Gupta, N.; Fan, G.; Gelinas, C. Current insights into the regulation of programmed cell death by NF-κ B. Oncogene 2006, 25, 6800-6816. [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells 2019, 8, 957. [CrossRef]
- Abdel-Aziz, A.M.; El-Tahawy, N.F.G.; Mohammed, M.M.; Ali, A.I.; Ibrahim, Y.F. Amelioration of testosterone-induced benign prostatic hyperplasia using febuxostat in rats: The role of VEGF/TGFβ and iNOS/COX-2. Eur. J. Pharmacol. 2020, 889, 173631. [CrossRef]
- Terzuoli, E.; Bellan, C.; Aversa, S.; Ciccone, V.; Morbidelli, L.; Giachetti, A.; Donnini, S.; Ziche, M. ALDH3A1 overexpression in melanoma and lung tumors drives cancer stem cell expansion, impairing immune surveillance through enhanced PD-L1 output. Cancers 2019, 11, 1963. [CrossRef]
- Fan, X.; Jin, J.; Yan, L.; Liu, L.; Li, Q.; Xu, Y. The impaired anti-tumoral effect of immune surveillance cells in the immune microenvironment of gastric cancer. Clin. Immunol. 2020, 108551. [CrossRef]
- Calabrese, L.H.; Calabrese, C.; Cappelli, L.C. Rheumatic immune-related adverse events from cancer immunotherapy. Nature Reviews Rheumatology 2018, 14, 569-579. [CrossRef]
- Dhodapkar, K.M. Autoimmune complications of cancer immunotherapy. Curr. Opin. Immunol. 2019, 61, 54-59. [CrossRef]
- Banerji, A.; Lax, T.; Guyer, A.; Hurwitz, S.; Camargo Jr, C.A.; Long, A.A. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. The Journal of Allergy and Clinical Immunology: In Practice 2014, 2, 428-433.
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; De Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13-26. [CrossRef]
- Marin, J.J.; Macias, R.I.; Monte, M.J.; Herraez, E.; Peleteiro-Vigil, A.; Blas, B.S.d.; Sanchon-Sanchez, P.; Temprano, A.G.; Espinosa-Escudero, R.A.; Lozano, E. Cellular mechanisms accounting for the refractoriness of colorectal carcinoma to pharmacological treatment. Cancers 2020, 12, 2605. [CrossRef]
- Lien, K.; Georgsdottir, S.; Sivanathan, L.; Chan, K.; Emmenegger, U. Low-dose metronomic chemotherapy: a systematic literature analysis. European Journal of Cancer 2013, 49, 3387-3395. [CrossRef]
- Loven, D.; Hasnis, E.; Bertolini, F.; Shaked, Y. Low-dose metronomic chemotherapy: from past experience to new paradigms in the treatment of cancer. Drug discovery today 2013, 18, 193-201. [CrossRef]
- Gong, L.-H.; Chen, X.-X.; Wang, H.; Jiang, Q.-W.; Pan, S.-S.; Qiu, J.-G.; Mei, X.-L.; Xue, Y.-Q.; Qin, W.-M.; Zheng, F.-Y. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxidative medicine and cellular longevity 2014, 2014. [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: potential lead molecules for MDR reversal. Frontiers in Pharmacology 2020, 11, 832. [CrossRef]
- Organization, W.H. WHO global report on traditional and complementary medicine 2019; World Health Organization: 2019.
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K. Targeting oncogenic transcription factors by polyphenols: A novel approach for cancer therapy. Pharmacol. Res. 2018, 130, 273-291. [CrossRef]
- Zangui, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: state-of-the-art. Pharmacol. Res. 2019, 141, 343-356. [CrossRef]
- Zhu, F.; Xu, Y.; Pan, J.; Li, M.; Chen, F.; Xie, G. Epigallocatechin Gallate Protects against MNNG-Induced Precancerous Lesions of Gastric Carcinoma in Rats via PI3K/Akt/mTOR Pathway. Evidence-Based Complementary and Alternative Medicine 2021, 2021. [CrossRef]
- Lopez, G.; McQuade, J.; Cohen, L.; Williams, J.T.; Spelman, A.R.; Fellman, B.; Li, Y.; Bruera, E.; Lee, R.T. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. Journal of Cancer 2017, 8, 395. [CrossRef]
- Jadhav, N.V.; Prasad, A.I.; Kumar, A.; Mishra, R.; Dhara, S.; Babu, K.; Prajapat, C.; Misra, N.; Ningthoujam, R.; Pandey, B. Synthesis of oleic acid functionalized Fe3O4 magnetic nanoparticles and studying their interaction with tumor cells for potential hyperthermia applications. Colloids Surf. B. Biointerfaces 2013, 108, 158-168. [CrossRef]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2019, 234, 5728-5740. [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules 2020, 25, 4553. [CrossRef]
- Kamel, K.M.; Khalil, I.A.; Rateb, M.E.; Elgendy, H.; Elhawary, S. Chitosan-coated cinnamon/oregano-loaded solid lipid nanoparticles to augment 5-fluorouracil cytotoxicity for colorectal cancer: extract standardization, nanoparticle optimization, and cytotoxicity evaluation. J. Agric. Food Chem. 2017, 65, 7966-7981. [CrossRef]
- Chimbetete, N.; Verghese, M.; Sunkara, R.; Walker, L.T. Phytochemical content, radical scavenging ability & enzyme inhibiting activities of selected spices (cinnamon, cardamom and cloves). Food and Nutrition Sciences 2019, 10, 266-275. [CrossRef]
- Han, B.; Huang, H.; Li, Z.; Gong, M.; Shi, W.; Zhu, C.; Gu, Z.; Zou, Z. Therapeutic effects of chinese medicine herb pair, huzhang and guizhi, on monosodium urate crystal-induced gouty arthritis in rats revealed by anti-inflammatory assessments and NMR-based metabonomics. Evidence-Based Complementary and Alternative Medicine 2016, 2016. [CrossRef]
- Baghel, U.; Nagar, A.; Pannu, M.; Singh, D.; Yadav, R. HPLC and HPTLC methods for simultaneous estimation of quercetin and curcumin in polyherbal formulation. Indian Journal of Pharmaceutical Sciences 2017, 79, 197-203. [CrossRef]
- Dallas, N.A.; Xia, L.; Fan, F.; Gray, M.J.; Gaur, P.; Van Buren, G.; Samuel, S.; Kim, M.P.; Lim, S.J.; Ellis, L.M. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009, 69, 1951-1957. [CrossRef]
- McDermott, M.; Eustace, A.; Busschots, S.; Breen, L.; Clynes, M.; O'Donovan, N.; Stordal, B. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Frontiers in oncology 2014, 4, 40. [CrossRef]
- Fan, Y.; Abulimiti, P.; Zhang, H.; Zhou, Y.; Zhu, L. Mechanism of reversal of multidrug resistance by curcumin in human colorectal cancer cell line HCT-8/5-FU. Gen. Mol. Res. 2017, 16.
- Toxicity–Up, A.O. OECD GUIDELINE FOR TESTING OF CHEMICALS. 2001.
- Nicol, B.; Prasad, S. The effects of cyclophosphamide alone and in combination with ascorbic acid against murine ascites Dalton's lymphoma. Indian J Pharmacol 2006, 38, 260. [CrossRef]
- Kleniewska, P.; Hoffmann, A.; Pniewska, E.; Pawliczak, R. The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxidative medicine and cellular longevity 2016, 2016.
- Wen, J.J.; Garg, N.J. Manganese superoxide dismutase deficiency exacerbates the mitochondrial ROS production and oxidative damage in Chagas disease. PLoS neglected tropical diseases 2018, 12, e0006687. [CrossRef]
- Banchroft, J.; Stevens, A.; Turner, D. Theory and practice of histological techniques. 1996.
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1-3. [CrossRef]
- Zhong, J.-H.; Huang, D.-H.; Chen, Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget 2017, 8, 75381. [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L.(licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186.
- Kamran, S.; Sinniah, A.; Alshawsh, M.A. Synergistic effects of 5-fluorouracil in combination with diosmetin in colorectal cancer cells. In Proceedings of the Presented at the 1st International Electronic Conference on Biomedicine, 2021; p. 26.
- Mawalizadeh, F.; Mohammadzadeh, G. Quercetin Potentiates the Chemosensitivity of Human Breast Cancer Cells to 5-Fluorouracil. 2021.
- Chen, S.; Zhang, Z.; Zhang, J. Emodin enhances antitumor effect of paclitaxel on human non-small-cell lung cancer cells in vitro and in vivo. Drug design, development and therapy 2019, 13, 1145. [CrossRef]
- Ko, G.; Kim, T.; Ko, E.; Park, D.; Lee, Y. Synergistic enhancement of paclitaxel-induced inhibition of cell growth by metformin in melanoma cells. Development & reproduction 2019, 23, 119. [CrossRef]
- Tang, H.; Zeng, L.; Wang, J.; Zhang, X.; Ruan, Q.; Wang, J.; Cui, S.; Yang, D. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget 2017, 8, 82842. [CrossRef]
- Larkins, T.L.; Nowell, M.; Singh, S.; Sanford, G.L. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer 2006, 6, 1-12. [CrossRef]
- Lau, T.Y.; Leung, L.K. Soya isoflavones suppress phorbol 12-myristate 13-acetate-induced COX-2 expression in MCF-7 cells. Br. J. Nutr. 2006, 96, 169-176. [CrossRef]
- Huang, C.-Y.; Lee, C.-H.; Tu, C.-C.; Wu, C.-H.; Huang, M.-T.; Wei, P.-L.; Chang, Y.-J. Glucose-regulated protein 94 mediates progression and metastasis of esophageal squamous cell carcinoma via mitochondrial function and the NF-kB/COX-2/VEGF axis. Oncotarget 2018, 9, 9425.
- Tong, D.; Liu, Q.; Wang, L.-a.; Xie, Q.; Pang, J.; Huang, Y.; Wang, L.; Liu, G.; Zhang, D.; Lan, W. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 2018, 37, 355-368.
- Pauzas, H.; Gyvyte, U.; Latkauskas, T.; Kairevice, L.; Lizdenis, P.; Svagzdys, S.; Birgiolaite, E.; Kuliaviene, I.; Kupcinskas, J.; Tamelis, A. The Role of VEGFA, COX2, HUR and CUGBP2 in predicting the response to neoadjuvant therapy in rectal cancer patients. Medicina (Mex). 2020, 56, 192.
- Shimizu, K.; Okita, R.; Saisho, S.; Maeda, A.; Nojima, Y.; Nakata, M. Impact of COX2 inhibitor for regulation of PD-L1 expression in non-small cell lung cancer. Anticancer Res. 2018, 38, 4637-4644. [CrossRef]
- Qi, F.; Zhou, S.; Li, L.; Wei, L.; Shen, A.; Liu, L.; Wang, Y.; Peng, J. Pien Tze Huang inhibits the growth of hepatocellular carcinoma cells by upregulating miR-16 expression. Oncology letters 2017, 14, 8132-8137. [CrossRef]
- Peng, W.; Zhang, S.; Zhang, Z.; Xu, P.; Mao, D.; Huang, S.; Chen, B.; Zhang, C.; Zhang, S. Jianpi Jiedu decoction, a traditional Chinese medicine formula, inhibits tumorigenesis, metastasis, and angiogenesis through the mTOR/HIF-1α/VEGF pathway. J. Ethnopharmacol. 2018, 224, 140-148.
- Spranger, S.; Sivan, A.; Corrales, L.; Gajewski, T.F. Tumor and host factors controlling antitumor immunity and efficacy of cancer immunotherapy. Adv. Immunol. 2016, 130, 75-93.
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl. J. Med. 2017, 377, 1345-1356. [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 1-15. [CrossRef]
- Rawangkan, A.; Iida, K.; Sakai, R.; Fujiki, H.; Suganuma, M. Green tea catechin, EGCG, enhances antitumor immunity by down-regulation of PD-L1 expression in non-small human lung cancer cell lines. 2017. [CrossRef]
- Duan, J.; Pan, L.; Yang, M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine (Baltimore). 2018, 97.
| Groups | Breast site | Peritoneum site (Ascites) | |||
|---|---|---|---|---|---|
| Prophylactic | Therapeutic | Prophylactic | Therapeutic | ||
| Tumor size (cm)/ volume (mL) | T1 | 1.69 ± 0.03### | 2.42 ± 0.09### | 18.12 ± 0.09### | 17.50 ± 0.11### |
| T_LDS2 | 1.21 ± 0.02*** | 1.75 ± 0.05*** | 16.18 ± 0.22*** | 13.96 ± 0.34*** | |
| T_HDS3 | 0.75 ± 0.01*** | 1.02 ± 0.05*** | 11.68 ± 0.07*** | 09.89 ± 0.09*** | |
| T_HDF4 | - | 0.78 ± 0.03*** | - | 7.71 ± 0.20*** | |
| Packed cell volume (mL) | T | - | - | 11.95 ± 0.12### | 11.46 ± 0.26### |
| T_LDS | - | - | 07.98 ± 0.09*** | 08.03 ± 0.14*** | |
| T_HDS | - | - | 05.22 ± 0.33*** | 05.06 ± 0.19*** | |
| T_HDF | - | - | - | 04.65 ± 0.11*** | |
| Mean survival time | T | 15.83±0.30### | 17.66±0.21### | ||
| T_LDS | 20 ±0.44*** | 22.83 ±0.30*** | |||
| T_HDS | 23.16±0.30*** | 26.16±0.30*** | |||
| T_HDF | - | 20.66±0.21*** | |||
| WBC (103 cells/mL) | Healthy | 9.69 ± 0.13 | 9.32 ± 0.06 | 9.59 ± 0.18 | 9.32 ± 0.06 |
| T | 19.56 ± 0.26### | 21.21 ± 0.24### | 20.41± 0.41### | 21.34 ± 0.47### | |
| T_LDS | 17.75 ± 0.11*** | 16.61 ± 0.24*** | 18.87 ± 0.21*** | 19.07 ± 0.14*** | |
| T_HDS | 15.44 ± 0.12*** | 14.34 ± 0.18*** | 16.27 ± 0.21*** | 15.97 ± 0.15*** | |
| T_HDF | - | 18.26 ± 0.27*** | - | 18.20 ± 0.16*** | |
| RBC (106 cells/mL) | Healthy | 10.67 ± 0.11 | 10.51 ± 0.20 | 10.46 ± 0.22 | 10.51 ± 0.20 |
| T | 4.11 ± 0.16### | 3.94 ± 0.21### | 4.01 ± 0.12### | 3.84 ± 0.06### | |
| T_LDS | 5.23 ± 0.11*** | 5.44 ± 0.16*** | 4.40 ± 0.18*** | 5.32 ± 0.08*** | |
| T_HDS | 6.34 ± 0.20*** | 6.95 ± 0.08*** | 5.98 ± 0.09*** | 7.08 ± 0.31*** | |
| T_HDF | - | 5.83 ± 0.08*** | - | 5.45 ± 0.12*** | |
| Groups | No. of living animals (out of 6) | Viable tumor cells (%) | Necrotic cells (%) | Apoptotic cells (%) | Remarks |
|---|---|---|---|---|---|
| T1 | 2 | 20-25% | 75-80% | 25-30% | Mild TILS2 |
| T_SR033L3 | 5 | 0-30% | 70-100% | - | No TILS, presence of Foamy cells. The tumor was replaced by mixed inflammation on the surface |
| T_SR033L + LDF4 | 4 | 20-30% | 30-50% | 10-30% | Presence of mixed inflammation around the tumor |
| T_SR033L + HDF5 | 4 | 10-25% | 80-90% | 20-35% | No TILS, presence of Foamy cells at margin, one lobe was replaced by inflammation |
| T_HDF6 | 3 | 10-20% | 90-100% | 20-30% | No inflammation or Foamy cells present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





