Submitted:
21 March 2024
Posted:
22 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Hydrogels synthesis
2.2. Characterization of the Hydrogels
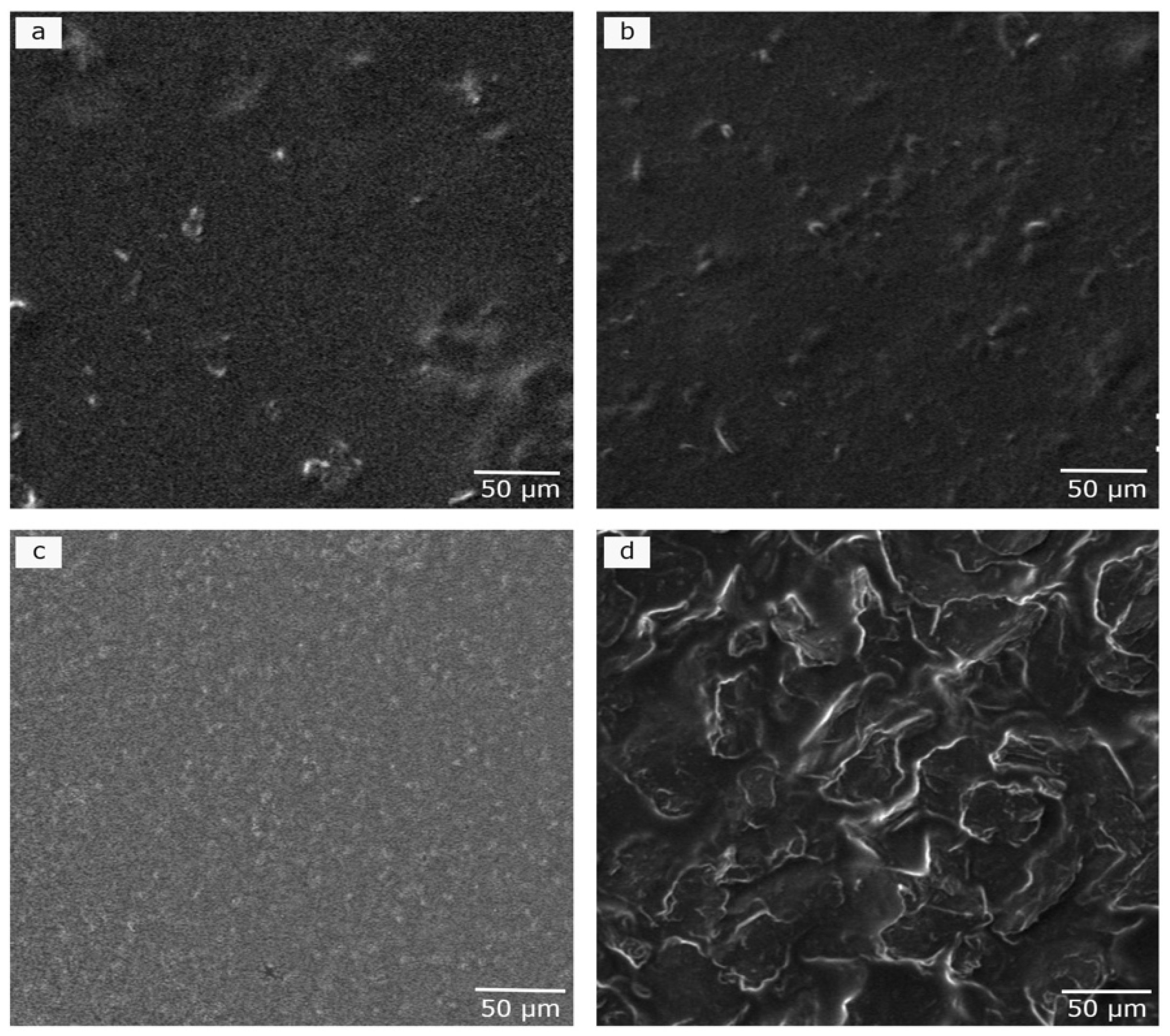
2.2.1. Scanning Electron Microscopy (SEM)
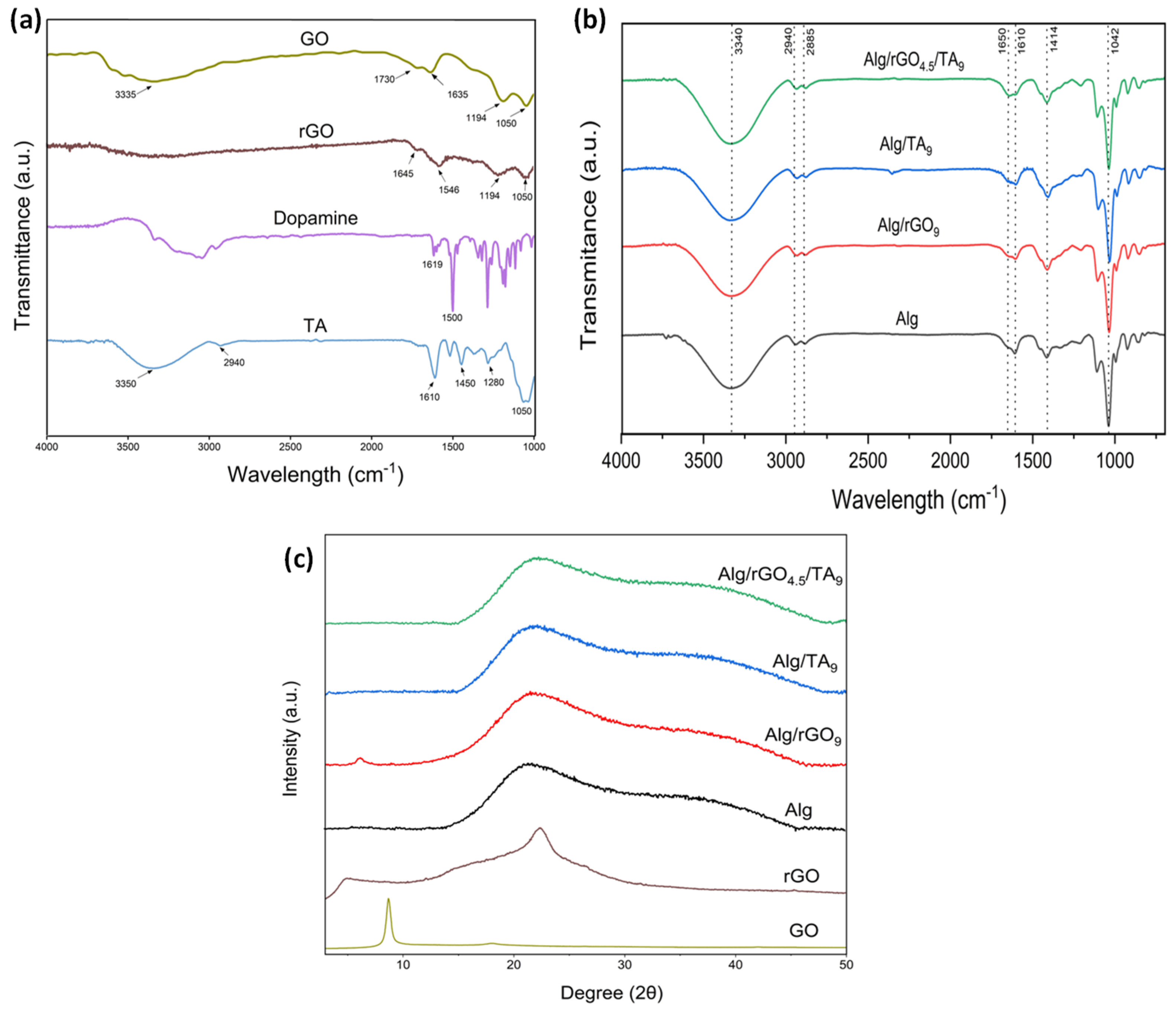
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.3. X-ray diffraction (XRD)
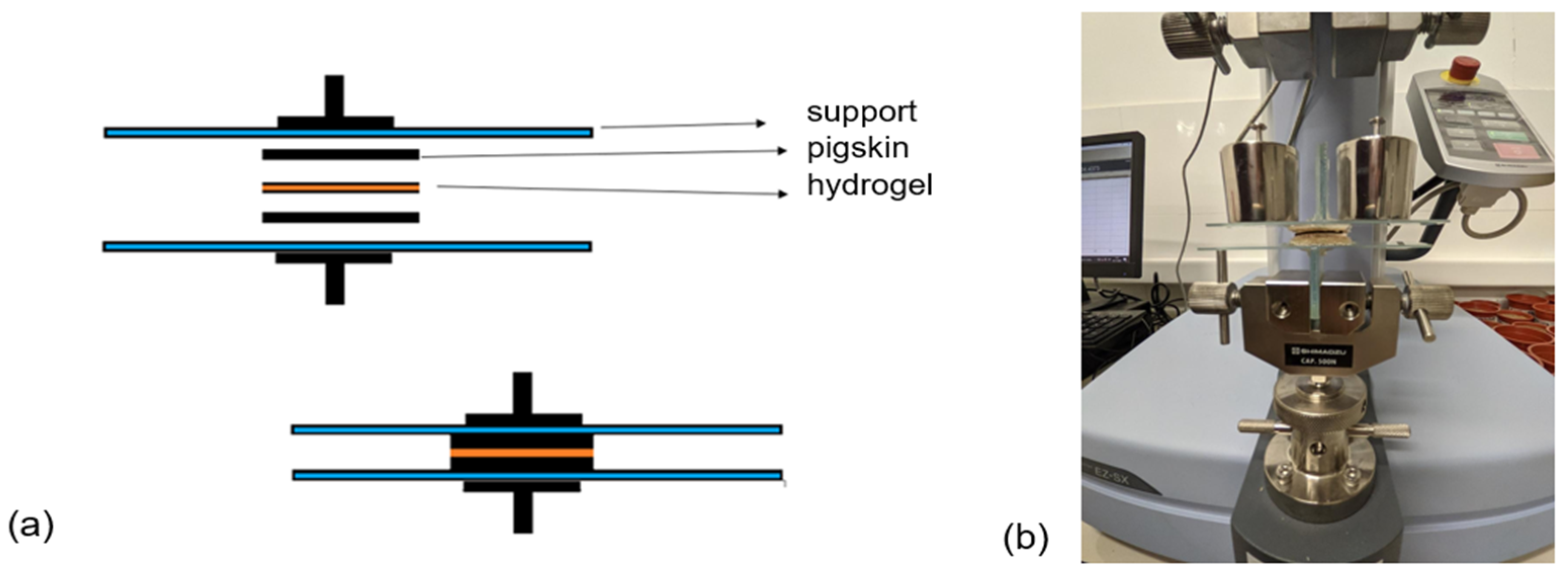
2.2.4. Mechanical Characterization
2.2.4. Adhesive Properties
2.3.5. Cytotoxicity Assay
2.3.6. In Vitro Wound Healing Assay (Scratch Test)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphological Studies
3.2. Chemical Characterization of the Hydrogels
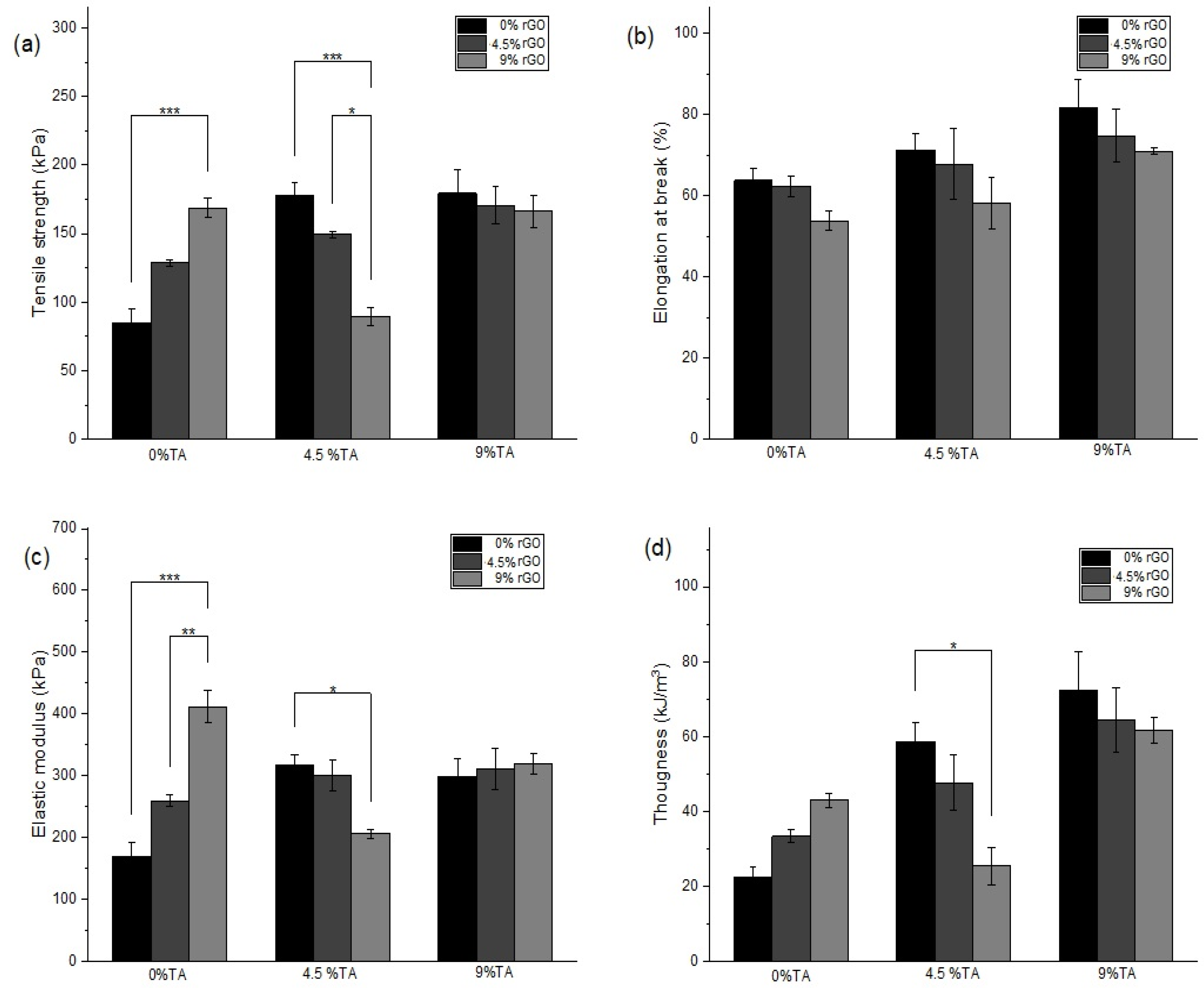
3.3. Mechanical Characterization of the Hydrogels
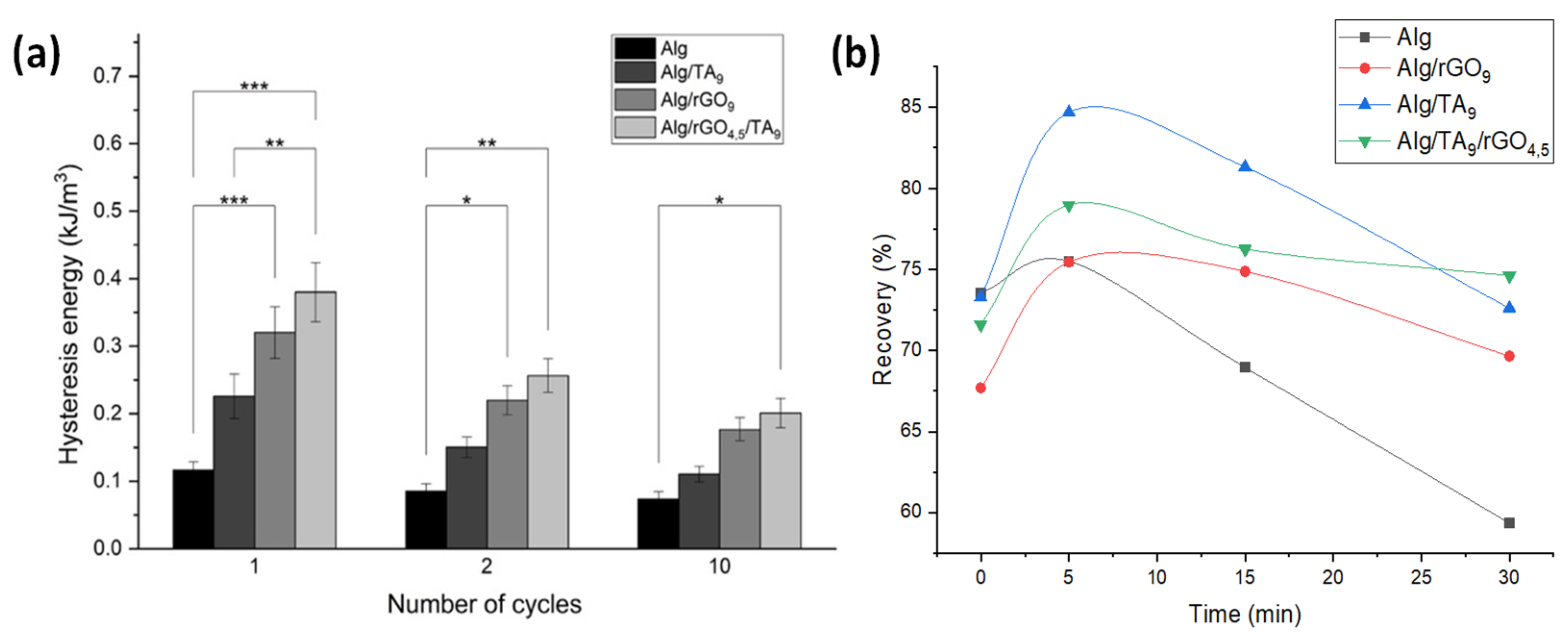
3.3.1. Hysteresis and Self-Recovery of the Hydrogels
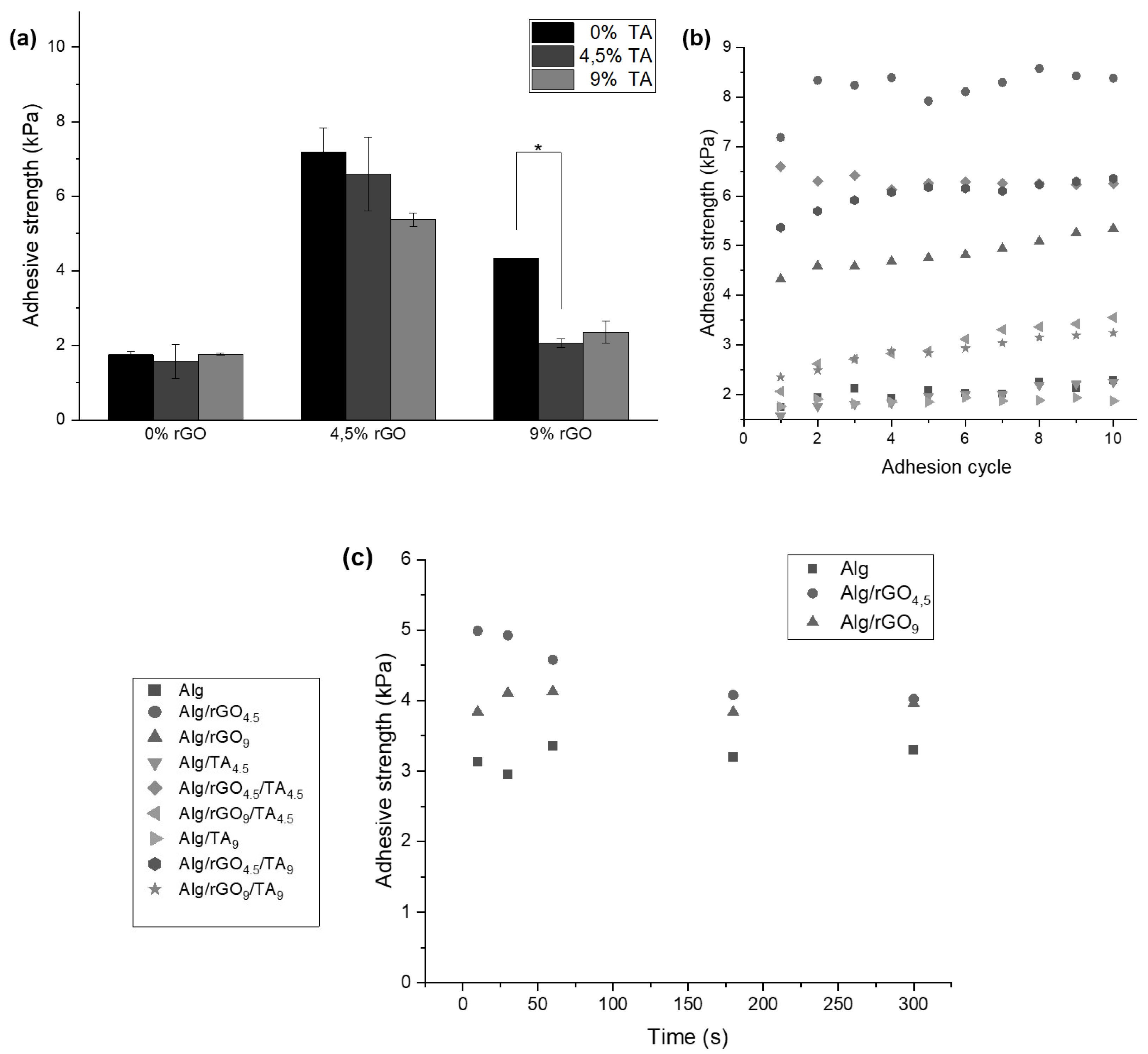
3.3.2. Adhesive Properties of the Hydrogels
3.4. Biological Characterization
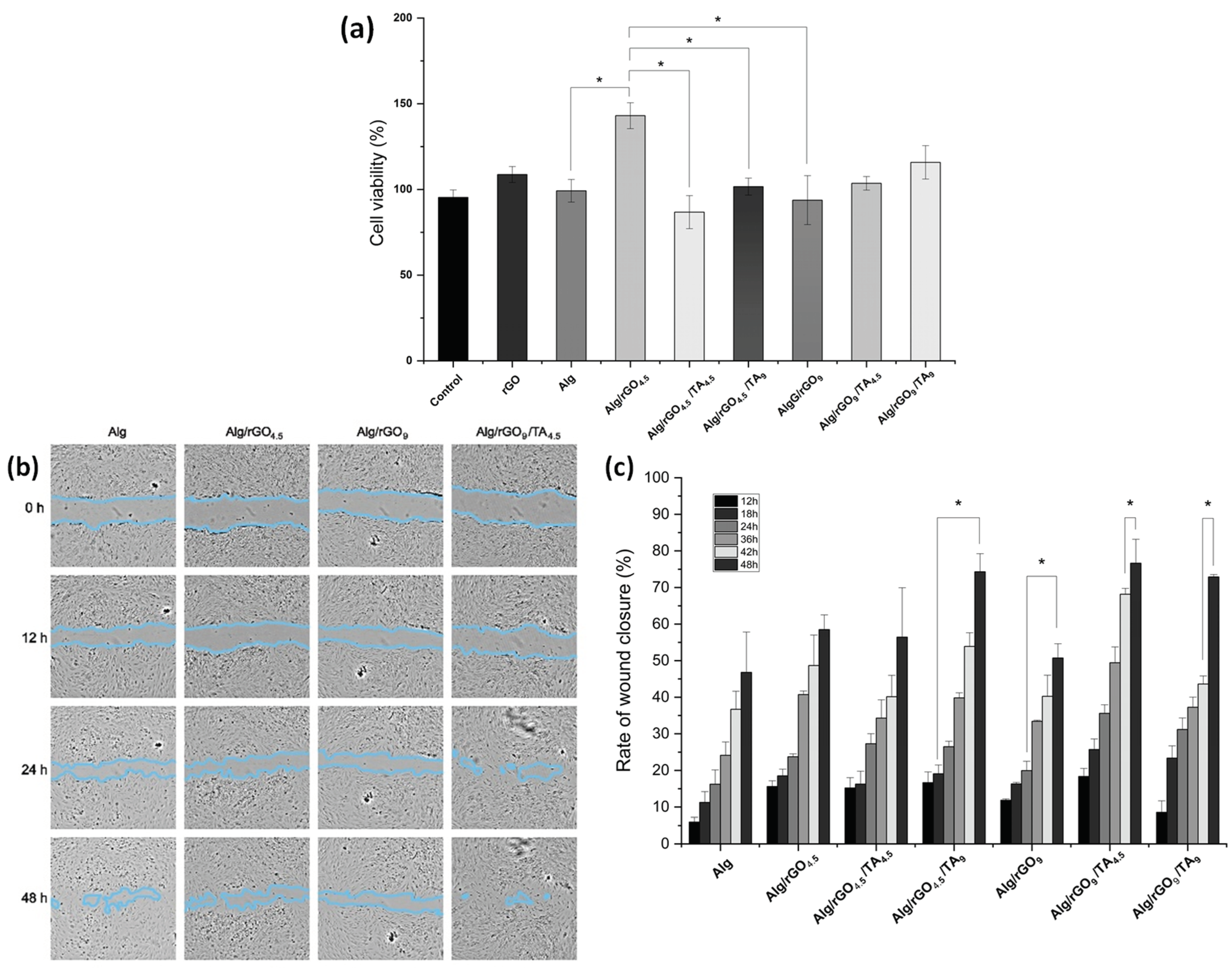
3.4.1. Cytotoxicity Assays
3.4.2. In Vitro Wound Healing Assay (Scratch Test)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef University Journal of Basic and Applied Sciences 2022, 11. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.L.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12. [Google Scholar] [CrossRef]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Advances in Polymer Technology 2018, 37, 3665–3679. [Google Scholar] [CrossRef]
- Raslan, A.; Ciriza, J.; Ochoa de Retana, A.M.; Sanjuan, M.L.; Toprak, M.S.; Galvez-Martin, P.; Saenz-del-Burgo, L.; Pedraz, J.L. Modulation of Conductivity of Alginate Hydrogels Containing Reduced Graphene Oxide through the Addition of Proteins. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Yu, W. Functionalized Graphene Oxide-Reinforced Chitosan Hydrogel as Biomimetic Dressing for Wound Healing. Macromolecular Bioscience 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble collagen based biodegradable hybrid hydrogel for biomedical scaffold. Journal of Biomaterials Science-Polymer Edition 2020, 31, 2199–2219. [Google Scholar] [CrossRef]
- Agnieszka, K.-P. Alginate-Based Hydrogels in Regenerative Medicine. In Alginates, Leonel, P., Ed. IntechOpen: Rijeka, 2019; Ch. 5.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Progress in Polymer Science 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Zhu, Y.-J.; Li, H.; Zhang, Y.-G.; Shen, Y.-Q.; Sun, T.-W.; Chen, F. Preparation and enhanced mechanical properties of hybrid hydrogels comprising ultralong hydroxyapatite nanowires and sodium alginate. Journal of Colloid and Interface Science 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef]
- Moghadam, M.N.; Pioletti, D.P. Improving hydrogels' toughness by increasing the dissipative properties of their network. Journal of the Mechanical Behavior of Biomedical Materials 2015, 41, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yang, J.; Suo, Z. Fatigue of hydrogels. European Journal of Mechanics - A/Solids 2019, 74, 337–370. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Materials Today Bio 2022, 16. [Google Scholar] [CrossRef]
- Jing, Z.; Dai, X.; Xian, X.; Du, X.; Liao, M.; Hong, P.; Li, Y. Tough, stretchable and compressive alginate-based hydrogels achieved by non-covalent interactions. Rsc Advances 2020, 10, 23592–23606. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mandavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Science and Engineering of Composite Materials 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chemical Reviews 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. Journal of Materials Chemistry C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Tang, T.; Goossens, K.; Lu, S.J.; Meng, D.; Bielawski, C.W. Agar-reduced graphene oxide selectively adsorbs organic dyes and strengthens double-network hydrogels. RSC Advances 2020, 10, 29287–29295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Pradhan, A.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B. Thermally exfoliated graphene oxide reinforced stress responsive conductive nanocomposite hydrogel. Polymers for Advanced Technologies 2019, 30, 2392–2401. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; Garcia-San-Martin, N.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Donderis Quiles, V.; Serrano-Aroca, A.; Sabater i Serra, R. Electroactive calcium-alginate/polycaprolactone/reduced graphene oxide nanohybrid hydrogels for skeletal muscle tissue engineering. Colloids and Surfaces B-Biointerfaces 2022, 214. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, M.; Liu, Y.; Xiong, L.; Zhang, Q.; Wei, L.; Li, L.; Yu, X. Strong alginate/reduced graphene oxide composite hydrogels with enhanced dye adsorption performance. Polymer Bulletin 2020, 77, 6609–6623. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chemical Reviews 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; González-Mora, J.L. Polydopamine-modified surfaces in biosensor applications.
- Cheng, C.; Li, S.; Zhao, J.; Li, X.; Liu, Z.; Ma, L.; Zhang, X.; Sun, S.; Zhao, C. Biomimetic assembly of polydopamine-layer on graphene: Mechanisms, versatile 2D and 3D architectures and pollutant disposal. Chemical Engineering Journal 2013, 228, 468–481. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. Rsc Advances 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-Inspired Tissue-Adhesive Hydrogel Based on the Polydopamine-Chondroitin Sulfate Complex for Growth-Factor-Free Cartilage Regeneration. Acs Applied Materials & Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef]
- Gonzalez, N.; Elissetche, J.; Pereira, M.; Fernandez, K. Extraction of polyphenols from Eucalyptus nitens and Eucalyptus globulus: Experimental kinetics, modeling and evaluation of their antioxidant and antifungical activities. Industrial Crops and Products 2017, 109, 737–745. [Google Scholar] [CrossRef]
- Molino, S.; Andrea Casanova, N.; Rufian Henares, J.A.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. Journal of Agricultural and Food Chemistry 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Soledad Parada, M.; Fernandez, K. Modelling the hydrophilic extraction of the bark of Eucalyptus nitens and Eucalyptus globulus: Adsorption isotherm and thermodynamic studies. Industrial Crops and Products 2017, 109, 558–569. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodriguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Huesing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Facile Synthesis of Soluble Graphene via a Green Reduction of Graphene Oxide in Tea Solution and Its Biocomposites. Acs Applied Materials & Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, Z.; Xu, H.; Ma, X.; Yin, J.; Tian, M. Fabrication of Super Extensible and Highly Tough Graphene Composite Hydrogels by Thermal Treatment Strategy for the Mixture of Tannin and Graphene Oxide. Macromolecular Chemistry and Physics 2017, 218. [Google Scholar] [CrossRef]
- Facchi, D.P.; Lima, A.C.; de Oliveira, J.H.; Lazarin-Bidoia, D.; Nakamura, C.V.; Canesin, E.A.; Bonafe, E.G.; Monteiro, J.P.; Visentainer, J.V.; Muniz, E.C.; et al. Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. International Journal of Biological Macromolecules 2017, 103, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. Journal of the American Chemical Society 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Olad, A.; Gordani, R.; Eslamzadeh, M.; Mollaei, M. Graphene Oxide Nanocomposite Hydrogels Based on Mucilage Extracted from<i> Ocimum</i><i> basilicum</i> Seeds Grafted by Acrylate Polymers; Assay on Physicochemical Properties. Journal of Polymers and the Environment 2023, 31, 2399–2414. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing-Know-how. INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES 2021, 186, 656–685. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conference Proceedings 2017, 1892. [Google Scholar] [CrossRef]
- Khosravi, H.; Naderi, R.; Ramezanzadeh, B. Designing an epoxy composite coating having dual-barrier-active self- healing anti-corrosion functions using a multi-functional GO/PDA/MO nano-hybrid. Materials Today Chemistry 2023, 27. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhen, M.; Wu, Y.; Ma, M.; Cheng, Y.; Jin, Y. Effect of catechin and tannins on the structural and functional properties of sodium alginate/gelatin/ poly(vinylalcohol) blend films. Food Hydrocolloids 2023, 135, 108141. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. South African Journal of Botany 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Hydroxyapatite Mineralization. Journal of Physical Chemistry C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Cui, W.; Li, M.; Liu, J.; Wang, B.; Zhang, C.; Jiang, L.; Cheng, Q. A Strong Integrated Strength and Toughness Artificial Nacre Based on Dopamine Cross-Linked Graphene Oxide. Acs Nano 2014, 8, 9511–9517. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, J.U.; Jung, B.M.; Byun, J.-H.; Yi, J.-W.; Lee, S.-B.; Kim, B.-S. Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon 2013, 65, 296–304. [Google Scholar] [CrossRef]
- Li, W.; Shang, T.; Yang, W.; Yang, H.; Lin, S.; Jia, X.; Cai, Q.; Yang, X. Effectively Exerting the Reinforcement of Dopamine Reduced Graphene Oxide on Epoxy-Based Composites via Strengthened Interfacial Bonding. Acs Applied Materials & Interfaces 2016, 8, 13037–13050. [Google Scholar] [CrossRef]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Analytical Chemistry 2011, 83, 8787–8793. [Google Scholar] [CrossRef] [PubMed]
- Gonultas, O.; Ucar, M.B. Chemical Composition of Some Commercial Tannins.
- Produced in Turkey. In Proceedings of 55th International Convention of Society of Wood Science and Technology.
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel based on an alginate-Ca<SUP>2+</SUP>/chondroitin sulfate matrix as a potential colon-specific drug delivery system. Rsc Advances 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Gu, R.P.; Xu, W.Z.; Charpentier, P.A. Synthesis of polydopamine-coated graphene-polymer nanocomposites via RAFT polymerization. Journal of Polymer Science Part a-Polymer Chemistry 2013, 51, 3941–3949. [Google Scholar] [CrossRef]
- Rasyida, A.; Halimah, S.; Wijayanti, I.D.; Wicaksono, S.T.; Nurdiansah, H.; Silaen, Y.M.T.; Ni'mah, Y.L.; Ardhyananta, H.; Purniawan, A. A Composite of Hydrogel Alginate/PVA/r-GO for Scaffold Applications with Enhanced Degradation and Biocompatibility Properties. Polymers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Q.; Zhao, Y.; Yuan, J.; Zha, X.; Xie, H.; Kong, F.; Xiong, X. Strong and Tough PAm/SA Hydrogel with Highly Strain Sensitivity. Journal of Renewable Materials 2022, 10, 415–430. [Google Scholar] [CrossRef]
- Liao, M.; Zhao, Y.; Pan, Y.; Pan, J.; Yao, Q.; Zhang, S.; Zhao, H.; Hu, Y.; Zheng, W.; Zhou, W.; et al. A good adhesion and antibacterial double-network composite hydrogel from PVA, sodium alginate and tannic acid by chemical and physical cross-linking for wound dressings. Journal of Materials Science 2023, 58, 5756–5772. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. Acs Applied Materials & Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surgical Technology International-International Developments in Surgery and Surgical Research 2019, 35. [Google Scholar]
- Marrella, A.; Lagazzo, A.; Barberis, F.; Catelani, T.; Quarto, R.; Scaglione, S. Enhanced mechanical performances and bioactivity of cell laden-graphene oxide/alginate hydrogels open new scenario for articular tissue engineering applications. Carbon 2017, 115, 608–616. [Google Scholar] [CrossRef]
- Wang, W.; Lin, S.; Ye, Z.; Zhou, Y.; Zou, Q.; Zheng, T.; Ding, M. Electrospun egg white protein/polyvinyl alcohol/graphene oxide fibrous wound dressing: Fabrication, antibacterial, cytocompatibility and wound healing assay. Colloids and Surfaces a-Physicochemical and Engineering Aspects 2023, 658. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-bohlouli, P.; Podstawczyk, D.; Nie, L.; Shavandi, A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydrate Polymers 2022, 295. [Google Scholar] [CrossRef]
- Zou, M.L.; Teng, Y.Y.; Wu, J.J.; Liu, S.Y.; Tang, X.Y.; Jia, Y.; Chen, Z.H.; Zhang, K.W.; Sun, Z.L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef]
- Wan, L.; He, Y.; Wang, A.; Pan, J.; Xu, C.; Fu, D.; Ye, Q.; Wu, F. Development of an integrated device utilizing exosome-hyaluronic acid-based hydrogel and investigation of its osteogenic and angiogenic characteristics. Materials & Design 2024, 237, 112565. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhang, W.; Xue, W.; Jiang, Y.; Li, S.; Wu, X.; Xu, H.; Ren, J.; Chi, B. Mechanoadaptive injectable hydrogel based on poly(γ-glutamic acid) and hyaluronic acid regulates fibroblast migration for wound healing. Carbohydr Polym 2021, 273, 118607. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic Biol Med 2001, 31, 38–42. [Google Scholar] [CrossRef]
| Nomenclature | 0% rGO | 4.5%rGO | 9%rGO |
|---|---|---|---|
| 0% TA | Alg | Alg/rGO4.5 | Alg/rGO9 |
| 4.5% TA | Alg/TA4.5 | Alg/rGO4.5/TA4.5 | Alg/rGO9/TA4.5 |
| 9% TA | Alg/TA9 | Alg/rGO4.5/TA9 | Alg/rGO9/TA9 |
| Hydrogels | Tensile strength (kPa) | Elongation (%) | Elastic modulus (kPa) | Toughness (kJ/m3) |
|---|---|---|---|---|
| Alg | 84.9 ± 17.6 a | 63.8 ± 4.9 a | 169.2 ± 41.5 a | 22.4 ± 4.5 a |
| Alg/rGO9 | 168.8 ± 12.4 b | 53.9 ± 4.2 a | 412 ± 45.7 b | 42.9 ± 3.2 a,b |
| Alg/TA9 | 179.4 ± 30.5 b | 81.8 ± 8.1 b | 298.7 ± 49.3 b | 72.4 ± 17.9 b |
| Alg/rGO4.5/TA9 | 170.7 ± 23.16 b | 74.8 ± 11.4 b | 310.5 ± 57.5 b | 64.3 ± 15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





