1. Introduction
The infection induced by the novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and referred to as COVID-19, is characterized by an exacerbated inflammatory response. Although most COVID-19 patients exhibit mild symptoms, approximately 5% develop severe manifestations, including acute respiratory distress syndrome, septic shock, and multiple organ failure. Kidney involvement is common, presenting clinically from mild proteinuria to advancing acute kidney injury (AKI) requiring kidney replacement therapy (KRT)[
1].
The incidence of COVID-19-associated acute kidney injury (C19-AKI) varies across different regions. A good illustration is the finding of an international meta-analysis conducted in 2020, involving 49,048 patients. It revealed that 28.6% of individuals hospitalized with COVID-19 in Europe and the USA were diagnosed with AKI, whereas the rate was only 5.5% for inpatients in China [
2]. In contrast, data from the UK indicated that the incidence of C19-AKI in intensive care exceeded 45% during the period from February to July 2020. Regardless of these differences, it remains evident that the development of AKI serves as a poor prognostic indicator for COVID-19 patients, with a risk ratio (RR) of 4.6 for mortality compared to COVID-19 patients without AKI [
3].
The primary risk factors for COVID-19 patients developing acute kidney injury are categorized into patient-related and disease-related factors. Patient-related factors include obesity, advancing age, underlying diseases, record of renal transplant, and pre-existing chronic kidney disease. Conversely, disease-related factors involve the use of invasive mechanical ventilation, severe COVID-19 condition, exposure to nephrotoxic drugs, and the necessity for vasopressor [
3].
The etiology of kidney involvement in COVID-19 is indeed multifaceted as it implicates various factors such as cardiovascular comorbidity and predisposing conditions (e.g., sepsis, hypovolemia, and nephrotoxins). Autopsy findings suggest that acute tubular injury becomes the predominantly cause of kidney injury in COVID-19 patients [
4]. In addition, glomerular injury were documented from the presence of proteinuria in up to 60% patients [
5]. Besides, some vascular causes, such as macrovascular thrombosis, microthrombi, endothelialitis, and thrombotic microangiopathies (atypical hemolytic uremic syndrome, thrombotic thrombocytopenic purpura) also contribute to this kidney injury.
Some studies showed that SARS-CoV-2 could exhibit viral tropism and directly affect the kidney. Some COVID-19 patients experienced endothelial dysfunction, coagulopathy and complement activation leading to AKI. Apart from direct pathophysiological mechanisms, renal dysfunction in COVID-19 may result from the systemic impacts of SARS-CoV-2 infection and critical illness. For instance, significant fluid losses may occur due to hyperpyrexia, and the gastrointestinal symptoms of COVID-19, such as diarrhea, could lead to volume depletion—an important potential factor contributing to AKI in other scenarios. Additionally, critically ill patients may be exposed to nephrotoxins as part of their clinical care, especially antibiotics, which can induce tubular injury or acute interstitial nephritis. Furthermore, those who develop secondary infections, regardless of their nature (bacterial, fungal, or viral), face an elevated risk of secondary sepsis-associated AKI. Patients with severe COVID-19-related pneumonia and/or ARDS also face an increased risk of AKI as a complication of mechanical ventilation [
6].
Thromboelastography (TEG), as a comprehensive whole blood assay, offers a more inclusive assessment for the collective involvement of blood cells, platelets, and plasma in the clot formation process, simulating in vivo coagulation mechanisms. TEG is capable of detecting significant hemostatic abnormalities, pinpointing crucial dysfunctional elements from the initial stages of blood clotting to the fibrinolysis phase. Consequently, TEG can be regarded as another valuable instrument in addressing the challenges posed by the current pandemic [
7].
The main objective of this research was to evaluate the coagulation profiles, including the TEG parameters, in COVID-19 patients who developed acute kidney injury compared to those who did not during their intensive care unit (ICU) stay. This analysis is useful to identify early predictors of acute kidney injury.
2. Materials and Methods
2.1. Study Design and Participants
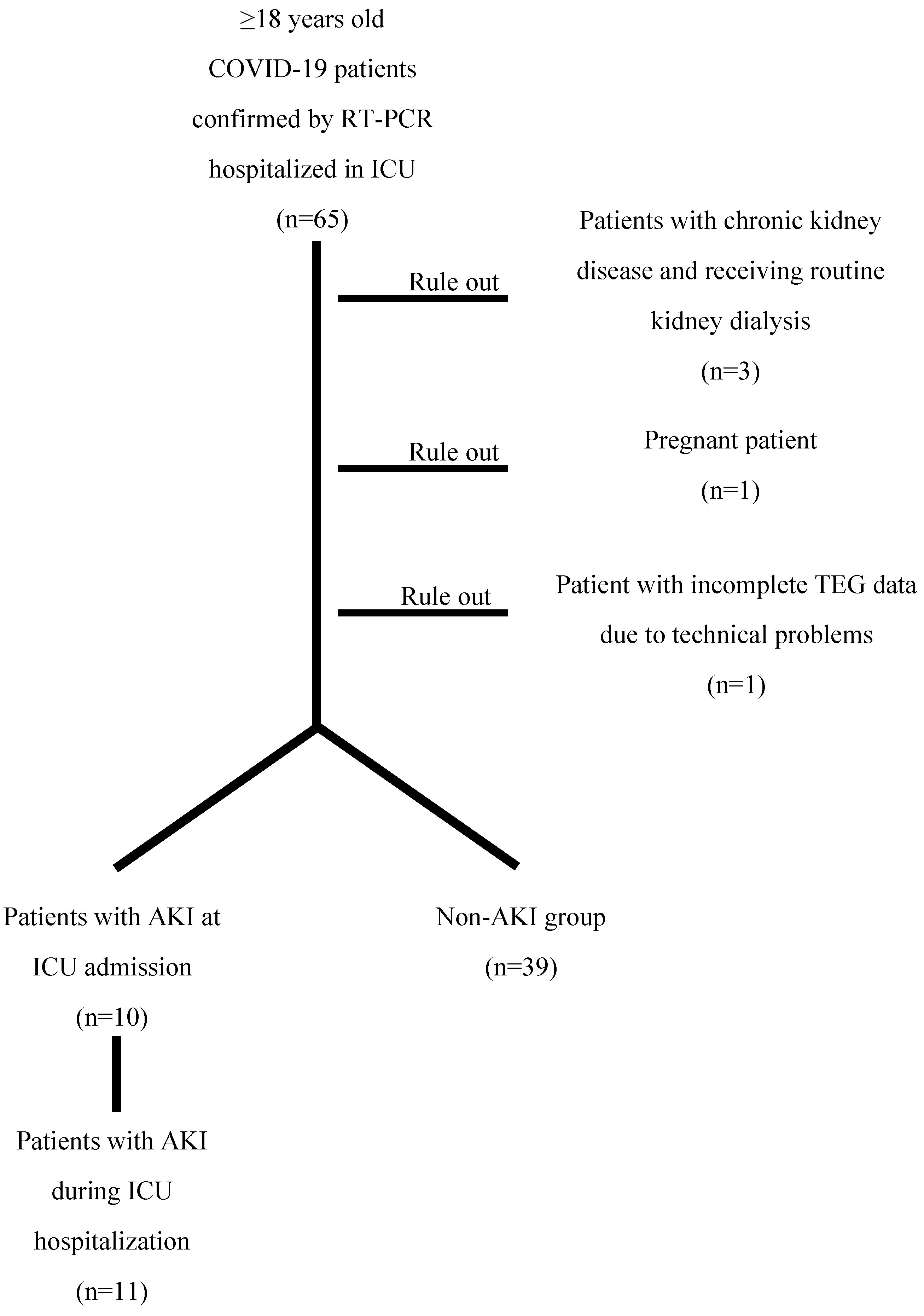
This single-center cohort retrospective study involved patients aged ≥18 years in the intensive care unit (ICU) of Sardjito Hospital in Yogyakarta, Indonesia between November 2020 and September 2021. This hospital was specifically designated for the exclusive treatment of patients with COVID-19 in Yogyakarta, Indonesia. The research subjects must meet inclusion criteria as follows: (1) patients diagnosed with COVID-19 infection, confirmed by a positive result of COVID-19 on reverse transcription polymerase chain reaction (RT-PCR) assay; (2) patients who is performed a TEG examination upon ICU admission as monitoring for coagulation status and anticoagulant therapy. While the exclusion criteria are as follows: (1) patients with chronic kidney disease (CKD) diagnosed by physician before admission or having chronic hemodialysis or renal transplantation; (2) patients with acute limb injury; (3) patients with thromboembolism, such as deep vein thrombosis (DVT) and pulmonary embolism (PE); (4) pregnancy. Approval for this study was obtained from Ethics Committee of Medicine, Public Health, and Nursing Faculty of Universitas Gadjah Mada, and research permission was granted by Sardjito Hospital.
2.2. Definitions
The diagnosis of acute kidney injury (AKI) applied the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition and the elaboration provided by 2023 Acute Kidney Injury Consensus of Indonesian Society of Nephrology. KDIGO defines AKI by any of the following criteria: (1) an increase in serum creatinine by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or (2) an increase in serum creatinine to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or (3) urine volume <0.5 ml/kg/h for 6 hours [
8]. Subsequently, the consensus explained that the baseline value refers to the serum creatinine obtained from prior data within 7 days. In cases where a baseline value is unavailable, a decrease of ≥0.3 mg/dL in creatinine serum within 48 hours to 7 days after treatment can be classified as AKI [
9].
2.3. Data Collection and Measurement
Data about demographic, clinical, and laboratory characteristics at the time of admission were obtained from electronic medical records. ICU monitoring data were extracted from paper-based medical records, if necessary, to diagnose acute kidney injury (AKI) based on urine output monitoring data. Over the eleven months of this study, a total of 65 patients were enrolled. Among the research subjects, three patients diagnosed with chronic kidney disease (CKD) and having chronic hemodialysis, along with the pregnant patient, were excluded from this study. Additionally, one patient was also excluded due to incomplete TEG data resulting from technical problems. In short, 60 patients were included in the cohort.
Laboratory data included complete blood counts, creatinine serum levels for assessing renal function, and coagulation function such as fibrinogen and D-Dimer. TEG analysis was performed using TEG
®5000 Thromboelastograph
® Hemostasis System. The completion of TEG provided measurements: R (minutes), K (minutes), α-angle (degrees), maximum amplitude (MA, mm), LY30 (percentage), and Coagulation Index (CI). R represents the time taken for initial measurable clot formation, measured from the sample’s beginning until the amplitude reaches 2 mm, representing thrombus formation initiation. K indicates the duration from R until the clot stiffness reaches an amplitude of 20 mm, representing the dynamics of clot formation. The α-angle (degrees) refers to the angle between the horizontal axis and the slope of the line starting at R and running tangent to the TEG tracing, measuring the kinetics of clot development. Maximum amplitude (MA) indicates the maximum width of the tracing, reflecting the greatest clot strength and is considered the parameter most indicative of platelet function in TEG. LY30 represents the percentage of clot lyses that occurs 30 minutes after reaching the maximum amplitude. Coagulation Index (CI) is the linear combination of R, K, α-angle, and MA. All personnel conducting TEG processing had undergone training for the procedure. Quality assurance for the TEG machine was maintained through daily quality control procedures using both normal and abnormal controls.
Figure 1.
Flowchart of the study.
Figure 1.
Flowchart of the study.
2.4. Statistical Analysis
Continuous data with normal distributions were presented as mean ± SD, and variables with skewed distribution were summarized using the median and interquartile range. Categorical variables were summarized as percentages. To compare differences between AKI group and non-AKI group in demographics, medical history, baseline laboratory values, and TEG parameters, the Wilcoxon rank-sum test (or 2-sample t-test as appropriate) was employed for continuous variables, and the chi-squared test was used for categorical variables. The variables showing a statistically significant difference in the two groups were then categorized as at-risk and not-at-risk, allowing for preliminary univariate binary logistic regression analyses to be conducted. Specifically, parameters with P < 0.25 in the univariate analyses were incorporated into the multivariate binary logistic regression analysis to select significant predictors. Results are presented as coefficient associated with an independent variable (B), odds ratios (ORs) with 95% confidence intervals (95% CIs), and P values. Statistical analyses were conducted using SPSS version 25.0 software (IBM Corp.).
3. Results
A total of 60 patients had participated in the study, with a median age of 61 (53-67) years. 65.0% (39 of 60) of patients were men, with the mean body mass index (BMI) of 26.00 ± 4.58 kg/m
2. The median length of their hospital stay was 16 (9-23) days. Upon admission of these 60 patients of COVID-19, 6.7% (4 of 60) patients were classified as having a moderate condition; 80.0% (48 of 60) patients were presented with severe degree; and 13.3% (8 of 60) patients were critically ill. The prevalence of comorbid of hypertension, diabetes mellitus, and coronary artery disease was 65.0% (39 of 60), 46.7% (28 of 60), and 21.7% (13 of 60), respectively. Coagulopathies were frequent in individuals with COVID-19, as observed in 76.7% (46 of 60) patients. This was further supported by the median D-Dimer levels, which measured 760 (525-1408) ng/mL, exceeding the 500 ng/mL threshold indicative of coagulopathy. The incidence of acute kidney injury (AKI) in the cohort was 35.0% (21 of 60). In comparison with non-AKI patients, those with AKI exhibited a greater prevalence of diabetes mellitus, higher D-Dimer levels, as well as higher values for MA and CI in TEG parameters (
Table 1).
Furthermore, the 60 patients were classified as either at-risk or not-at-risk based on the comorbidity of diabetes mellitus, D-Dimer levels, and specific TEG parameters, namely, MA and CI. A patient is considered at-risk if they have comorbid diabetes mellitus, if their D-Dimer levels exceed 500 ng/mL, or if their TEG parameters indicate a hypercoagulable state. TEG measurements suggest a hypercoagulable state when maximum amplitude (MA) is higher than 70 mm or coagulation index (CI) is greater than 3.
Univariate binary logistic analyses of the 4 variables were then conducted to examine the effect of each variable individually on the emergence of AKI. It is observed that comorbid diabetes mellitus alone was associated with 3.57-fold increased odds (95% CI, 1.17 to 10.93) of developing AKI. Compared with patients with D-Dimer level of ≤500 ng/mL, those with D-Dimer level >500 ng/mL had independently 4.22-fold odds (95% CI, 0.85 to 21.08) of emerging AKI. Moreover, the hypercoagulable state, as indicated by MA >70 mm orac CI >3, had individually 7.20-fold odds (95%CI, 2.14 to 24.21) or 5.10-fold odds (95% CI, 1.42 to 18.27) of AKI development, respectively (
Table 2).
Subsequently, all 4 parameters were incorporated into the multivariate analysis to identify significant predictor(s), because they all exhibited P-values of <0.25 in the univariate binary logistic regression analyses. It was revealed that the TEG parameter of MA emerged as the sole significant predictor for the development of AKI (OR, 6.33; 95% CI, 1.56 to 25.64) (
Table 2).
4. Discussion
COVID-19 infection primarily results in a respiratory disease although it frequently affects various organs, such as the kidneys. The ability of SARS-CoV-2 to directly invade kidney cells is attributed to the existence of angiotensin-converting enzyme (ACE-2) on the renal cell membrane [
10]. In this study, it was observed that 35.0% of COVID-19 patients admitted to the intensive care unit (ICU) developed acute kidney injury (AKI) during their ICU stay. This rate is comparable to studies with larger sample sizes conducted by Hirsch et al. and Shchepalina et al., who found AKI incidence rates of 36.6% [
11] and 38.0% [
12], respectively. However, Cheruiyot et al. found a lower AKI incidence rate of 21.6% [
13]. Specifically, concerning patients in the ICU, Chan et al. reported a higher occurrence rate of AKI, at 76%, for COVID-19-related AKI in ICU patients [
14].
In the meta-analysis in patients affected with COVID-19, diabetes mellitus (DM) is among the most commonly occurring comorbidities, with an average prevalence of 9.7% [
15], a rate lower than that observed in this study, which is 46.7%. A significant difference was observed in the prevalence of comorbid DM among COVID-19 patients in the AKI group (66.7%) compared to those in the non-AKI group (35.9%). Further, the findings demonstrated that COVID-19 patients with comorbid diabetes mellitus (DM) are 3.57 times more likely to develop AKI compared to those without comorbid DM. These result align with findings reported in meta-analyses conducted by Tahereh et al. and Zhang et al., which identified DM as one of the significant risk factors for the development of AKI in COVID-19 patients [
16,
17].
The multifaceted causes underlying kidney damage in diabetes patients involve various pathophysiological mechanisms. One hypothesis proposes that changes in both structure and function of renal blood vessels and tubular epithelial cells trigger an increase in the generation of cytokines and chemokines. As a result, inflammation, ischemia, and a specific type of tubular dysfunction known as isolated proximal tubulopathy occur. Another primary mechanism contributing to diabetic nephropathy (DN) is the dysfunction of endothelial cells. In diabetic kidneys, there is a reduced production of nitric oxide (NO), a molecule synthesized by the enzyme endothelial nitric oxide synthase (eNOS). The altered metabolism of NO in diabetes makes the renal blood vessels more susceptible to stimuli that induce vasoconstriction [
18].
In this study, we noted a frequent occurrence of coagulopathies in COVID-19 patients, regardless of whether they developed acute kidney injury (AKI). The findings align with those obtained by Silva et al., who found that 96.7% of COVID-19 patients had coagulopathy [
19], a notably higher rate than our findings (76.7%). Our result also showed an elevated D-Dimer levels >500 ng/mL in COVID-19 patients. D-dimers serve as the indicative of the formation of fibrin clots, crosslinking of clots by F-XIIIa, and the process of fibrinolysis. The significant increase in D-dimers observed in COVID-19 suggests the activation of coagulation due to viremia and cytokine storm. However, it is worth noting that superinfection and organ dysfunction could also be potential contributing factors [
20].
One of the complexities of COVID-19 that had received little attention involves the microvascular thrombosis in organs including the kidneys, lungs, arteries and veins. Severe inflammation, dysfunction of the endothelium (the lining of blood vessels), activation of platelets, reduced mobility, and stagnant blood flow may serve as the potential triggers for this phenomenon. These factors collectively increase the likelihood of patients developing a pro-thrombotic condition. The clotting issues linked to COVID-19 typically start with significant elevations in fibrin and fibrinogen levels, coupled with the presence of fibrin degradation products. There are also lower platelet counts, abnormalities in blood clotting tests like prothrombin time and partial thromboplastin time, and the appearance of fragmented red blood cells in peripheral blood samples. Oxygen deprivation caused by COVID-19-induced pneumonia directly amplifies blood viscosity, thereby promoting clot formation through a specific biological pathway activated by low oxygen levels [
21].
If summarized, the pathophysiology of COVID-19 infection is divided into three phases, namely (1) infection, (2) hypercoagulation and thrombosis, then (3) coagulopathy (bleeding tendency) and multiple organ failure (MOF). Firstly, the transmission of SARS-CoV-2 to the infected host by ACE-2 in the epithelial cells in the respiratory tract leads to injury in the endothelial cells as well as the activation of platelets and immune system. Secondly, there is an occurrence of endothelial dysfunction that triggers an inflammatory response and platelet activation in order to repair the damaged endothelium. The activation of platelets subsequently leads to the development of microthrombi containing fibrin. Additionally, the immune defense and cytokine storm also play a role in this process through immunothrombosis [
22]. These incidents of thrombotic microangiopathy (TMA) impaired kidney function, potentially resulting in the development of acute kidney injury (AKI). Moreover, the blockage of major blood vessels within the kidneys, such as the renal artery and renal vein, by thrombotic occlusion, may additionally contribute to kidney injury [
23]. These conditions involving microvascular and macrovascular clotting, along with compromised blood flow, are referred to as suppressed-fibrinolytic-type disseminated intravascular coagulation (DIC) [
24]. Patients at this phase would exhibit significantly increased levels of D-Dimer (3 to 6 times higher than normal), slightly decreased platelet count (ranging between 100-150 × 10
9/L), and a slight prolongation in PT, even when undergoing thromboprophylaxis [
25]. Thirdly, the condition known as enhanced-fibrinolytic-type DIC, which causes hemorrhage, is linked to impairment of endothelial function, depletion of coagulation factors (including fibrinogen and others), and the consumption of platelets. Ultimately, all of these alterations seen in COVID-19 contribute to the development of multiple organ failure (MOF) in various organs such as the kidney, lung, liver, brain, and heart [
22,
24].
The mechanism of coagulopathy causing AKI strengthens our findings, which revealed that individuals with a D-Dimer level >500 ng/mL, indicative of coagulopathy, are 4.22 times more prone to experience AKI compared to those who had a D-Dimer level ≤500 ng/mL. Moreover, our results also indicated that COVID-19 patients with a hypercoagulable state have a greater risk of developing AKI; namely, patients with TEG parameter of maximum amplitude (MA) >70 mm are 7.2 times more at risk, while patients with TEG parameter of coagulation index (CI) >3 are 5.1 times more at risk.
The risk factors and causes of AKI in COVID-19 are varied and complex. Our multivariate binary regression analysis highlights that the hypercoagulable state, indicated solely by TEG parameter of MA >70 mm, is the predominant factor in AKI development among COVID-19 patients. The patients having TEG parameter of MA > 70 mm are 6.33 times more at risk of developing AKI. This finding aligns with that mentioned by Meier et al., who argue that in ambulatory patients with renal sufficiency, the predominant anomaly observed in TEG examinations is an increased MA [
26]. This is supported by the fact that MA is a parameter of TEG that represents the greatest clot strength and is believed to be indicative of platelet activity. Moreover, it is commonly understood that platelets and fibrinogen play essential roles in clot formation and clot strength [
27]. Thus, it could be summed up that TEG examinations are essential to predict the occurrence of AKI in COVID-19 patients.
The present study has several limitations. First, this research had the limited number of patients as it is conducted at a single center. Hence, it is essential to validate the study findings through a more extensive cohort. Second, given that this is an observational study, we cannot establish the causal relationships between exposures and AKI. Third, there remain undisclosed variables despite the effort to consider possible confounding factors. Fourth, our primary data depended on electronic health information systems. However, the analysis of AKI identification was supported by paper-based medical records of ICU monitoring data to corroborate the AKI condition.
5. Conclusions
Our cohort validates the kidney involvement of COVID-19 infection, as it is evident in the occurrence of acute kidney injury (AKI) among the patients. Diabetes mellitus, high D-Dimer levels, and hypercoagulability serve as prominent risk factors in the development of AKI in COVID-19 patients. Since coagulopathy is one of the direct mechanisms contributing to AKI, it was identified that TEG parameter of maximum amplitude (MA) exceeding 70 mm is the single independent significant predictor of AKI.
Author Contributions
Conceptualization, U.S.; methodology, U.S., I.P. and Y.W.; software, M.R.M.; validation, U.S., I.P. and Y.W.; formal analysis, M.R.M.; investigation, I.P. and Y.W.; resources, U.S.; data curation, M.R.M.; writing—original draft preparation, M.R.M.; writing—review and editing, U.S., I.P., and Y.W.; supervision, U.S.; project administration, M.R.M.; funding acquisition, U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
The study was approved by Ethics Committee of Medicine, Public Health, and Nursing Faculty of Universitas Gadjah Mada, protocol code KE/FK/0400/EC/2021 on April 28th, 2021.
Data Availability Statement
The dataset will be made available upon reasonable request.
Acknowledgments
We are grateful to the entire team of medical practitioners and healthcare professionals responsible for COVID-19 patient management at Sardjito Hospital, especially to Ika Trisnawati and Nur Rahmi Ananda (Division of Pulmonology, Department of Internal Medicine, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Sardjito Hospital, Yogyakarta, Indonesia), as well as Akhmad Yun Jufan (Department of Anesthesiology and Intensive Care, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Sardjito Hospital, Yogyakarta, Indonesia). We acknowledge the Clinical Pathology residents and laboratory staff of Sardjito Hospital for their support.
Conflicts of Interest
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- K. Ramanathan et al., “Management of Acute Kidney Injury in Patients with COVID-19,” Lancet Respir. Med., vol. 8, pp. 738–742, 2020. [CrossRef]
- E. L. Fu et al., “Acute kidney injury and kidney replacement therapy in COVID-19: A systematic review and meta-analysis,” Clin. Kidney J., vol. 13, no. 4, pp. 550–563, 2020. [CrossRef]
- J. Hilton, N. Boyer, M. K. Nadim, L. G. Forni, and J. A. Kellum, “COVID-19 and Acute Kidney Injury,” no. 38, pp. 473–489, 2022. [CrossRef]
- J. H. Ng, V. Bijol, M. A. Sparks, M. E. Sise, H. Izzedine, and K. D. Jhaveri, “Pathophysiology and Pathology of Acute Kidney Injury in Patients With COVID-19,” Adv. Chronic Kidney Dis., vol. 27, no. 5, pp. 365–376, 2020. [CrossRef]
- Z. Li et al., “Caution on Kidney Dysfunctions of COVID-19 Patients,” medRxiv, 2020. [CrossRef]
- M. K. Nadim et al., “COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup,” Nat. Rev. Nephrol., vol. 16, no. 12, pp. 747–764, 2020. [CrossRef]
- T. Hranjec et al., “Integral Use of Thromboelastography With Treatment, Avoid Complications, and Improve Survival of Patients With Coronavirus Disease 2019-Related Coagulopathy,” Crit. Care Explor., vol. 2, p. e0287, 2020. [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group, “Summary of clinical practice guidelines for acute kidney injury.,” Kidney Int. Suppl., vol. 2, no. 1, 2012. [CrossRef]
- Indonesian Society of Nephrology, Konsensus Gangguan Ginjal Akut. 2023.
- V. G. Puelles et al., “Multiorgan and Renal Tropism of SARS-CoV-2,” New Engl. Med. J., vol. 383, no. 6, pp. 590–592, 2020. [CrossRef]
- J. S. Hirsch et al., “Acute kidney injury in patients hospitalized with COVID-19,” Kidney Int., vol. 98, no. 1, pp. 209–218, 2020. [CrossRef]
- A. Shchepalina et al., “Acute Kidney Injury in Hospitalized Patients with COVID-19: Risk Factors and Serum Biomarkers,” Biomedicines, vol. 11, no. 5, 2023. [CrossRef]
- S. Cheruiyot, J. Shabani, J. Shah, C. Gathu, and A. Sokwala, “Associated Factors and Outcomes of Acute Kidney Injury in COVID-19 Patients in Kenya,” Can. J. Kidney Heal. Dis., vol. 11, 2024. [CrossRef]
- L. Chan et al., “AKI in Hospitalized Patients with COVID-19,” J. Am. Soc. Nephrol., vol. 32, no. 1, pp. 151–160, 2021. [CrossRef]
- J. Yang, Y. Zheng, X. Gou, K. Pu, Z. Chen, and Q. Guo, “Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis,” Int. J. Infect. Dis., vol. 94, pp. 91–95, 2020. [CrossRef]
- S. Tahereh, R. S. Tahereh, R. Foad, O. Fatemeh, H. Bahareh, N. M. Javad, and M. Mehdi, “COVID-19 in patients with and without acute kidney injury,” vol. 123, no. 5, pp. 372–380, 2022. [CrossRef]
- J. Zhang et al., “Risk factors for acute kidney injury in COVID-19 patients : an updated systematic review and meta-analysis,” Ren. Fail., vol. 45, no. 1, 2023. [CrossRef]
- Sharma, and D. R. Kumbala, “Acute kidney injury in diabetic patients: A narrative review,” Medicine (Baltimore)., vol. 102, no. 21, p. e33888, 2023. [CrossRef]
- C. da Silva et al., “COVID-19-associated coagulopathy and acute kidney injury in critically ill patients,” einstein, vol. 21, p. eAO0119, 2023. [CrossRef]
- G. D. Wool and J. L. Miller, “The Impact of COVID-19 Disease on Platelets and Coagulation,” vol. 88, no. 1, pp. 15–27, 2021. [CrossRef]
- T. Sadighpour, M. Mubarak, P. Sabaeifard, S. Saeifar, and F. Kenari, “COVID-19 and renal involvement; evolving role of thromboinflammation, vascular and glomerular disease in the pathogenesis,” J. Nephropathol., vol. 10, no. 3, p. e23, 2021. [CrossRef]
- H. Liu et al., “Thrombosis and Coagulopathy in COVID-19 : Current Understanding and Implications for Antithrombotic Treatment in Patients Treated With Percutaneous Coronary Intervention,” Front. Cardiovasc. Med., vol. 7, no. 599334, 2021. [CrossRef]
- G. M. Rossi et al., “Kidney Biopsy Findings in a Critically Ill COVID-19 Patient With Dialysis-Dependent Acute Kidney Injury: A Case Against ‘SARS-CoV-2 Nephropathy,’” Kidney Int. Reports, vol. 5, no. 7, pp. 1100–1105, 2020. [CrossRef]
- H. Asakura and H. Ogawa, “COVID - 19 - associated coagulopathy and disseminated intravascular coagulation,” Int. J. Hematol., vol. 113, pp. 45–57, 2021. [CrossRef]
- J. Thachil, F. M. Cushman, and A. Srivastava, “A proposal for staging COVID-19 coagulopathy,” Res. Pract. Thromb. Haemost., vol. 4, pp. 731–736, 2020. [CrossRef]
- K. Meier et al., “Thrombelastography Suggests Hypercoagulability in Patients with Renal Dysfunction and Intracerebral Hemorrhage,” J. Stroke Cerebrovasc. Dis., vol. 27, no. 5, pp. 1350–1356, 2018. [CrossRef]
- X. Guan, L. Li, H. Li, M. Gong, H. Zhang, and X.-L. Wang, “Risk factor prediction of severe postoperative acute kidney injury at stage 3 in patients with acute type A aortic dissection using thromboelastography,” Front. Cardiovasc. Med., 2023. [CrossRef]
Table 1.
Demographics, clinical, and laboratory characteristics of COVID-19 patients having TEG examinations in ICU.
Table 1.
Demographics, clinical, and laboratory characteristics of COVID-19 patients having TEG examinations in ICU.
| Variable |
All Patients |
AKI |
Non-AKI |
P value |
| N |
60 |
21 (35.0%) |
39 (65.0%) |
|
| Age, years |
61 (53-67) |
64 (55-67) |
61 (47-66) |
0.364 |
| Male patient, % |
39 (65.0%) |
16 (76.2%) |
23 (59.0%) |
0.206 |
| Body Mass Index, kg/m2
|
26.00 ± 4.58 |
26.12 ± 4.10 |
25.93 ± 4.87 |
0.872 |
| Length of Stay, days |
16 (9-23) |
12 (8-23) |
17 (9-24) |
0.140 |
COVID-19 Severity, %
Moderate
Severe
Critical |
|
|
|
0.229 |
| 4 (6.7%) |
0 (0.0%) |
4 (10.0%) |
|
| 48 (80.0%) |
17 (81.0%) |
31 (79.5%) |
|
| 8 (13.3%) |
4 (19.0%) |
4 (10.3%) |
|
| Obese, % |
9 (15.0%) |
4 (19.0%) |
5 (12.8%) |
0.519 |
| Hypertension, % |
39 (65.0%) |
16 (76.2%) |
23 (59.0%) |
0.182 |
| Diabetes mellitus, % |
28 (46.7%) |
14 (66.7%) |
14 (35.9%) |
0.023* |
| Coronary artery disease, % |
13 (21.7%) |
6 (28.6%) |
7 (17.9%) |
0.341 |
| Coagulopathy, % |
46 (76.7%) |
19 (90.5%) |
27 (69.2%) |
0.063 |
| PT, seconds |
16.8 (15.5-18.7) |
16.9 (15.7-18.8) |
16.7 (15.4-18.7) |
0.883 |
| APTT, seconds |
37.2 (32.8-40.3) |
37.8 (34.0-42.9) |
35.9 (32-38.7) |
0.110 |
| INR |
1.32 (1.20-1.44) |
1.32 (1.13-1.46) |
1.32 (1.20-1.44) |
0.828 |
| D-Dimer, ng/mL |
760 (525-1408) |
970 (666-1733) |
685 (427-1371) |
0.045* |
| Fibrinogen, mg/dL |
460.8 ± 192.6 |
473.8 ± 201.4 |
453.9 ± 190.0 |
0.712 |
| WBC count, 103/μL |
10.2 (6.9-14.5) |
10.2 (7.5-14.9) |
9.5 (6.3-14.4) |
0.407 |
| RBC count, 106/μL |
4.52 ± 0.74 |
4.36 ± 0.87 |
4.61 ± 0.65 |
0.258 |
| Hemoglobin, g/dL |
13.0 (11.4-14.0) |
12.4 (11.0-14.1) |
13.0 (11.6-14.0) |
0.500 |
| Hematocrit, % |
37.81 ± 6.21 |
37.04 ± 7.01 |
38.23 ± 5.79 |
0.510 |
| Platelet Count, 103/μL |
258 (178-369) |
234 (153-289) |
270 (178-411) |
0.314 |
| TEG – R, minutes |
5.3 ± 1.9 |
5.3 ± 2.1 |
5.3 ± 1.8 |
0.987 |
| TEG – K, minutes |
1.5 (1.2-2.2) |
1.2 (1.2-2.4) |
1.7 (1.2-2.2) |
0.301 |
| TEG – α-angle, degrees |
68.1 (59.8-73.4) |
72.2 (60.0-74.3) |
65.7 (58.5-72.3) |
0.152 |
| TEG – MA, mm |
69.0 ± 9.3 |
74.6 ± 9.6 |
65.9 ± 7.8 |
0.001* |
| TEG – LY30, % |
0.1 (0.0-1.6) |
0.1 (0.0-1.0) |
0.2 (0.0-1.9) |
0.643 |
| TEG – CI |
1.4 ± 2.1 |
2.3 ± 2.3 |
1.0 ± 1.9 |
0.033* |
Table 2.
Univariate and multivariate logistic regression analyses of risk factors associated with the development of AKI.
Table 2.
Univariate and multivariate logistic regression analyses of risk factors associated with the development of AKI.
| Variable |
Univariate Analyses |
Multivariate analysis |
| B |
P |
OR (95% CI) |
B |
P |
OR (95% CI) |
| Diabetes mellitus |
1.273 |
0.026* |
3.57 (1.17-10.93) |
1.157 |
0.084 |
3.18 (0.86-11.79) |
| D-Dimer |
1.440 |
0.079 |
4.22 (0.85-21.08) |
1.708 |
0.071 |
5.52 (0.86-35.28) |
| TEG – MA |
1.974 |
0.001* |
7.20 (2.14-24.21) |
1.845 |
0.010* |
6.33 (1.56-25.64) |
| TEG – CI |
1.629 |
0.012* |
5.10 (1.42-18.27) |
0.891 |
0.261 |
2.44 (0.51-11.53) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).