Submitted:
04 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Orchard
2.2. Phenological Growth Stage
2.3. Climatic Data and Soil Moisture Content Measurement
2.4. Measurement of Ecophysiological Parameters
2.4.1. Leaf Area Index, Sap Flow and Gas Exchange
2.4.2. Water Potential
2.5. Stem Water Storage
2.6. Statistical Analysis
3. Results
3.1. Bioclimatic Context
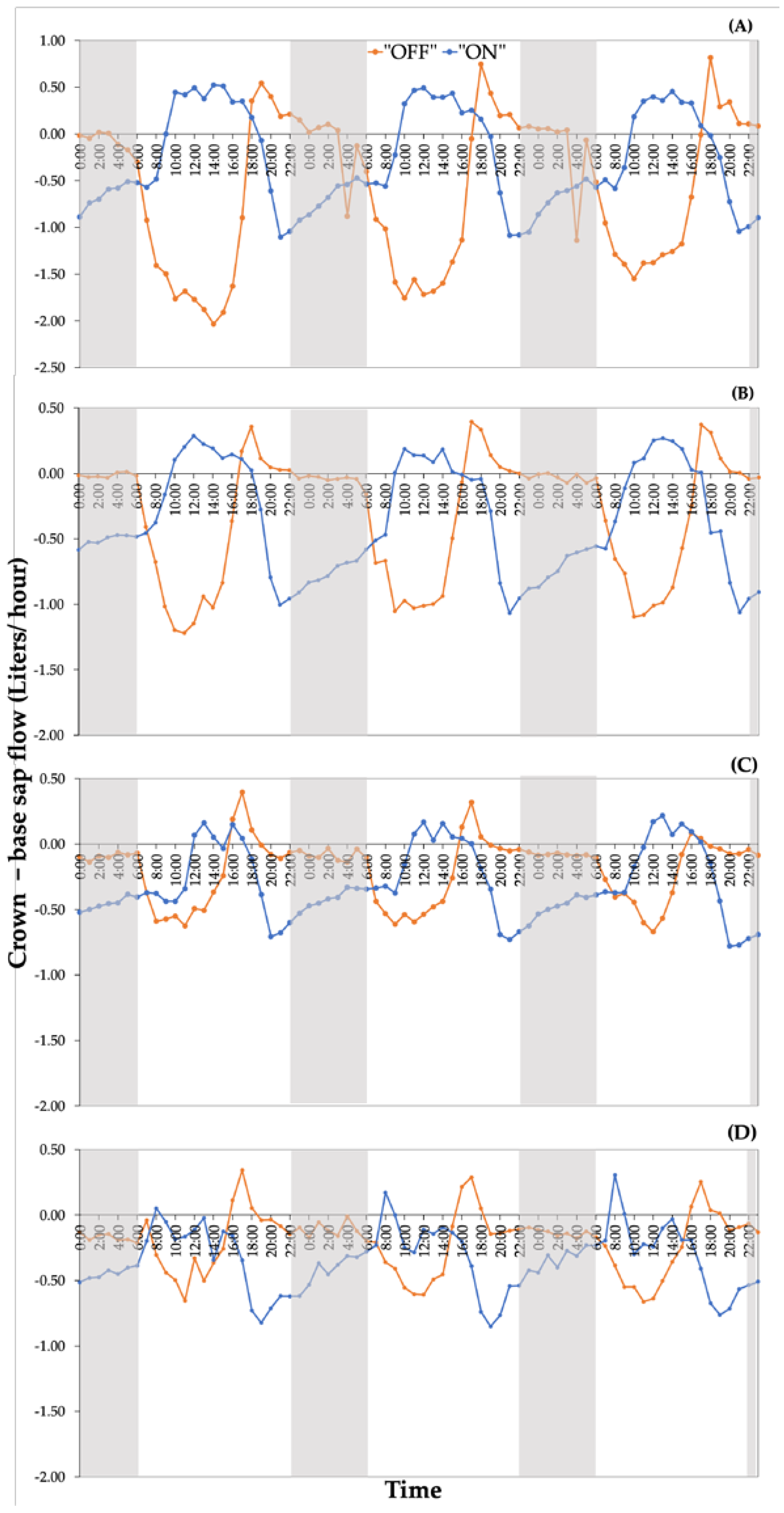
3.2. Sap Flow during the Nocturnal Period
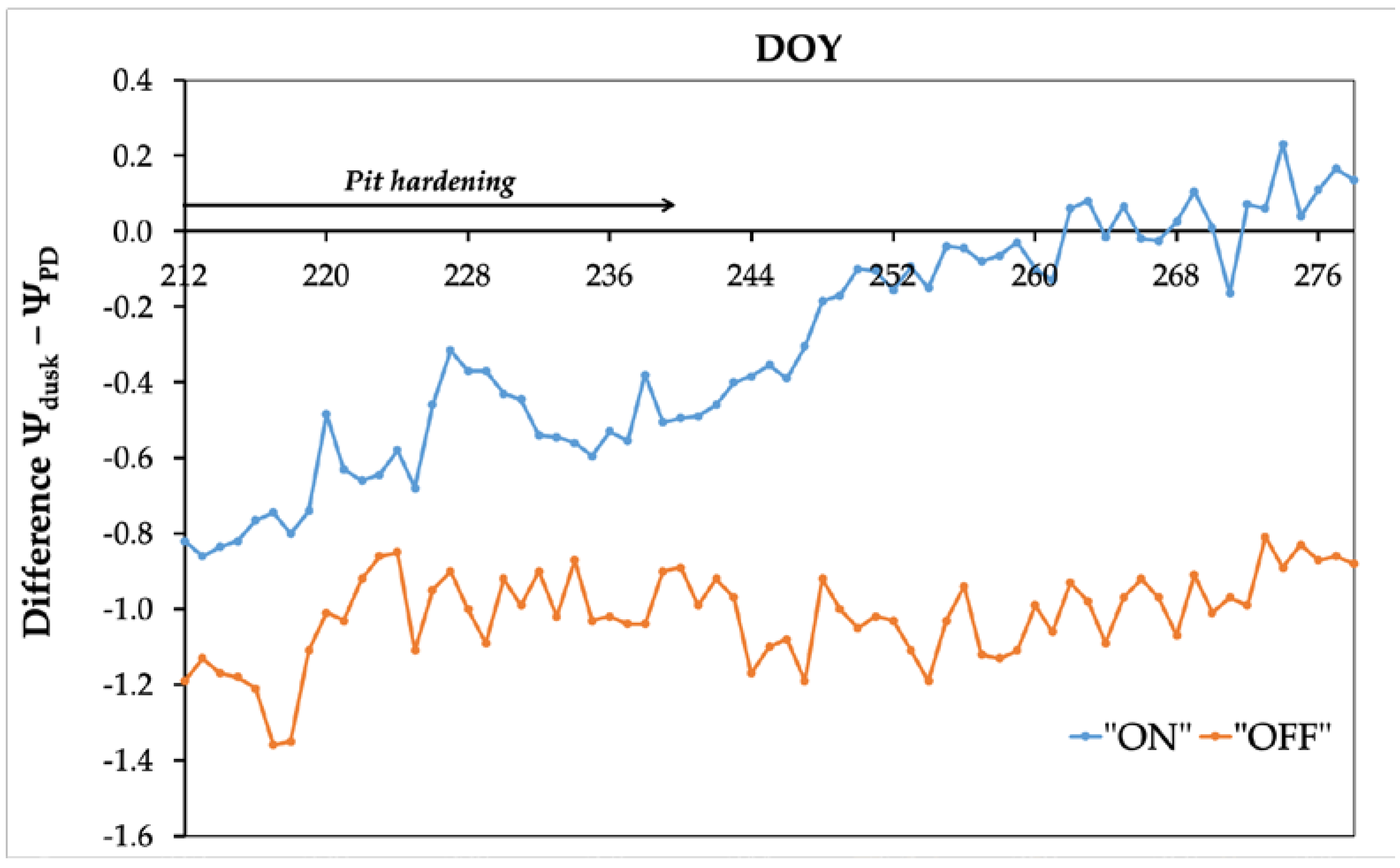
3.3. Assessment of Replenishment and Depletion of Internal Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sillmann, J.; Kharin, V.V.; Zwiers, F.W.; Zhang, X.; Bronaugh, D. Climate Extremes Indices in the CMIP5 Multimodel Ensemble: Part 2. Future Climate Projections. Journal of Geophysical Research: Atmospheres 2013, 118, 2473–2493. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Hsiao, T.C. Significant Transpirational Water Loss Occurs throughout the Night in Field-Grown Tomato. Functional Plant Biology 2007, 34, 172–177. [Google Scholar] [CrossRef]
- Sadok, W.; Jagadish, S.V.K. The Hidden Costs of Nighttime Warming on Yields. Trends Plant Sci 2020, 25, 644–651. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The Role of Nighttime Water Balance on Olea Europaea Plants Subjected to Contrasting Water Regimes. J Plant Physiol 2018, 226, 56–63. [Google Scholar] [CrossRef]
- Escalona, J.M.; Fuentes, S.; Tomás, M.; Martorell, S.; Flexas, J.; Medrano, H. Responses of Leaf Night Transpiration to Drought Stress in Vitis Vinifera L. Agric Water Manag 2013, 118, 50–58. [Google Scholar] [CrossRef]
- Dayer, S.; Herrera, J.C.; Dai, Z.; Burlett, R.; Lamarque, L.J.; Delzon, S.; Bortolami, G.; Cochard, H.; Gambetta, G.A. Nighttime Transpiration Represents a Negligible Part of Water Loss and Does Not Increase the Risk of Water Stress in Grapevine. Plant Cell Environ 2021, 44, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.R.; Donovan, L.A. Helianthus Nighttime Conductance and Transpiration Respond to Soil Water But Not Nutrient Availability. Plant Physiol 2007, 143, 145–155. [Google Scholar] [CrossRef] [PubMed]
- de Dios, V.R.; Roy, J.; Ferrio, J.P.; Alday, J.G.; Landais, D.; Milcu, A.; Gessler, A. Processes Driving Nocturnal Transpiration and Implications for Estimating Land Evapotranspiration. Sci Rep 2015, 5, 10975. [Google Scholar] [CrossRef]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime Stomatal Conductance and Transpiration in C3 and C4 Plants. Plant Physiol 2007, 143, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Removal of Nutrient Limitations by Long-Term Fertilization Decreases Nocturnal Water Loss in Savanna Trees. Tree Physiol 2007, 27, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.J.; Phillips, N.G. Interspecific Variation in Nighttime Transpiration and Stomatal Conductance in a Mixed New England Deciduous Forest. Tree Physiol 2006, 26, 411–419. [Google Scholar] [CrossRef]
- Donovan, L.; Linton, M.; Richards, J. Predawn Plant Water Potential Does Not Necessarily Equilibrate with Soil Water Potential under Well-Watered Conditions. Oecologia 2001, 129, 328–335. [Google Scholar] [CrossRef]
- Kozlowski, T.; Pallardy, S. Transpiration and Plant Water Balance. In Physiology of Woody Plants; Kozlowski, T.T., Pallardy, S.G., Eds.; Academic Press: San Diego, California, USA, 1997; pp. 294–296. [Google Scholar]
- Huang, C.-W.; Domec, J.-C.; Ward, E.J.; Duman, T.; Manoli, G.; Parolari, A.J.; Katul, G.G. The Effect of Plant Water Storage on Water Fluxes within the Coupled Soil–Plant System. New Phytologist 2017, 213, 1093–1106. [Google Scholar] [CrossRef]
- Fang, W.; Lu, N.; Zhang, Y.; Jiao, L.; Fu, B. Responses of Nighttime Sap Flow to Atmospheric and Soil Dryness and Its Potential Roles for Shrubs on the Loess Plateau of China. Journal of Plant Ecology 2018, 11, 717–729. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Veneklaas, E.J.; Lambers, H.; Burgess, S.S.O. Using Multiple Trait Associations to Define Hydraulic Functional Types in Plant Communities of South-Western Australia. Oecologia 2008, 158, 385–397. [Google Scholar] [CrossRef]
- Goldstein, G.; Andrade, J.L.; Meinzer, F.C.; Holbrook, N.M.; Cavelier, J.; Jackson, P.; Celis, A. Stem Water Storage and Diurnal Patterns of Water Use in Tropical Forest Canopy Trees. Plant Cell Environ 1998, 21, 397–406. [Google Scholar] [CrossRef]
- Steppe, K.; Lemeur, R. An Experimental System for Analysis of the Dynamic Sap-Flow Characteristics in Young Trees: Results of a Beech Tree. CSIRO Publishing 2004, 31, 83–92. [Google Scholar] [CrossRef]
- Zeppel, M.; Tissue, D.; Taylor, D.; Macinnis-Ng, C.; Eamus, D. Rates of Nocturnal Transpiration in Two Evergreen Temperate Woodland Species with Differing Water-Use Strategies. Tree Physiol 2010, 30, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Feng, Q.; Si, J.; Mitchell, P.J.; Forster, M.A.; Zhang, X.; Zhao, C. Depressed Hydraulic Redistribution of Roots More by Stem Refilling than by Nocturnal Transpiration for Populus Euphratica Oliv. in Situ Measurement. Ecol Evol 2018, 8, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Moriana, A.; Orgaz, F.; Pastor, M.; Fereres, E. Yield Responses of a Mature Olive Orchard to Water Deficits. Journal of the American Society for Horticultural Science jashs 2003, 128, 425–431. [Google Scholar] [CrossRef]
- Kokkotos, E.; Zotos, A.; Patakas, A. The Ecophysiological Response of Olive Trees under Different Fruit Loads. Life 2024, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Naor, A.; Schneider, D.; Ben-Gal, A.; Zipori, I.; Dag, A.; Kerem, Z.; Birger, R.; Peres, M.; Gal, Y. The Effects of Crop Load and Irrigation Rate in the Oil Accumulation Stage on Oil Yield and Water Relations of “Koroneiki” Olives. Irrig Sci 2013, 31, 781–791. [Google Scholar] [CrossRef]
- Bustan, A.; Dag, A.; Yermiyahu, U.; Erel, R.; Presnov, E.; Agam, N.; Kool, D.; Iwema, J.; Zipori, I.; Ben-Gal, A. Fruit Load Governs Transpiration of Olive Trees. Tree Physiol 2016, 36, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Goldhamer, D.A. Regulated Deficit Irrigation for California Canning Olives. Acta Hortic 1999, 369–372. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Moriana, A.; Corell, M.; Girón, I.F.; Morales, D.; Torrecillas, A.; Moreno, F. Low Water Stress Conditions in Table Olive Trees (Olea Europaea L.) during Pit Hardening Produced a Different Response of Fruit and Leaf Water Relations. Agric Water Manag 2012, 114, 11–17. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; FAO, 2012; ISBN 9789251072745. [Google Scholar]
- Oteros, J.; García-Mozo, H.; Vázquez, L.; Mestre, A.; Domínguez-Vilches, E.; Galán, C. Modelling Olive Phenological Response to Weather and Topography. Agric Ecosyst Environ 2013, 179, 62–68. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Pérez-López, D.; Hammami, S.B.M.; Agüera, J.; Moriana, A. Fruit Pit Hardening: Physical Measurement during Olive Fruit Growth. Annals of Applied Biology 2013, 163, 200–208. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and Drainage Paper No. 56 - Crop Evapotranspiration, 1998.
- Kokkotos, E.; Zotos, A.; Patakas, A. Evaluation of Water Stress Coefficient Ks in Different Olive Orchards. Agronomy 2020, 10, 11. [Google Scholar] [CrossRef]
- Kokkotos, E.; Zotos, A.; Tsirogiannis, G.; Patakas, A. Prediction of Olive Tree Water Requirements under Limited Soil Water Availability, Based on Sap Flow Estimations. Agronomy 2021, 11. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An Improved Heat Pulse Method to Measure Low and Reverse Rates of Sap Flow in Woody Plants. Tree Physiol 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Nadezhdin, V.; Ferreira, M.I.; Pitacco, A. Variability with Xylem Depth in Sap Flow in Trunks and Branches of Mature Olive Trees. Tree Physiol 2007, 27, 105–113. [Google Scholar] [CrossRef]
- Moreno, F.; Fernández, J.E.; Clothier, B.E.; Green, S.R. Transpiration and Root Water Uptake by Olive Trees. Plant Soil 1996, 184, 85–96. [Google Scholar] [CrossRef]
- Fuentes, S.; Mahadevan, M.; Bonada, M.; Skewes, M.A.; Cox, J.W. Night-Time Sap Flow Is Parabolically Linked to Midday Water Potential for Field-Grown Almond Trees. Irrig Sci 2013, 31, 1265–1276. [Google Scholar] [CrossRef]
- López-Bernal, Á.; García-Tejera, O.; Vega, V.A.; Hidalgo, J.C.; Testi, L.; Orgaz, F.; Villalobos, F.J. Using Sap Flow Measurements to Estimate Net Assimilation in Olive Trees under Different Irrigation Regimes. Irrig Sci 2015, 33, 357–366. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Fernández, J.E.; Rodriguez-Dominguez, C.M.; Romero, R.; Diaz-Espejo, A. The Dynamics of Radial Sap Flux Density Reflects Changes in Stomatal Conductance in Response to Soil and Air Water Deficit. Agric For Meteorol 2016, 218–219, 92–101. [Google Scholar] [CrossRef]
- Phillips, N.G.; Lewis, J.D.; Logan, B.A.; Tissue, D.T. Inter- and Intra-Specific Variation in Nocturnal Water Transport in Eucalyptus. Tree Physiol 2010, 30, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Baldocchi, D.D.; Misson, L.; Dawson, T.E.; Goldstein, A.H. What the Towers Don’t See at Night: Nocturnal Sap Flow in Trees and Shrubs at Two AmeriFlux Sites in California. Tree Physiol 2007, 27, 597–610. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding Olive Adaptation to Abiotic Stresses as a Tool to Increase Crop Performance. Environ Exp Bot 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Connor, D.J. Adaptation of Olive (Olea Europaea L.) to Water-Limited Environments. Aust J Agric Res 2005, 56, 1181–1189. [Google Scholar] [CrossRef]
- Connor, D.J.; Fereres, E. The Physiology of Adaptation and Yield Expression in Olive. In Horticultural Reviews; 2005; Vol. 31, pp. 155–229. ISBN 9780470650882. [Google Scholar]
- Chartzoulakis, K.; Patakas, A.; Bosabalidis, A.M. Changes in Water Relations, Photosynthesis and Leaf Anatomy Induced by Intermittent Drought in Two Olive Cultivars. Environ Exp Bot 1999, 42, 113–120. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M.G. Sclerophylly and Leaf Anatomical Traits of Five Field-Grown Olive Cultivars Growing under Drought Conditions. Tree Physiol 2004, 24, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.M. Regulatory Mechanisms in Source Sink Relationships in Plants-a Review. In Proceedings of the International Symposium on Source-Sink Relationships in Plants; Ron’zhina, E.S., Blanke, M., Eds.; ISHS, Acta Horticulturae 835, 2007; pp. 13–20.
- Haouari, A.; Van Labeke, M.-C.; Steppe, K.; Mariem, F. Ben; Braham, M.; Chaieb, M. Fruit Thinning Affects Photosynthetic Activity, Carbohydrate Levels, and Shoot and Fruit Development of Olive Trees Grown under Semiarid Conditions. Functional Plant Biology 2013, 40, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Paoletti, A.; Al Hariri, R.; Morelli, A.; Famiani, F. Resource Investments in Reproductive Growth Proportionately Limit Investments in Whole-Tree Vegetative Growth in Young Olive Trees with Varying Crop Loads. Tree Physiol 2018, 38, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Trentacoste, E.R.; Sadras, V.O.; Puertas, C.M. Effects of the Source:Sink Ratio on the Phenotypic Plasticity of Stem Water Potential in Olive (Olea Europaea L.). J Exp Bot 2011, 62, 3535–3543. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Fernández, J.E.; Torres-Ruiz, J.M.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Hernandez-Santana, V. Chapter 18 - The Olive Tree Under Water Stress: Fitting the Pieces of Response Mechanisms in the Crop Performance Puzzle. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; García Tejero, I.F., Durán Zuazo, V.H., Eds.; Academic Press, 2018; pp. 439–479. ISBN 978-0-12-813164-0. [Google Scholar]
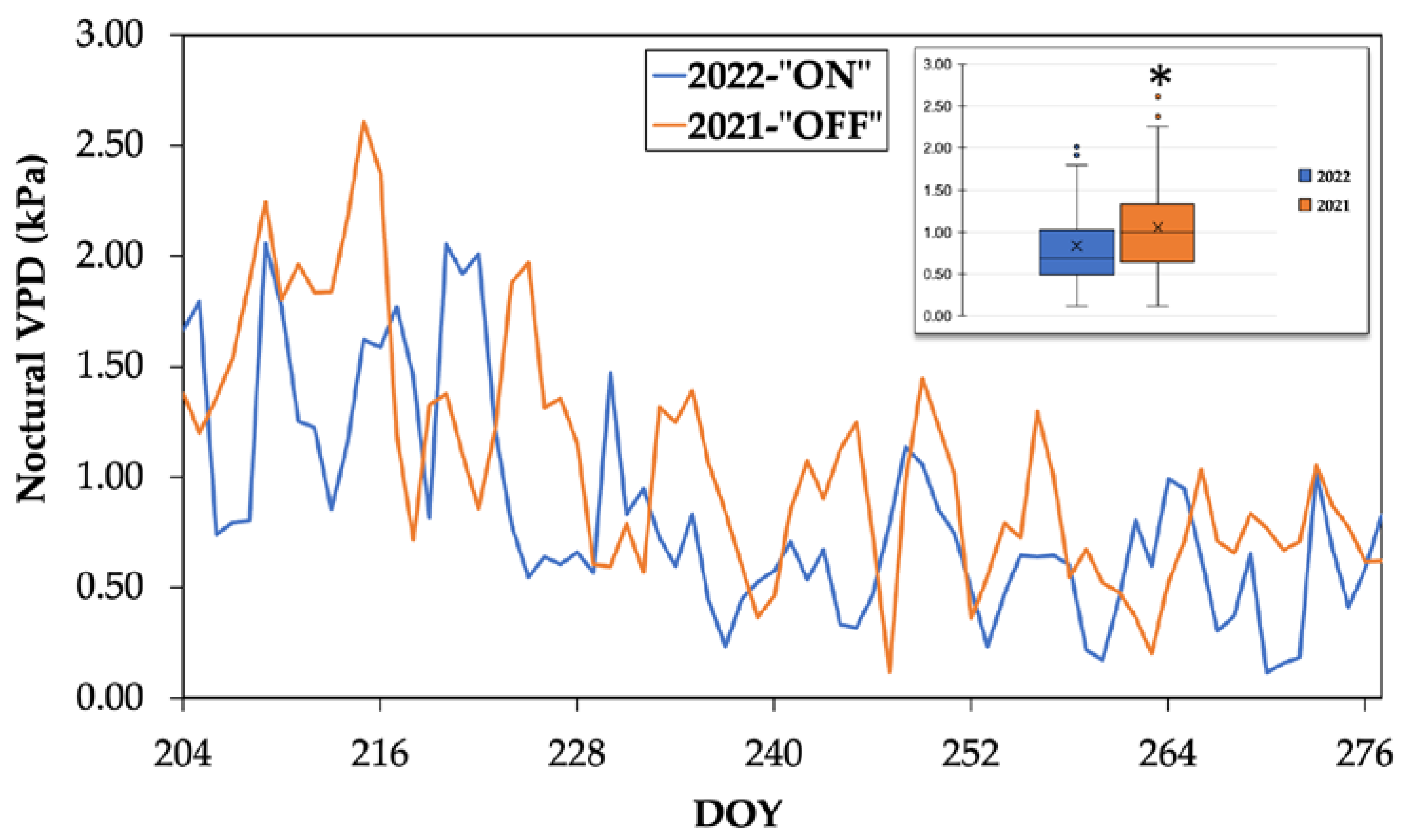
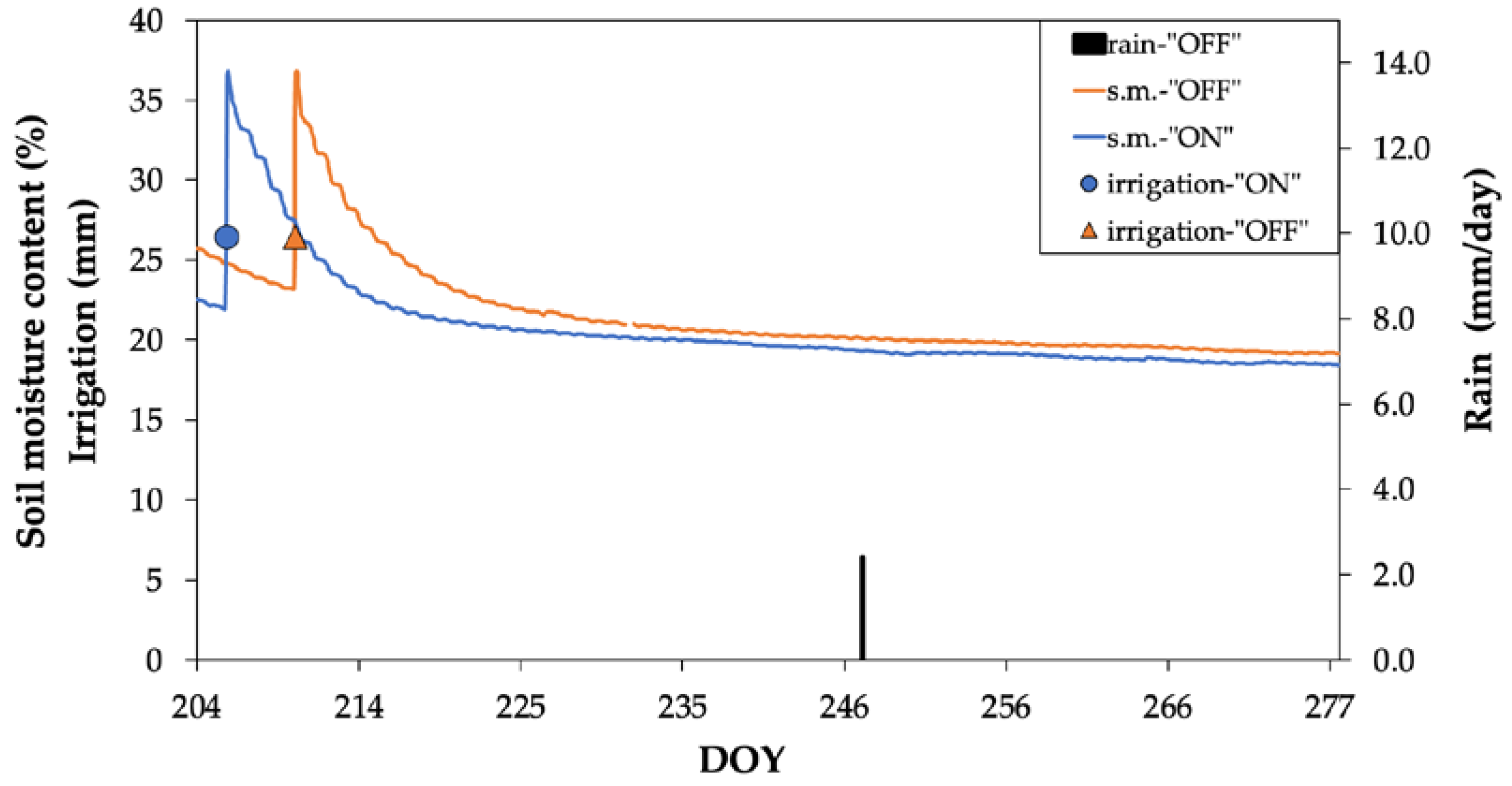
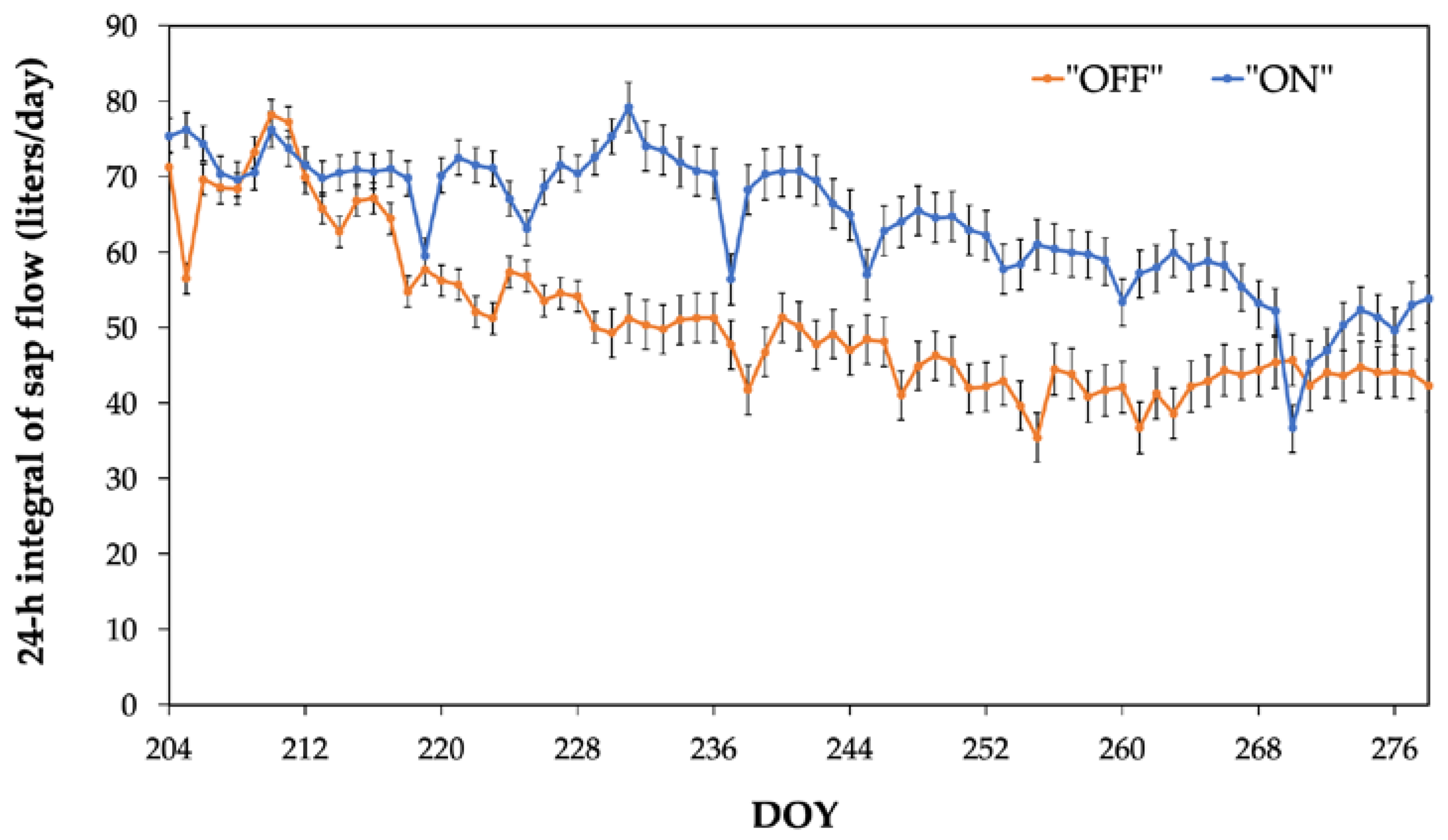
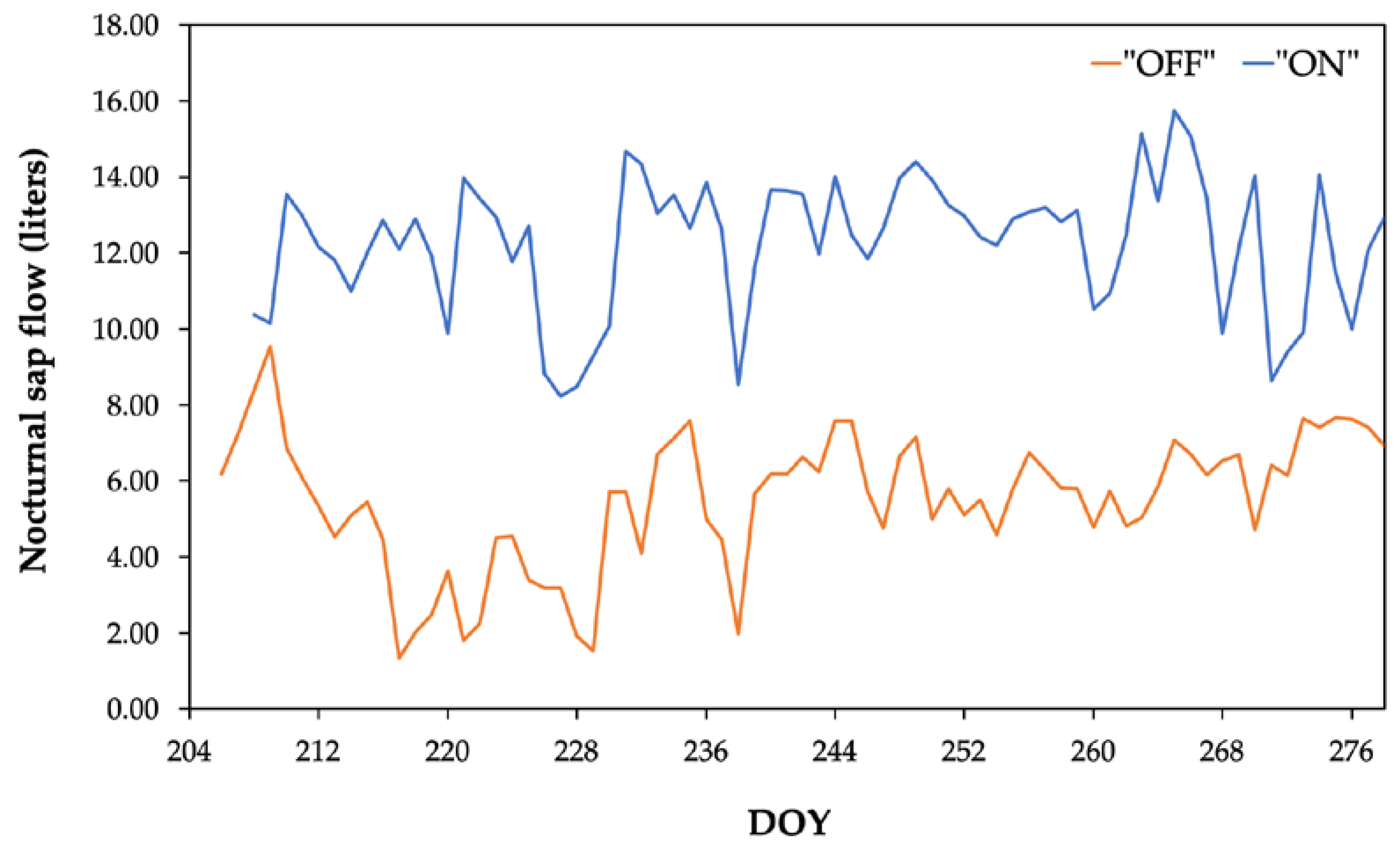
| Experimental year | Average VPD during the experimental period | Minimum VPD value | Maximum VPD value |
|---|---|---|---|
| 2021- “OFF” | 1.06±0.53 a | 0.12 | 2.61 |
| 2022- “ON” | 0.83±0.49 b | 0.11 | 2.06 |
| Experimental year | Average SFnight during the experimental period (Liter) | Minimum SFnight value (Liter) | Maximum SFnight value (Liter) |
|---|---|---|---|
| 2021- “OFF” | 5.33±1.70 a | 1.33 | 7.67 |
| 2022- “ON” | 12.31±1.76 b | 8.23 | 15.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





