Submitted:
28 February 2024
Posted:
29 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of 9"-O-tert-butyldimethylsilyl-silybin A (9a) and 9"-O-tert-buthyldimethylsilyl-silybin B (9b)
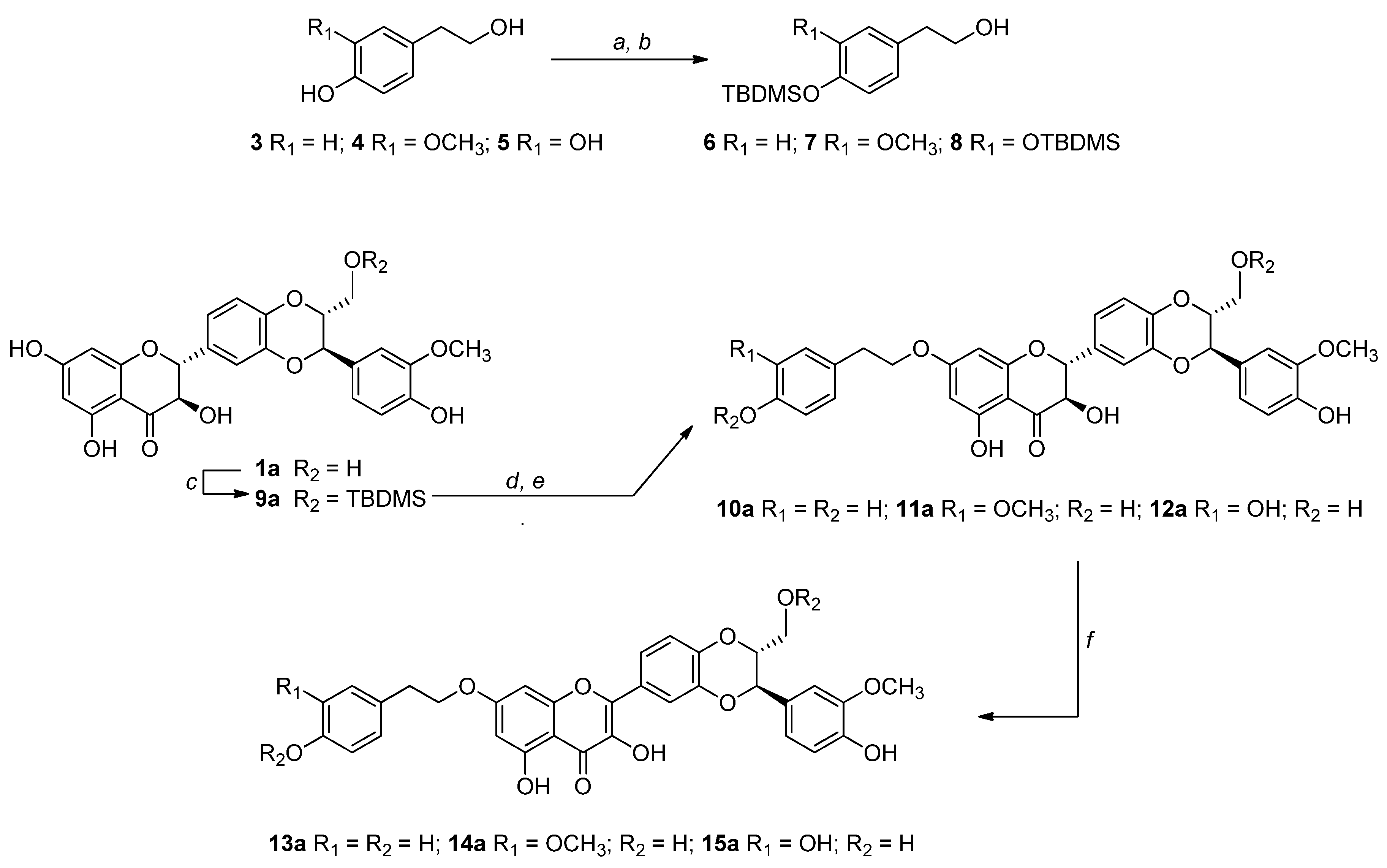
2.3. Alkylation by Mitsunobu Reaction: General Procedure for the Synthesis of 10a – 12a.
2.4. Synthesis of 7-O-tyrosyl-2,3-dehydro silybin derivatives (13a − 15a): General procedure
2.5. Medium and Chemical Stability
2.6. DPPH assay
2.7. ORAC Assay
2.8. Cell culture, Reagents, and treatments
2.9. Cell Growth and Death Assay
2.10. Flow cytometry for apoptosis and cell cycle determination
2.11. Lysate Preparation and Immunoblot Analysis
2.12. Statistical analysis
3. Results
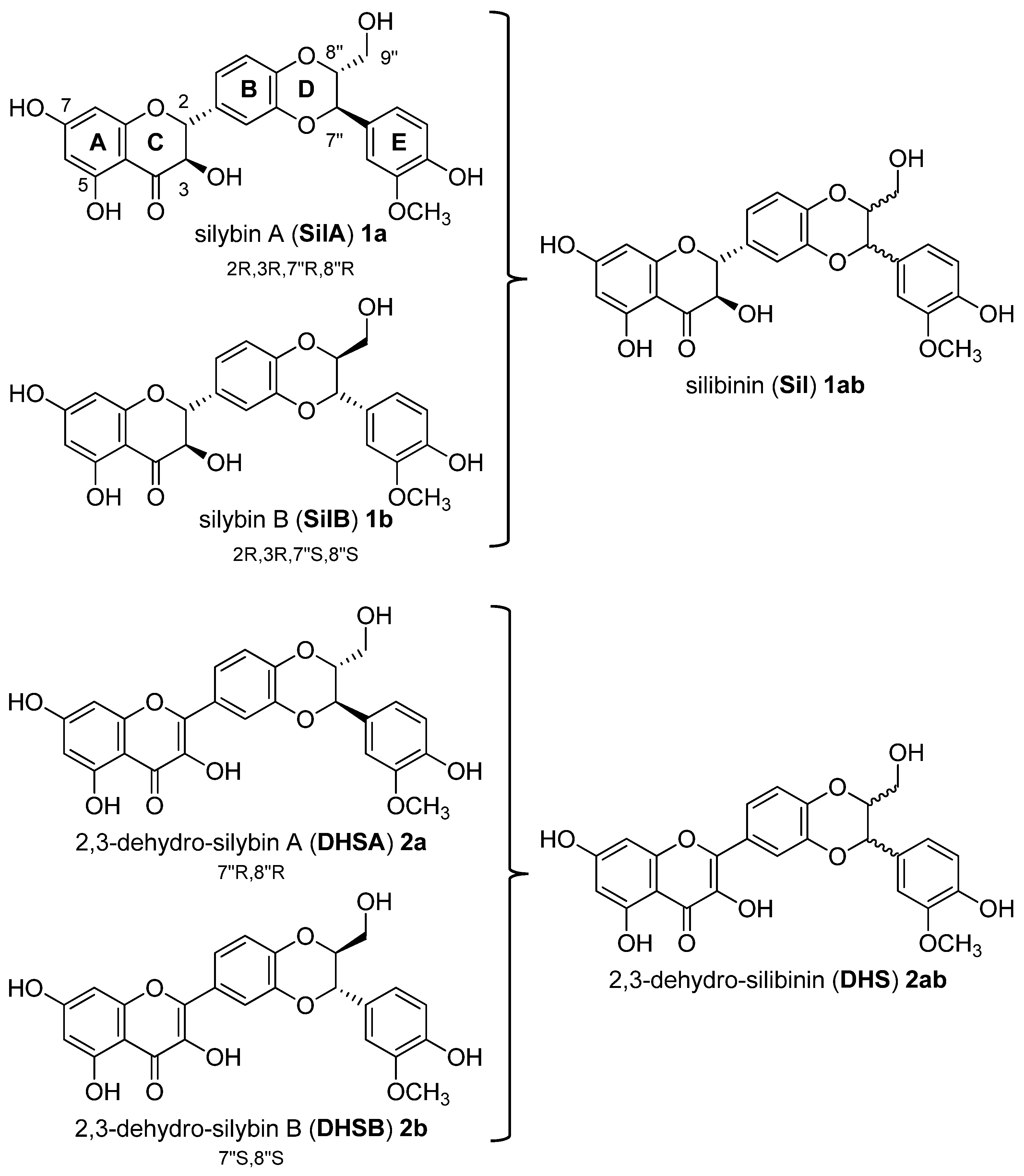
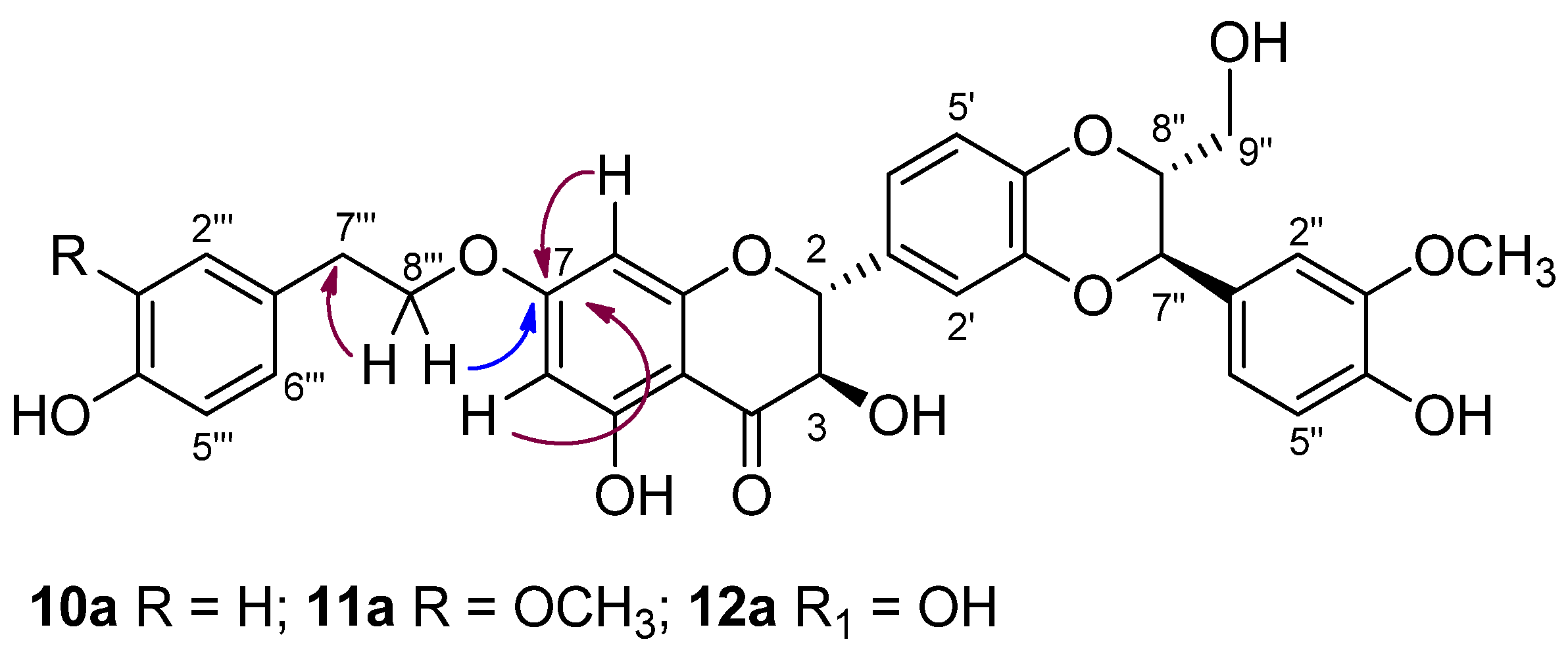
3.1. Synthesis, NMR Characterization and Chemical Stability
3.2. Radical Scavenger Properties (DPPH and ORAC Assays)
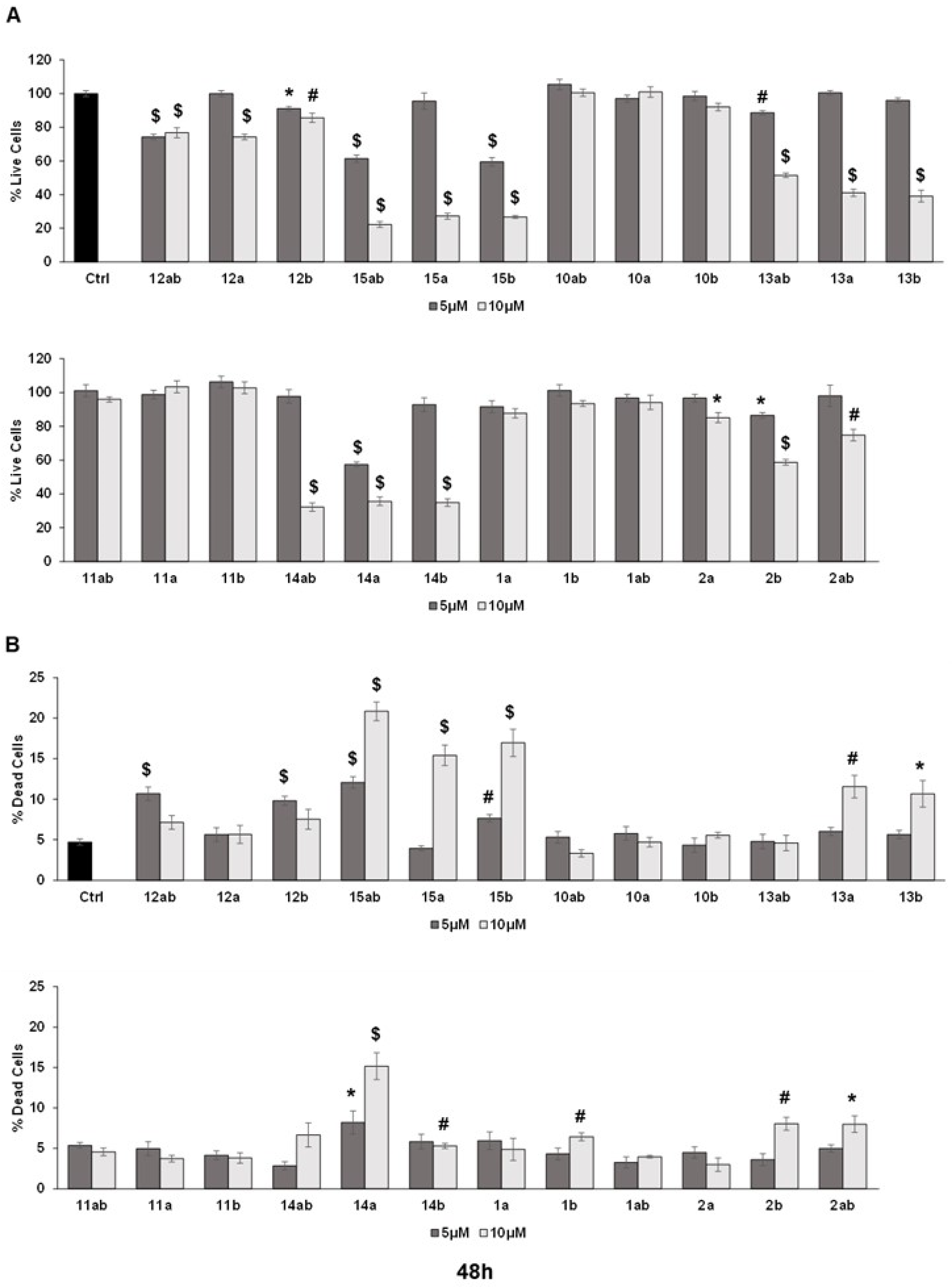
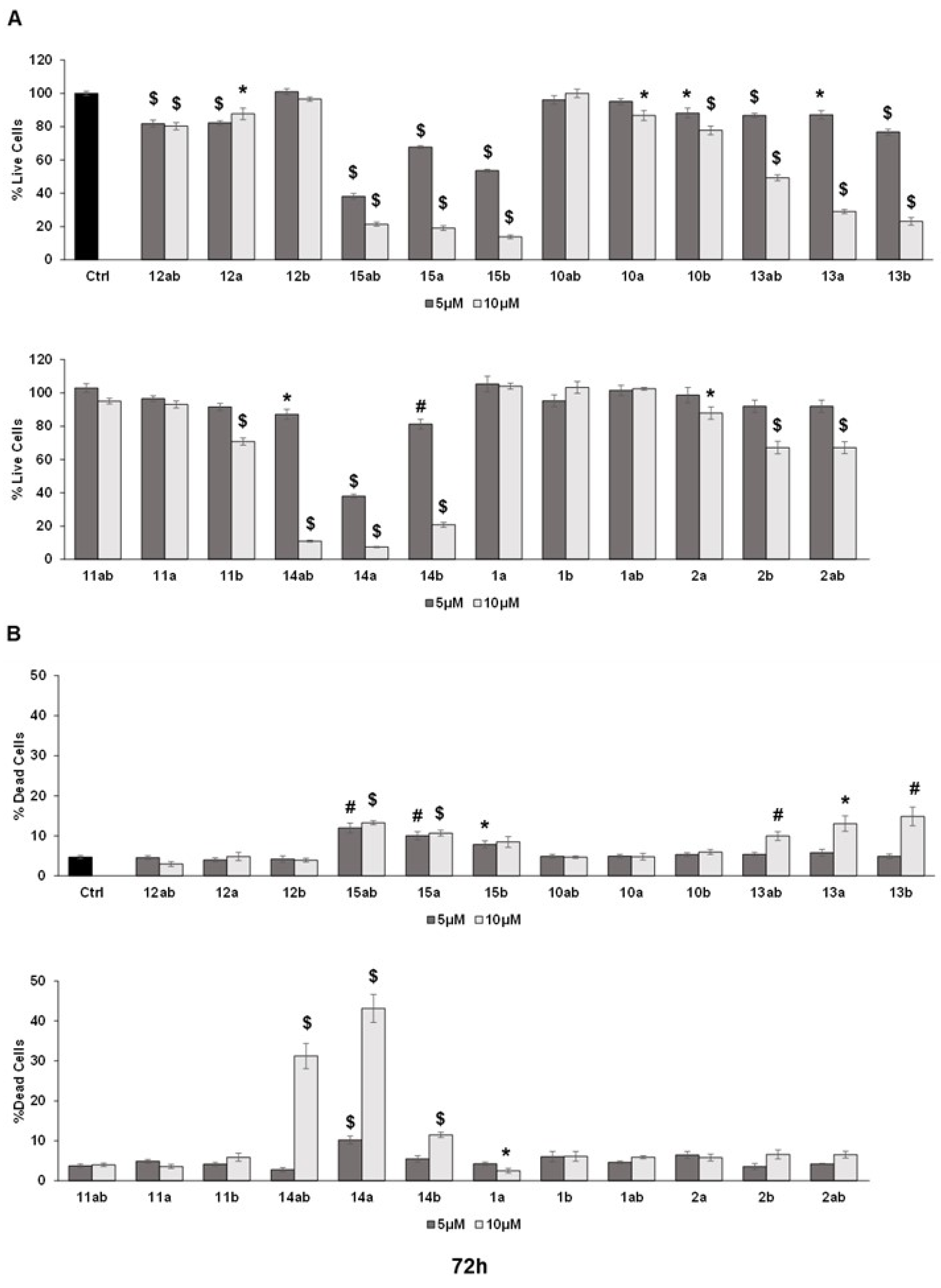
3.3. Anti-Proliferative Effects towards Prostate Cancer Cell Lines
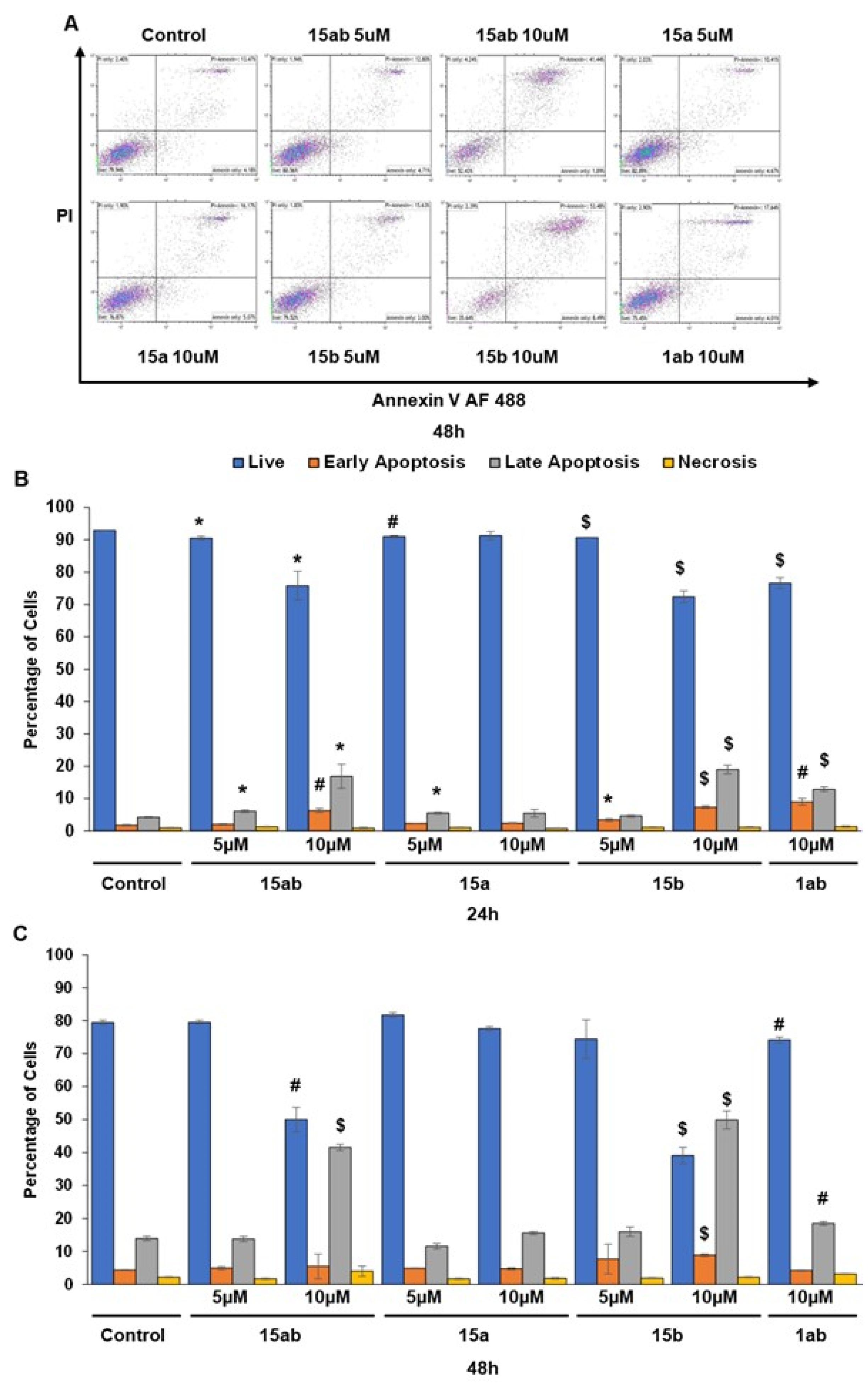
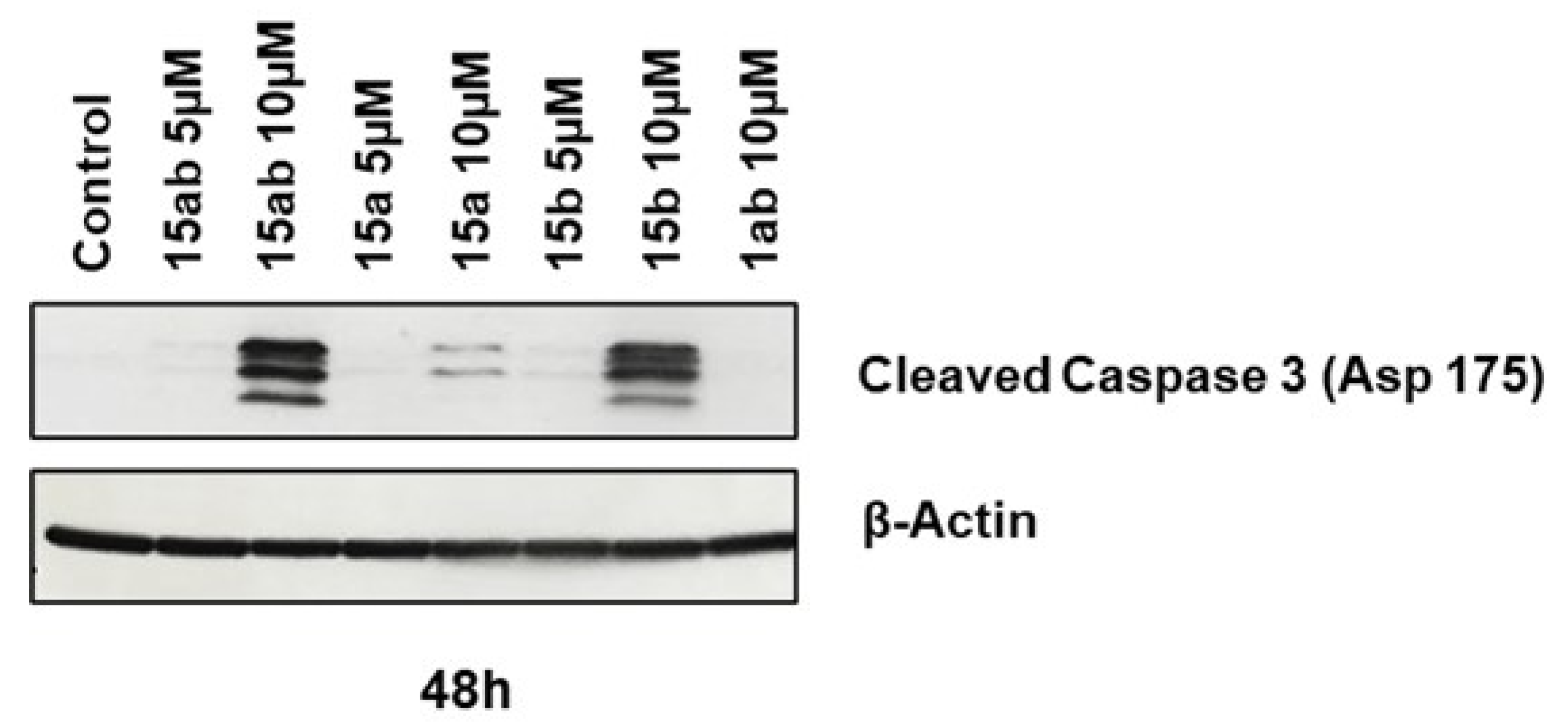
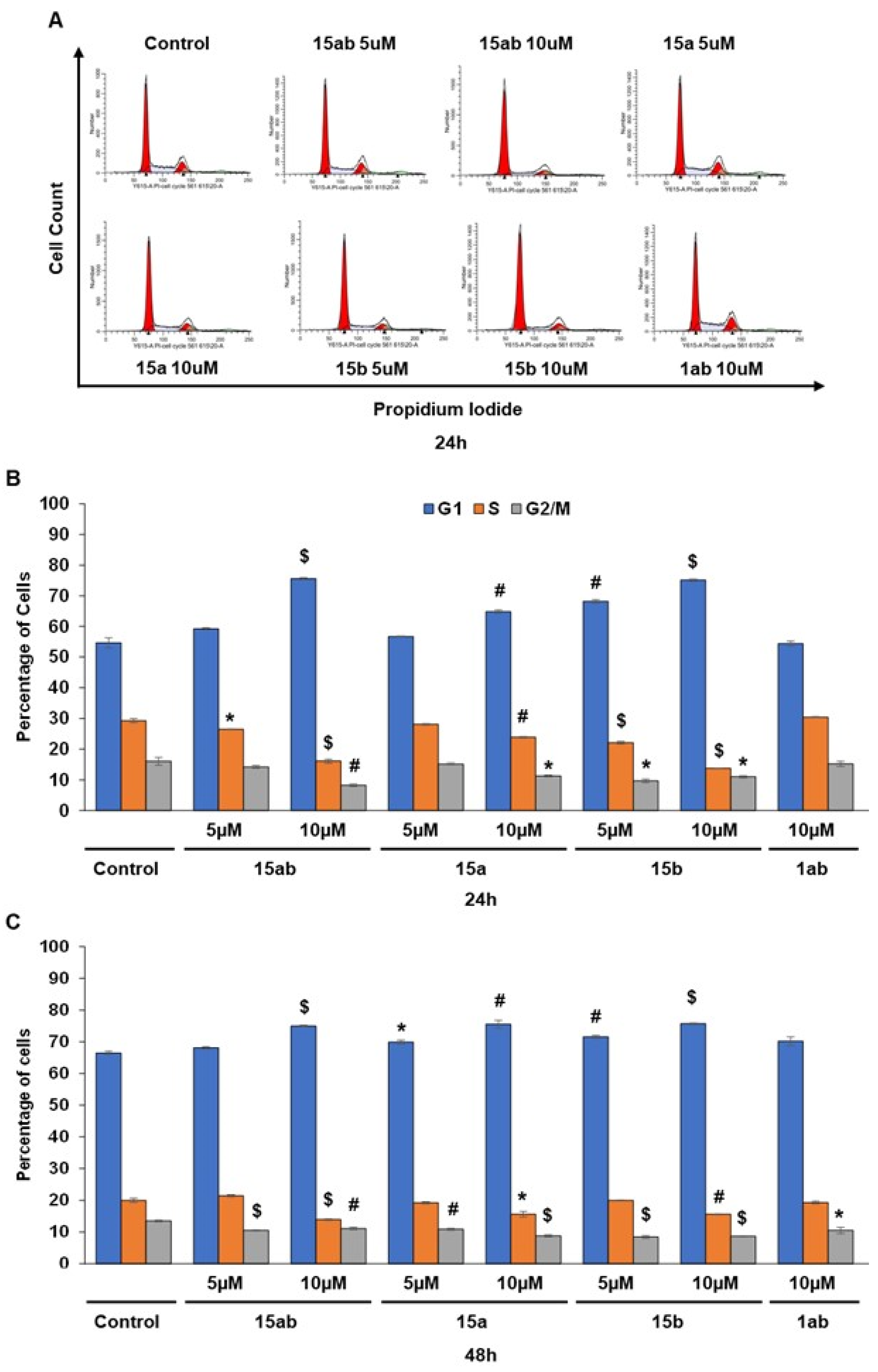
3.4. Effects of 7-O-tyrosyl 2,3-dehydro-silybin Derivatives on PC-3 Apoptosis and Cell Cycle Progression
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J. Natural Products as Leads to Potential Drugs: An Old Process or the New Hope for Drug Discovery? J Med Chem 2008, 51, 2589–2599. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat Rev Drug Discov 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie International Edition 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat Rev Mol Cell Biol 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sassetti, E.; Clausen, M.H.; Laraia, L. Small-Molecule Inhibitors of Reactive Oxygen Species Production. J Med Chem 2021, 64, 5252–5275. [Google Scholar] [CrossRef]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative Stress in Alzheimer’s Disease: Are We Connecting the Dots? J Med Chem 2014, 57, 2821–2831. [Google Scholar] [CrossRef]
- Kirtonia, A.; Sethi, G.; Garg, M. The Multifaceted Role of Reactive Oxygen Species in Tumorigenesis. Cellular and Molecular Life Sciences 2020, 77, 4459–4483. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and Silymarin - New and Emerging Applications in Medicine. Curr Med Chem 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat Prod Rep 2014, 31, 1138. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete Isolation and Characterization of Silybins and Isosilybins from Milk Thistle (Silybum Marianum)Electronic Supplementary Information (ESI) Available: HPLC Chromatograms of Isolates and Extracts. See Http://Www.Rsc.Org/Suppdata/Ob/B3/B300099k/. Org Biomol Chem 2003, 1, 1684. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojović, M.; Popović-Bijelić, A.; et al. Flavonolignan 2,3-Dehydroderivatives: Preparation, Antiradical and Cytoprotective Activity. Free Radic Biol Med 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Polachi, N.; Bai, G.; Li, T.; Chu, Y.; Wang, X.; Li, S.; Gu, N.; Wu, J.; Li, W.; Zhang, Y.; et al. Modulatory Effects of Silibinin in Various Cell Signaling Pathways against Liver Disorders and Cancer – A Comprehensive Review. Eur J Med Chem 2016, 123, 577–595. [Google Scholar] [CrossRef]
- Flaig, T.W.; Glodé, M.; Gustafson, D.; van Bokhoven, A.; Tao, Y.; Wilson, S.; Su, L.-J.; Li, Y.; Harrison, G.; Agarwal, R.; et al. A Study of High-Dose Oral Silybin-Phytosome Followed by Prostatectomy in Patients with Localized Prostate Cancer. Prostate 2010, 70, 848–855. [Google Scholar] [CrossRef]
- Flaig, T.W.; Gustafson, D.L.; Su, L.-J.J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A Phase I and Pharmacokinetic Study of Silybin-Phytosome in Prostate Cancer Patients. Invest New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef]
- Gažák, R.; Svobodová, A.; Psotová, J.; Sedmera, P.; Přikrylová, V.; Walterová, D.; Křen, V. Oxidised Derivatives of Silybin and Their Antiradical and Antioxidant Activity. Bioorg Med Chem 2004, 12, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Di Fabio, G.; Romanucci, V.; Di Marino, C.; De Napoli, L.; Zarrelli, A. A Rapid and Simple Chromatographic Separation of Diastereomers of Silibinin and Their Oxidation to Produce 2,3-Dehydrosilybin Enantiomers in an Optically Pure Form. Planta Med 2013, 79, 1077–1080. [Google Scholar] [CrossRef]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-Cancer Efficacy of Silybin Derivatives - A Structure-Activity Relationship. PLoS One 2013, 8, e60074. [Google Scholar] [CrossRef]
- Han, Y.H.; Lou, H.X.; Ren, D.M.; Sun, L.R.; Ma, B.; Ji, M. Stereoselective Metabolism of Silybin Diastereoisomers in the Glucuronidation Process. J Pharm Biomed Anal 2004, 34, 1071–1078. [Google Scholar] [CrossRef]
- Morazzoni, P.; Montalbetti, A.; Malandrino, S.; Pifferi, G. Comparative Pharmacokinetics of Silipide and Silymarin in Rats. Eur J Drug Metab Pharmacokinet 1993, 18, 289–297. [Google Scholar] [CrossRef]
- Wu, J.-W.; Lin, L.-C.; Hung, S.-C.; Chi, C.-W.; Tsai, T.-H. Analysis of Silibinin in Rat Plasma and Bile for Hepatobiliary Excretion and Oral Bioavailability Application. J Pharm Biomed Anal 2007, 45, 635–641. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Zhang, X.; Parisis, K.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.H. Silibinin Derivatives as Anti-Prostate Cancer Agents: Synthesis and Cell-Based Evaluations. Eur J Med Chem 2016, 109, 36–46. [Google Scholar] [CrossRef]
- Karas, D.; Gažák, R.; Valentová, K.; Chambers, C.S.; Pivodová, V.; Biedermann, D.; Křenková, A.; Oborná, I.; Kuzma, M.; Cvačka, J.; et al. Effects of 2,3-Dehydrosilybin and Its Galloyl Ester and Methyl Ether Derivatives on Human Umbilical Vein Endothelial Cells. J Nat Prod 2016, 79, 812–820. [Google Scholar] [CrossRef]
- Huber, A.; Thongphasuk, P.; Erben, G.; Lehmann, W.-D.; Tuma, S.; Stremmel, W.; Chamulitrat, W. Significantly Greater Antioxidant Anticancer Activities of 2,3-Dehydrosilybin than Silybin. Biochim Biophys Acta 2008, 1780, 837–847. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, X.; Lee, T.; Nair, N.; Zhang, S.; Chen, G.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. 5- or/and 20- O -Alkyl-2,3-Dehydrosilybins: Synthesis and Biological Profiles on Prostate Cancer Cell Models. Bioorg Med Chem 2017, 25, 4845–4854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Vue, B.; Huang, M.; Zhang, X.; Lee, T.; Chen, G.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.-H. 3- O -Alkyl-2,3-Dehydrosilibinins: Two Synthetic Approaches and in Vitro Effects toward Prostate Cancer Cells. Bioorg Med Chem Lett 2016, 26, 3226–3231. [Google Scholar] [CrossRef]
- Křen, V. Chirality Matters: Biological Activity of Optically Pure Silybin and Its Congeners. Int J Mol Sci 2021, 22, 7885. [Google Scholar] [CrossRef]
- Gažák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Křen, V. Molecular Mechanisms of Silybin and 2,3-Dehydrosilybin Antiradical Activity—Role of Individual Hydroxyl Groups. Free Radic Biol Med 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, Michele. F.M.; Romanucci, V.; Zarrelli, A.; Monaco, I.; Lolicato, F.; Spinella, N.; Galati, C.; Grasso, G.; D’Urso, L.; Romeo, M.; et al. Inhibition of Aβ Amyloid Growth and Toxicity by Silybins: The Crucial Role of Stereochemistry. ACS Chem Neurosci 2017, 8, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- García-Viñuales, S.; Ilie, I.M.; Santoro, A.M.; Romanucci, V.; Zarrelli, A.; Di Fabio, G.; Caflisch, A.; Milardi, D. Silybins Inhibit Human IAPP Amyloid Growth and Toxicity through Stereospecific Interactions. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 2022, 1870, 140772. [Google Scholar] [CrossRef]
- Persico, M.; García-Viñuales, S.; Santoro, A.M.; Lanza, V.; Tundo, G.R.; Sbardella, D.; Coletta, M.; Romanucci, V.; Zarrelli, A.; Di Fabio, G.; et al. Silybins Are Stereospecific Regulators of the 20S Proteasome. Bioorg Med Chem 2022, 66, 116813. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, D.; Saez, I.; Dillin, A. The Role of Protein Clearance Mechanisms in Organismal Ageing and Age-Related Diseases. Nat Commun 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tundo, G.R.; Sbardella, D.; Santoro, A.M.; Coletta, A.; Oddone, F.; Grasso, G.; Milardi, D.; Lacal, P.M.; Marini, S.; Purrello, R.; et al. The Proteasome as a Druggable Target with Multiple Therapeutic Potentialities: Cutting and Non-Cutting Edges. Pharmacol Ther 2020, 213. [Google Scholar] [CrossRef] [PubMed]
- García-Viñuales, S.; Ahmed, R.; Sciacca, M.F.M.; Lanza, V.; Giuffrida, M.L.; Zimbone, S.; Romanucci, V.; Zarrelli, A.; Bongiorno, C.; Spinella, N.; et al. Trehalose Conjugates of Silybin as Prodrugs for Targeting Toxic Aβ Aggregates. ACS Chem Neurosci 2020, 11, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Agarwal, C.; Agarwal, R.; Pannecouque, C.; Iuliano, M.; De Tommaso, G.; Caruso, T.; Di Fabio, G.; Zarrelli, A. Silibinin Phosphodiester Glyco-Conjugates: Synthesis, Redox Behaviour and Biological Investigations. Bioorg Chem 2018, 77, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Giordano, M.; Pagano, R.; Zimbone, S.; Giuffrida, M.L.; Milardi, D.; Zarrelli, A.; Di Fabio, G. Investigation on the Solid-Phase Synthesis of Silybin Prodrugs and Their Timed-Release. Bioorg Med Chem 2021, 50, 116478. [Google Scholar] [CrossRef]
- Romanucci, V.; Giordano, M.; De Tommaso, G.; Iuliano, M.; Bernini, R.; Clemente, M.; Garcia-Viñuales, S.; Milardi, D.; Zarrelli, A.; Di Fabio, G. Synthesis of New Tyrosol-Based Phosphodiester Derivatives: Effect on Amyloid β Aggregation and Metal Chelation Ability. ChemMedChem 2021, 16, 1172–1183. [Google Scholar] [CrossRef]
- Kandhari, K.; Mishra, J.P.N.; Agarwal, R.; Singh, R.P. Acacetin Induces Sustained ERK1/2 Activation and RIP1-Dependent Necroptotic Death in Breast Cancer Cells. Toxicol Appl Pharmacol 2023, 462. [Google Scholar] [CrossRef]
- Džubák, P.; Hajdúch, M.; Gažák, R.; Svobodová, A.; Psotová, J.; Walterová, D.; Sedmera, P.; Křen, V. New Derivatives of Silybin and 2,3-Dehydrosilybin and Their Cytotoxic and P-Glycoprotein Modulatory Activity. Bioorg Med Chem 2006, 14, 3793–3810. [Google Scholar] [CrossRef]
- Althagafy, H.S.; Graf, T.N.; Sy-Cordero, A.A.; Gufford, B.T.; Paine, M.F.; Wagoner, J.; Polyak, S.J.; Croatt, M.P.; Oberlies, N.H. Semisynthesis, Cytotoxicity, Antiviral Activity, and Drug Interaction Liability of 7-O-Methylated Analogues of Flavonolignans from Milk Thistle. Bioorg Med Chem 2013, 21, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Huang, K.X.; Li, H.B.; Gong, J.X.; Wang, F.; Feng, Y.B.; Tao, Q.F.; Wu, Y.H.; Li, X.K.; Wu, X.M.; et al. Design, Synthesis, and Examination of Neuron Protective Properties of Alkenylated and Amidated Dehydro-Silybin Derivatives †. J Med Chem 2009, 52, 7732–7752. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. The Mitsunobu Reaction in the 21 St Century. Organic Chemistry Frontiers 2015, 2, 739–752. [Google Scholar] [CrossRef]
- Zarrelli, A.; Sgambato, A.; Petito, V.; De Napoli, L.; Previtera, L.; Di Fabio, G. New C-23 Modified of Silybin and 2,3-Dehydrosilybin: Synthesis and Preliminary Evaluation of Antioxidant Properties. Bioorg Med Chem Lett 2011, 21, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Schramm, S.; Heilmann, J.; Biedermann, D.; Křen, V.; Decker, M. Unconventional Application of the Mitsunobu Reaction: Selective Flavonolignan Dehydration Yielding Hydnocarpins. Beilstein Journal of Organic Chemistry 2016, 12, 662–669. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gažák, R.; Hrbáč, J.; Sedmera, P.; Křen, V.; Lazzaroni, R.; Duroux, J.; et al. Mechanism of the Antioxidant Action of Silybin and 2,3-Dehydrosilybin Flavonolignans: A Joint Experimental and Theoretical Study. J Phys Chem A 2008, 112, 1054–1063. [Google Scholar] [CrossRef]
- Gažák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Křen, V. Molecular Mechanisms of Silybin and 2,3-Dehydrosilybin Antiradical Activity—Role of Individual Hydroxyl Groups. Free Radic Biol Med 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Pagano, R.; Lembo, A.; Capasso, D.; Di Gaetano, S.; Zarrelli, A.; Di Fabio, G. Phosphodiester Silybin Dimers Powerful Radical Scavengers: A Antiproliferative Activity on Different Cancer Cell Lines. Molecules 2022, 27, 1702. [Google Scholar] [CrossRef]
- Romanucci, V.; Gravante, R.; Cimafonte, M.; Marino, C. Di; Mailhot, G.; Brigante, M.; Zarrelli, A.; Fabio, G. Di Phosphate-Linked Silibinin Dimers (PLSd): New Promising Modified Metabolites. Molecules 2017, 22, 1323. [Google Scholar] [CrossRef]
- Zarrelli, A.; Romanucci, V.; Tuccillo, C.; Federico, A.; Loguercio, C.; Gravante, R.; Di Fabio, G. New Silibinin Glyco-Conjugates: Synthesis and Evaluation of Antioxidant Properties. Bioorg Med Chem Lett 2014, 24, 5147–5149. [Google Scholar] [CrossRef]
- Zarrelli, A.; Romanucci, V.; De Napoli, L.; Previtera, L.; Di Fabio, G. Synthesis of New Silybin Derivatives and Evaluation of Their Antioxidant Properties. Helv Chim Acta 2015, 98, 399–409. [Google Scholar] [CrossRef]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as Source of Bioactive Compounds with Multi-Targeting Anti-Cancer Profile. Eur J Med Chem 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.-W.; Jiang, J.-G.; Xu, X.-L. Hydroxytyrosol and Its Potential Therapeutic Effects. J Agric Food Chem 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Deep, G.; Singh, R.P.; Agarwal, C.; Kroll, D.J.; Agarwal, R. Silymarin and Silibinin Cause G1 and G2-M Cell Cycle Arrest via Distinct Circuitries in Human Prostate Cancer PC3 Cells: A Comparison of Flavanone Silibinin with Flavanolignan Mixture Silymarin. Oncogene 2006, 25, 1053–1069. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-Cancer Efficacy of Silybin Derivatives - A Structure-Activity Relationship. PLoS One 2013, 8, e60074. [Google Scholar] [CrossRef] [PubMed]
| Compound | Yield (%)a | ORAC (TE)b | DPPH (EC50, µM) |
|---|---|---|---|
| Sil (1ab) | – | 4.76±0.20 | 620 ± 0.2 |
| SilA (1a) | – | 4.21±0.16 | 360 ± 0.2 |
| SilB (1b) | – | 4.36±0.15 | 580 ± 0.5 |
| DHS (2ab) | – | 3.57±0.23 | 29.6±0.18 |
| DHSA (2a) | – | 3.88±0.20 | 15.5 ± 0.3 |
| DHSB (2b) | – | 3.86±0.13 | 11.6 ± 0.4 |
| TYR | – | 2.18±0.12 | >1000 |
| MTYR | – | 2.90±0.21 | 31.0 ± 2.6 |
| HTYR | – | 7.40±0.17 | 12.3 ± 1.0 |
| 10a | 35 | 4.81±0.45 | 290 ± 0.2 |
| 11a | 28 | 2.50±0.29 | 13.5 ± 0.6 |
| 12a | 26 | 5.57±0.17 | 6.53 ± 0.6 |
| 13a | 95 | 1.16±0.05 | 17.1 ± 0.1 |
| 14a | 78 | 1.25±0.02 | 9.01 ± 0.5 |
| 15a | 95 | 1.28±0.02 | 4.98 ± 0.4 |
| 10b | 83 | 4.89±0.22 | 220.0 ± 0.1 |
| 11b | 21 | 3.22±0.52 | 17.8 ± 0.6 |
| 12b | 65 | 5.38±0.17 | 5.65 ± 0.5 |
| 13b | 72 | 1.15±0.02 | 19.3 ± 0.1 |
| 14b | 82 | 1.32±0.04 | 9.10 ± 0.6 |
| 15b | 81 | 1.25±0.04 | 4.76 ± 0.4 |
| 10ab | 51 | 4.88±0.09 | 200 ± 0.2 |
| 11ab | 33 | 4.91±0.17 | 9.4 ± 0.5 |
| 12ab | 40 | 5.45±0.54 | 4.9 ± 0.6 |
| 13ab | 90 | 1.47±0.05 | 26.9 ± 0.2 |
| 14ab | 76 | 1.01±0.12 | 9.27 ± 0.6 |
| 15ab | 74 | 1.44±0.14 | 6.19 ± 0.7 |
| Position | 10a (DMSO-d6) | 11a (CD3OD) | 12a (CD3OD) | |||
|---|---|---|---|---|---|---|
| δH, (J in Hz) | δC type | δH, (J, Hz) | δC type | δH, (J, Hz) | δC type | |
| 2 | 5.13, d (11.3) | 83.1 | 4.99, d (11.3) | 83.4 | 4.97, d (11.5) | 83.1 |
| 3 | 4.65, dd (11.3) | 72.0 | 4.52, d (11.3) | 72.3 | 4.50, d (11.5) | 72.0 |
| 3-OH | 4.9, s | – | – | |||
| 4 | – | 198.7 | – | 197.3 | – | 198.7 |
| 4a | – | 101.8 | – | 101.1 | – | 101.7 |
| 5 | – | 163.4 | – | 163.5 | – | 163.4 |
| 6 | 6.10, d (2.3) | 95.7 | 6.06, d (2.1) | 95.2 | 6.05, d (2.0) | 95.7 |
| 7 | – | 167.2 | – | 167.6 | – | 167.2 |
| 8 | 6.08, d (2.3) | 94.6 | 6.01, d (2.1) | 94.1 | 6.00, d (2.0) | 94.6 |
| 8a | – | 162.8 | 162.8 | – | 162.8 | |
| 1' | – | 130.4 | 130.0 | – | 130.4 | |
| 2' | 7.09, s | 117.1 | 7.11, d (1.5) | 116.1 | 7.10, d (1.5) | 117.1 |
| 3' | – | 143.7 | – | 143.7 | – | 143.7 |
| 4' | – | 144.1 | – | 144.1 | – | 144.1 |
| 5' | 6.98, d (8.3) | 116.7 | 7.02-6.96, m | 116.4 | 6.98, d (8.3) | 116.7 |
| 6' | 7.04-7.00, m | 121.6 | 7.03, dd (8.2, 1.8) | 120.8 | 7.03, dd (8.3, 1.8) | 121.6 |
| 1'' | – | 127.9 | – | 127.9 | – | 127.9 |
| 2'' | 7.04-7.00, m | 112.0 | 7.02-6.96, m | 110.6 | 7.01, d (1.7) | 112.0 |
| 3'' | – | 148.0 | – | 147.5 | – | 148.0 |
| 4'' | – | 147.4 | – | 146.9 | – | 147.4 |
| 5'' | 6.80, d (8.1) | 115.9 | 6.87-6.83, m | 114.8 | 6.84, d (8.2) | 115.9 |
| 6'' | 6.87, dd (8.1, 1.7) | 120.9 | 6.91, dd (8.0, 1.7) | 120.2 | 6.90, dd (8.2, 1.7) | 120.9 |
| 7'' | 4.92, d (7.9) | 76.3 | 4.95-4.89, m | 76.2 | 4.93-4.83, m | 76.3 |
| 8" | 4.23-4.12, m | 78.5 | 4.09-4.03, m | 78.6 | 4.08-4.02, m | 78.5 |
| 9" | H-9"a 3.54, d (12.1) H-9"b 3.39-3.30, m |
60.6 | H-9"a 3.71, dd (12.4, 2.4) H-9"b 3.49, dd (12.3, 4.3) |
60.6 | H-9"a 3.70, dd (12.4, 2.2) H-9"b 3.49 dd (12.3, 4.4) |
60.6 |
| 3''-OMe | 3.78, s | 56.1 | 3.82, s | 54.9 | 3.86, s | 56.1 |
| 1''' | 128.2 | 129.3 | 129.0 | |||
| 2''' | 7.08, d (8.6) | 130.2 | 6.87–6.83, m | 112.2 | 6.71, d (1.7) | 116.7 |
| 3''' | 6.68, d (8.6) | 115.5 | – | 147.8 | – | 145.5 |
| 4''' | – | 156.3 | – | 144.7 | – | 144.2 |
| 5''' | 6.68, d (8.6) | 115.5 | 6.72, d (8.0) | 114.7 | 6.69, d (8.0) | 115.7 |
| 6''' | 7.08, d (8.6) | 130.2 | 6.69, dd (8.0, 1.6) | 121.1 | 6.57, dd (8.0, 1.7) | 120.0 |
| 7''' | 2.88, t (6.9) | 34.1 | 2.95, t (6.8) | 34.5 | 2.88, t (6.8) | 34.5 |
| 8''' | 4.23-4.12, m | 69.6 | 4.15, t, (6.8) | 69.3 | 4.10, t (6.8) | 69.6 |
| 3'''-OMe | – | - | 3.87, s | 55.0 | – | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





